Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Patients
2.1. Study Design and Participants
2.2. Data Collection and Assessment
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatments
3.3. Treatments in the Adjuvant Setting
3.4. Treatments in the Metastatic Setting
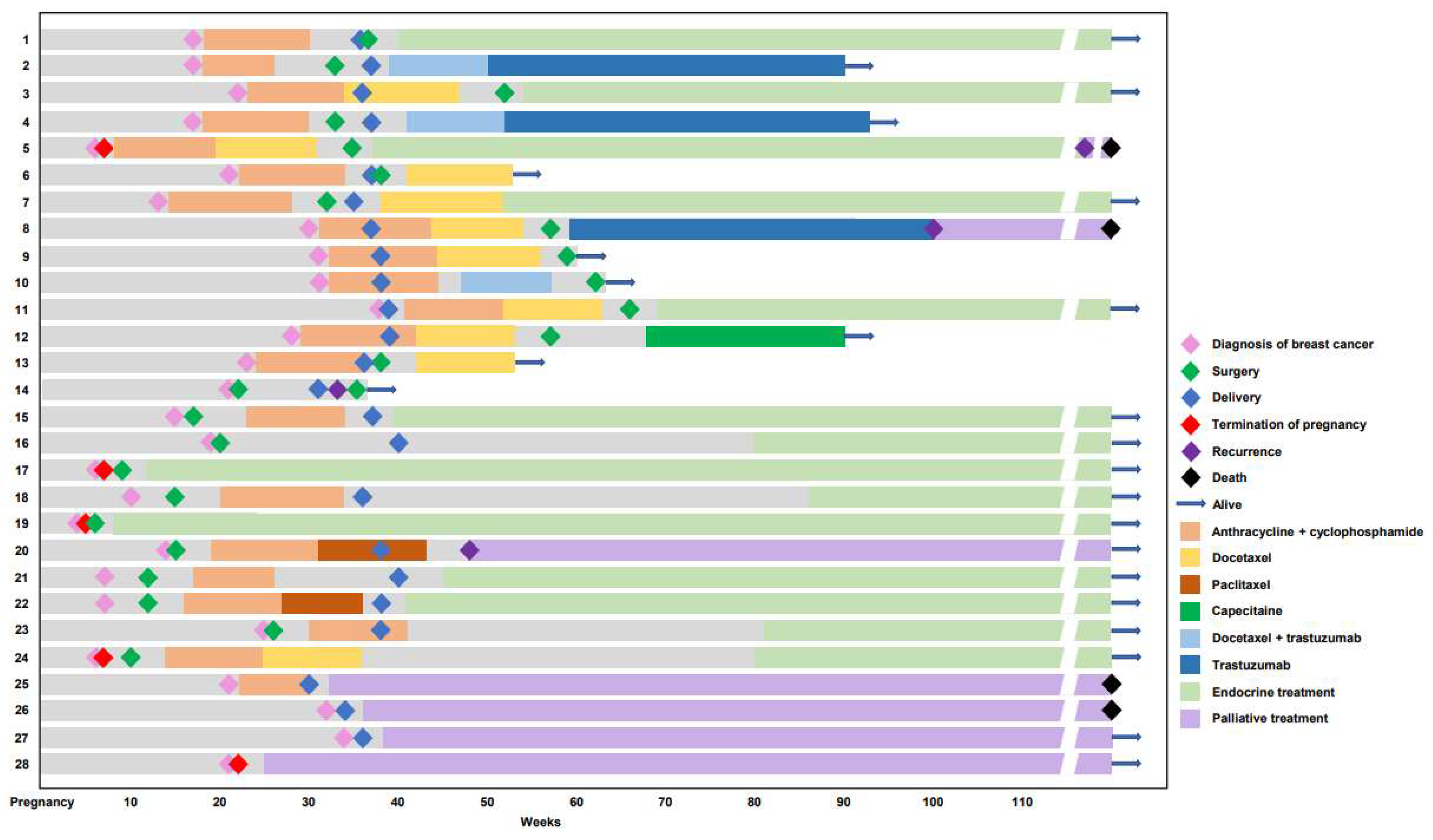
3.5. Subgroup Analysis of the PABC Group
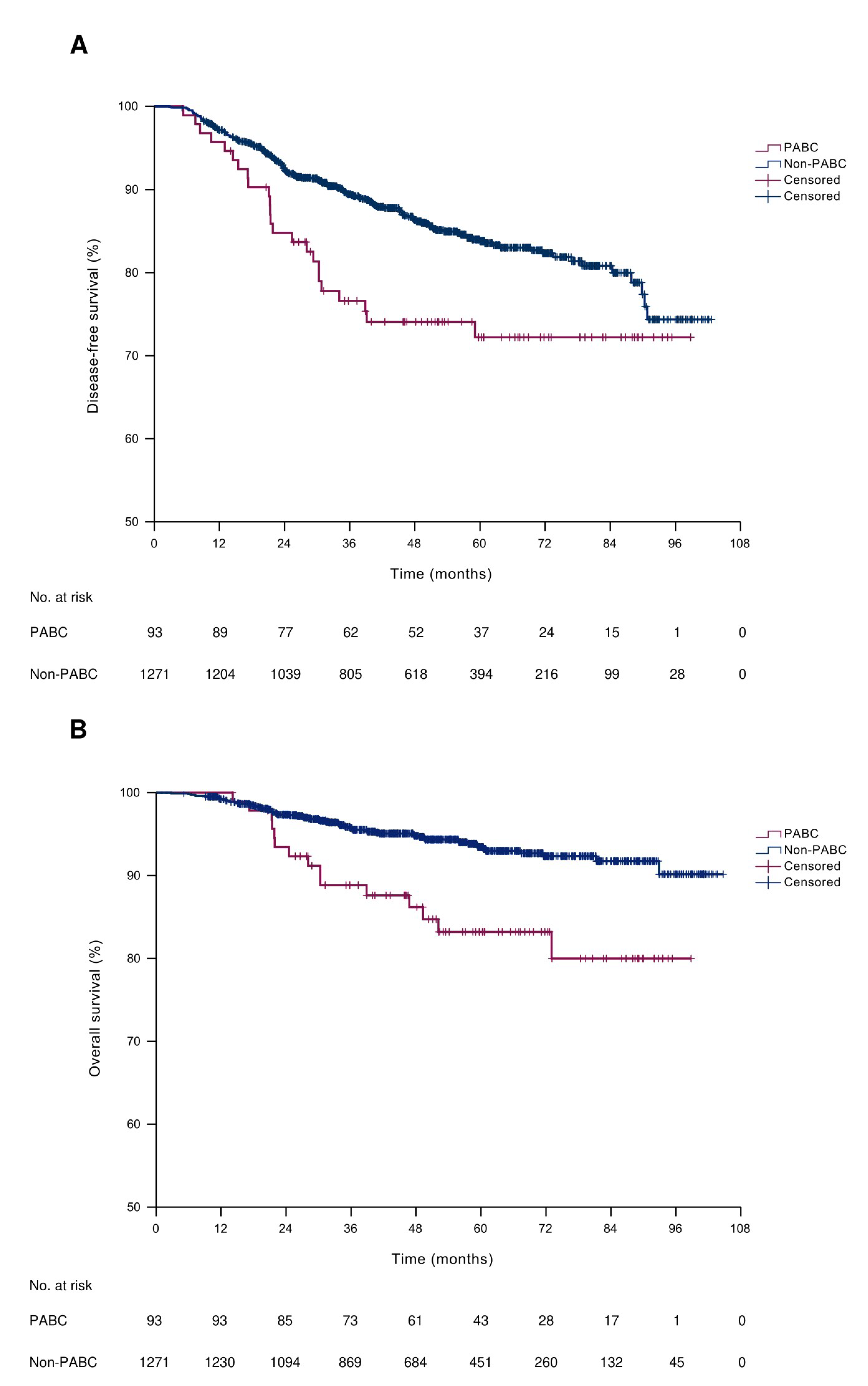
3.6. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K.; et al. Breast Cancer Statistics in Korea in 2017: Data from a Breast Cancer Registry. J. Breast Cancer 2020, 23, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yap, Y.S.; Lee, K.-H.; Im, S.-A.; Naito, Y.; Yeo, W.; Ueno, T.; Kwong, A.; Li, H.; Huang, S.-M.; et al. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. J. Natl. Cancer Inst. 2019, 111, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.-S.; Lu, Y.-S.; Tamura, K.; Lee, J.E.; Ko, E.Y.; Park, Y.H.; Cao, A.-Y.; Lin, C.-H.; Toi, M.; Wu, J. Insights into breast cancer in the east vs the west: A review. JAMA Oncol. 2019, 5, 1489–1496. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A., Jr.; Bianchi-Micheli, G.; Cardoso, M.-J.; Curigliano, G.; Gelmon, K.A.; Harbeck, N. ESO–ESMO 4th International consensus guidelines for breast cancer in young women (BCY4). Ann. Oncol. 2020, 31, 674–696. [Google Scholar] [CrossRef]
- Fredholm, H.; Eaker, S.; Frisell, J.; Holmberg, L.; Fredriksson, I.; Lindman, H. Breast Cancer in Young Women: Poor Survival Despite Intensive Treatment. PLoS ONE 2009, 4, e7695. [Google Scholar] [CrossRef]
- Ahn, S.H.; Son, B.H.; Kim, S.W.; Kim, S.I.; Jeong, J.; Ko, S.-S.; Han, W. Poor Outcome of Hormone Receptor–Positive Breast Cancer at Very Young Age Is Due to Tamoxifen Resistance: Nationwide Survival Data in Korea—A Report from the Korean Breast Cancer Society. J. Clin. Oncol. 2007, 25, 2360–2368. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, S.J.; Jung, H.A.; Kim, S.M.; Kim, M.J.; Kil, W.H.; Lee, J.E.; Nam, S.J.; Ahn, J.S.; Im, Y.-H. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast 2015, 24, 213–217. [Google Scholar] [CrossRef]
- Peto, J.; Collins, N.; Barfoot, R.; Seal, S.; Warren, W.; Rahman, N.; Easton, D.F.; Evans, C.; Deacon, J.; Stratton, M.R. Prevalence of BRCA1 and BRCA2 Gene Mutations in Patients with Early-Onset Breast Cancer. J. Natl. Cancer Inst. 1999, 91, 943–949. [Google Scholar] [CrossRef]
- Ryu, J.M.; Choi, H.J.; Kim, I.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; Choi, D.H.; Park, Y.H.; Kim, J.-W. Prevalence and oncologic outcomes of BRCA 1/2 mutations in unselected triple-negative breast cancer patients in Korea. Breast Cancer Res. Treat. 2019, 173, 385–395. [Google Scholar] [CrossRef]
- Phillips-Salimi, C.R.; Andrykowski, M.A. Physical and mental health status of female adolescent/young adult survivors of breast and gynecological cancer: A national, population-based, case-control study. Support. Care Cancer 2013, 21, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Christinat, A.; Pagani, O. Fertility after breast cancer. Maturitas 2012, 73, 191–196. [Google Scholar] [CrossRef]
- Tiedtke, C.; De Rijk, A.; De Casterlé, B.D.; Christiaens, M.-R.; Donceel, P. Experiences and concerns about ‘returning to work’ for women breast cancer survivors: A literature review. Psycho-Oncol. 2010, 19, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Peccatori, F.A.; Azim, J.H.A.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi160–vi170. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.G.; Mallam, D.; Stein, S.; Patil, S.; Howard, J.; Sklarin, N.; Hudis, C.A.; Gemignani, M.L.; Seidman, A.D. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer 2012, 118, 3254–3259. [Google Scholar] [CrossRef]
- Rodriguez, A.O.; Chew, H.; Cress, R.; Xing, G.; McElvy, S.; Danielsen, B.; Smith, L. Evidence of Poorer Survival in Pregnancy-Associated Breast Cancer. Obstet. Gynecol. 2008, 112, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Von Minckwitz, G.; Han, S.; Bontenbal, M.; Ring, A.E.; Giermek, J.; Wildiers, H.; Fehm, T.; Linn, S.C.; Schlehe, B.; et al. Prognosis of Women with Primary Breast Cancer Diagnosed During Pregnancy: Results from an International Collaborative Study. J. Clin. Oncol. 2013, 31, 2532–2539. [Google Scholar] [CrossRef]
- Bladström, A.; Anderson, H.; Olsson, H. Worse Survival in Breast Cancer Among Women with Recent Childbirth: Results from a Swedish Population-Based Register Study. Clin. Breast Cancer 2003, 4, 280–285. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Botteri, E.; Renne, G.; Dell’Orto, P.; Rotmensz, N.; Gentilini, O.; Sangalli, C.; Pruneri, G.; Di Nubila, B.; Locatelli, M. The biological features and prognosis of breast cancer diagnosed during pregnancy: A case-control study. Acta Oncol. 2012, 51, 653–661. [Google Scholar] [CrossRef]
- Litton, J.K.; Warneke, C.L.; Hahn, K.M.; Palla, S.L.; Kuerer, H.M.; Perkins, G.H.; Mittendorf, E.A.; Barnett, C.; Gonzalez-Angulo, A.M.; Hortobágyi, G.N.; et al. Case Control Study of Women Treated with Chemotherapy for Breast Cancer During Pregnancy as Compared with Nonpregnant Patients with Breast Cancer. Oncologist 2013, 18, 369–376. [Google Scholar] [CrossRef]
- Beadle, B.M.; Woodward, W.A.; Middleton, L.; Tereffe, W.; Strom, E.A.; Litton, J.K.; Meric-Bernstam, F.; Do, R.L.T.; Buchholz, T.A.; Perkins, G.H. The impact of pregnancy on breast cancer outcomes in women ≤35 years. Cancer 2009, 115, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Ondansetron Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020103s030,020605s014,020781s014lbl.pdf (accessed on 10 June 2022).
- Pasternak, B.; Svanström, H.; Hviid, A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N. Engl. J. Med. 2013, 368, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.K.; Eslick, G.D. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: A meta-analysis. Breast Cancer Res. Treat. 2016, 160, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yu, Z.; Xiao, J.; Liu, L.; Hong, F.; Zhang, Y.; Jia, H. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer 2020, 20, 746. [Google Scholar] [CrossRef] [PubMed]
- Schedin, P. Pregnancy-associated breast cancer and metastasis. Nat. Rev. Cancer 2006, 6, 281–291. [Google Scholar] [CrossRef]
- Lyons, T.R.; O’Brien, J.; Borges, V.F.; Conklin, M.; Keely, P.J.; Eliceiri, K.; Marusyk, A.; Tan, A.C.; Schedin, P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat. Med. 2011, 17, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Lefrère, H.; Borges, V.F.; Cardonick, E.; Lambertini, M.; Loibl, S.; Peccatori, F.; Partridge, A.; Schedin, P. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol. 2021, 22, 753–754. [Google Scholar] [CrossRef]
- Amant, F.; Deckers, S.; Van Calsteren, K.; Loibl, S.; Halaska, M.; Brepoels, L.; Beijnen, J.; Cardoso, F.; Gentilini, O.; Lagae, L.; et al. Breast cancer in pregnancy: Recommendations of an international consensus meeting. Eur. J. Cancer 2010, 46, 3158–3168. [Google Scholar] [CrossRef]
- Reed, W.; Sandstad, B.; Holm, R.; Nesland, J.M. The Prognostic Impact of Hormone Receptors and c-erbB-2 in Pregnancy-Associated Breast Cancer and Their Correlation with BRCAI and Cell Cycle Modulators. Int. J. Surg. Pathol. 2003, 11, 65–74. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Jiang, Z.; Zhao, J.; Mao, Y.; Liu, J.; Zhang, J. Clinicopathological characteristics, diagnosis, and prognosis of pregnancy-associated breast cancer. Thorac. Cancer 2019, 10, 1060–1068. [Google Scholar] [CrossRef]
- Ulery, M.; Carter, L.; McFarlin, B.L.; Giurgescu, C. Pregnancy-Associated Breast Cancer: Significance of Early Detection. J. Midwifery Women’s Health 2009, 54, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Venet, D.; Azim, H.A.; Brown, D.; Desmedt, C.; Lambertini, M.; Majjaj, S.; Pruneri, G.; Peccatori, F.; Piccart, M.; et al. Breast cancer diagnosed during pregnancy is associated with enrichment of non-silent mutations, mismatch repair deficiency signature and mucin mutations. Npj Breast Cancer 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Pennock, N.D.; Sun, D.; Horton, W.; Ozaki, M.K.; Narasimhan, J.; Bartlett, A.Q.; Weinmann, S.; Goss, P.E.; Borges, V.F.; et al. Postpartum breast cancer has a distinct molecular profile that predicts poor outcomes. Nat. Commun. 2021, 12, 6341. [Google Scholar] [CrossRef] [PubMed]
- van der Groep, P.; van der Wall, E.; van Diest, P.J. Pathology of hereditary breast cancer. Cell. Oncol. 2011, 34, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Group, A.B.C.S. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br. J. Cancer 2000, 83, 1301. [Google Scholar]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Pavlidis, N.; Peccatori, F.A. Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part II: Hematological tumors. Cancer Treat. Rev. 2010, 36, 110–121. [Google Scholar] [CrossRef]
- O’Laughlin, A.; So, S.; Fleischer, L.; Akoto, S.; Cardonick, E. Safety of Taxane Chemotherapy in Breast Cancer During Pregnancy [28O]. Obstet. Gynecol. 2019, 133, 169–170. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Azim, H.; Peccatori, F.A. Treatment of cancer during pregnancy with monoclonal antibodies: A real challenge. Expert Rev. Clin. Immunol. 2010, 6, 821–826. [Google Scholar] [CrossRef]
- Braems, G.; Denys, H.; De Wever, O.; Cocquyt, V.; Van den Broecke, R. Use of Tamoxifen Before and During Pregnancy. Oncologist 2011, 16, 1547–1551. [Google Scholar] [CrossRef]
| Characteristics | PABC Group (n = 93) | Non-PABC Group (n = 1271) | p Value |
|---|---|---|---|
| Age at diagnosis (years) | 34 (26–43) | 36 (19–40) | 0.001 |
| Family history of breast cancer | 14 (15.1) | 208 (16.4) | 0.775 |
| Histology | |||
| Ductal | 87 (93.5) | 1179 (92.8) | 0.009 * |
| Lobular | 1 (1.1) | 43 (3.4) | |
| Mixed ductal/lobular | 0 | 5 (0.4) | |
| Mucinous | 1 (1.1) | 38 (3.0) | |
| Others | 4 (4.3) | 6 (0.5) | |
| Receptor status | |||
| HR+/HER2− | 42 (45.2) | 802 (63.1) | <0.001 |
| HR+/HER2+ | 18 (19.4) | 146 (11.5) | |
| HR−/HER2+ | 7 (7.5) | 79 (6.1) | |
| Triple negative | 26 (28.0) | 244 (19.2) | |
| Ki67 semiquantitative | |||
| 1+ | 19 (20.4) | 563 (44.3) | <0.001 |
| 2+ | 26 (28.0) | 334 (26.3) | |
| 3+ | 26 (28.0) | 172 (13.5) | |
| 4+ | 22 (23.7) | 202 (15.9) | |
| BRCA1 | |||
| Pathogenic | 6 (6.5) | 51 (4.0) | 0.243 * |
| VUS/Equivocal | 23 (24.7) | 305 (24.0) | |
| Wild type | 64 (68.8) | 915 (72.0) | |
| BRCA2 | |||
| Pathogenic | 5 (5.4) | 76 (6.0) | 0.965 |
| VUS/equivocal | 22 (23.7) | 327 (25.7) | |
| Wild type | 66 (71.0) | 868 (68.3) | |
| Treatment setting | |||
| Neoadjuvant | 49 (52.7) | 446 (35.1) | <0.001 |
| Adjuvant | 33 (35.5) | 794 (62.5) | |
| Metastatic | 11 (11.8) | 31 (2.4) |
| Characteristics | PABC Group (n = 49) | Non-PABC Group (n = 446) | p Value |
|---|---|---|---|
| Receptor status | |||
| HR+/HER2− | 11 (22.4) | 169 (37.9) | 0.040 |
| HR+/HER2+ | 13 (26.5) | 67 (15.0) | |
| HR−/HER2+ | 6 (12.2) | 56 (12.6) | |
| Triple negative | 19 (38.8) | 154 (34.5) | |
| Neoadjuvant chemotherapy | |||
| AC | 7 (14.3) | 7 (1.6) | <0.001 * |
| AC + docetaxel | 26 (53.1) | 293 (65.7) | |
| AC + docetaxel + platinum | 1 (2.0) | 29 (6.5) | |
| AC + docetaxel + trastuzumab | 8 (16.3) | 27 (6.1) | |
| TCHP | 4 (8.2) | 66 (14.8) | |
| Others | 3 (6.1) | 24 (5.4) | |
| Surgery | |||
| BCS & SLNB | 21 (42.9) | 177 (39.7) | 0.574 |
| BCS & ALND | 5 (10.2) | 71 (15.9) | |
| TM & SLNB | 10 (20.4) | 81 (18.2) | |
| TM & ALND | 13 (26.5) | 103 (23.1) | |
| Others | 0 | 14 (3.1) | |
| pCR | 12 (24.5) | 140 (31.4) | 0.335 |
| Adjuvant therapy | |||
| Adjuvant chemotherapy | 15 (30.6) | 76 (17.0) | 0.031 |
| Capecitabine | 3 (20.0) | 47 (61.8) | |
| Docetaxel | 5 (33.3) | 2 (2.6) | |
| Cisplatin | 1 (6.7) | 3 (3.9) | |
| Others | 6 (40.0) | 24 (31.6) | |
| Adjuvant trastuzumab | 18 (36.7) | 121 (27.1) | 0.180 |
| Adjuvant endocrine therapy | 27 (55.1) | 236 (52.9) | 0.880 |
| Tamoxifen | 16 (59.3) | 89 (37.7) | |
| Tamoxifen + goserelin | 10 (37.0) | 122 (51.7) | |
| Letrozole + goserelin | 0 | 12 (5.1) | |
| Others | 1 (3.7) | 13 (5.5) | |
| Adjuvant radiotherapy | 44 (89.8) | 414 (92.8) | 0.396 * |
| ypT stage | |||
| ypT0 | 8 (16.3) | 108 (24.2) | 0.757 * |
| ypTis | 5 (10.2) | 42 (9.4) | |
| ypT1 | 19 (38.8) | 165 (37.0) | |
| ypT2 | 13 (26.5) | 98 (22.0) | |
| ypT3 | 4 (8.2) | 33 (7.4) | |
| ypN stage | |||
| ypN0 | 33 (67.3) | 280 (62.8) | 0.552 * |
| ypN1 | 8 (16.3) | 102 (22.9) | |
| ypN2 | 4 (8.2) | 42 (9.4) | |
| ypN3 | 4 (8.2) | 22 (4.9) | |
| Pathological stage | |||
| 0 | 12 (24.5) | 140 (31.4) | 0.356 |
| I | 18 (36.7) | 114 (25.6) | |
| II | 10 (20.4) | 114 (25.6) | |
| III | 9 (18.4) | 78 (17.5) |
| Characteristics | PABC Group (n = 33) | Non-PABC Group (n = 794) | p value |
|---|---|---|---|
| Receptor status | |||
| HR+/HER2− | 27 (81.8) | 618 (77.9) | 0.078 * |
| HR+/HER2+ | 2 (6.1) | 74 (9.3) | |
| HR−/HER2+ | 1 (3.0) | 20 (2.5) | |
| Triple negative | 3 (9.1) | 82 (10.3) | |
| Surgery | |||
| BCS & SLNB | 12 (36.4) | 426 (53.7) | 0.103 * |
| BCS & ALND | 2 (6.1) | 49 (6.2) | |
| TM & SLNB | 9 (27.3) | 210 (26.4) | |
| TM & ALND | 10 (30.3) | 101 (12.7) | |
| Others | 0 | 8 (1.0) | |
| Adjuvant therapy | |||
| Adjuvant chemotherapy | 26 (78.8) | 459 (57.8) | 0.033 |
| AC | 7 (27.0) | 131 (28.5) | |
| AC + docetaxel | 12 (46.2) | 144 (31.4) | |
| AC + weekly paclitaxel | 3 (11.5) | 32 (7.0) | |
| FAC | 2 (7.7) | 26 (5.7) | |
| TAC | 0 | 45 (9.8) | |
| TC | 1 (3.8) | 59 (12.9) | |
| TCH | 0 | 11 (2.4) | |
| Others | 1 (3.8) | 11 (2.4) | |
| Adjuvant trastuzumab | 2 (6.1) | 80 (10.1) | 0.567 * |
| Adjuvant endocrine therapy | 28 (84.8) | 687 (86.5) | 0.798 |
| Tamoxifen | 19 (67.9) | 298 (43.4) | |
| Tamoxifen + goserelin | 8 (28.6) | 367 (53.4) | |
| Letrozole + goserelin | 1 (3.6) | 10 (1.5) | |
| Others | 0 | 12 (1.7) | |
| Adjuvant radiotherapy | 16 (48.5) | 564 (71.0) | 0.012 |
| pT stage | |||
| pT0 | 0 | 2 (0.3) | 0.010 * |
| pTis | 1 (3.0) | 9 (1.1) | |
| pT1 | 11 (33.3) | 458 (57.7) | |
| pT2 | 19 (57.6) | 297 (37.4) | |
| pT3 | 2 (6.1) | 28 (3.5) | |
| pN stage | |||
| pN0 | 20 (60.6) | 549 (69.1) | 0.179 * |
| pN1 | 10 (30.3) | 191 (24.1) | |
| pN2 | 1 (3.0) | 42 (5.3) | |
| pN3 | 2 (6.1) | 12 (1.5) | |
| Pathological stage | |||
| 0 | 1 (3.0) | 11 (1.4) | 0.037 * |
| I | 9 (27.3) | 397 (50.0) | |
| II | 20 (60.6) | 322 (40.6) | |
| III | 3 (9.1) | 64 (8.1) |
| Variables | Disease-Free Survival | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age at diagnosis, years | 0.97 | 0.93–1.00 | 0.061 | 0.98 | 0.94–1.01 | 0.198 | 0.98 | 0.93–1.03 | 0.383 | 1.00 | 0.94–1.06 | 0.916 |
| PABC | 1.73 | 1.13–2.65 | 0.012 | 1.59 | 1.04–2.45 | 0.034 | 2.61 | 1.49–4.56 | 0.001 | 2.33 | 1.33–4.10 | 0.003 |
| HR positivity | 0.53 | 0.40–0.71 | <0.001 | 0.61 | 0.44–0.85 | 0.003 | 0.36 | 0.24–0.55 | <0.001 | 0.51 | 0.31–0.82 | 0.006 |
| HER2 positivity | 0.73 | 0.50–1.08 | 0.732 | 0.62 | 0.33–1.17 | 0.144 | ||||||
| Ki-67 4+ (76–100%) | 1.77 | 1.27–2.45 | 0.001 | 1.31 | 0.90–1.89 | 0.162 | 3.05 | 1.96–4.77 | <0.001 | 2.04 | 1.22–3.40 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Park, S.; Kim, H.R.; Kim, H.; Hong, J.; Lee, J.E.; Yu, J.; Chae, B.J.; Lee, S.K.; Ryu, J.M.; et al. Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort. Cancers 2022, 14, 4839. https://doi.org/10.3390/cancers14194839
Jo H, Park S, Kim HR, Kim H, Hong J, Lee JE, Yu J, Chae BJ, Lee SK, Ryu JM, et al. Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort. Cancers. 2022; 14(19):4839. https://doi.org/10.3390/cancers14194839
Chicago/Turabian StyleJo, Hyunji, Seri Park, Hye Ryeon Kim, Hongsik Kim, Joohyun Hong, Jeong Eon Lee, Jonghan Yu, Byung Joo Chae, Se Kyung Lee, Jai Min Ryu, and et al. 2022. "Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort" Cancers 14, no. 19: 4839. https://doi.org/10.3390/cancers14194839
APA StyleJo, H., Park, S., Kim, H. R., Kim, H., Hong, J., Lee, J. E., Yu, J., Chae, B. J., Lee, S. K., Ryu, J. M., Oh, S.-y., Choi, S. J., Kim, J.-Y., Ahn, J. S., Im, Y.-H., Nam, E. M., Nam, S. J., & Park, Y. H. (2022). Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort. Cancers, 14(19), 4839. https://doi.org/10.3390/cancers14194839







