Change of the Neutrophil-to-Lymphocyte Ratio during Treatment: A Potential Prognostic Biomarker in Metastatic Prostate Cancer Treated with Radium-223 Dichloride
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of the Study Population
3.2. Primary Outcome within Radium-Treated Population
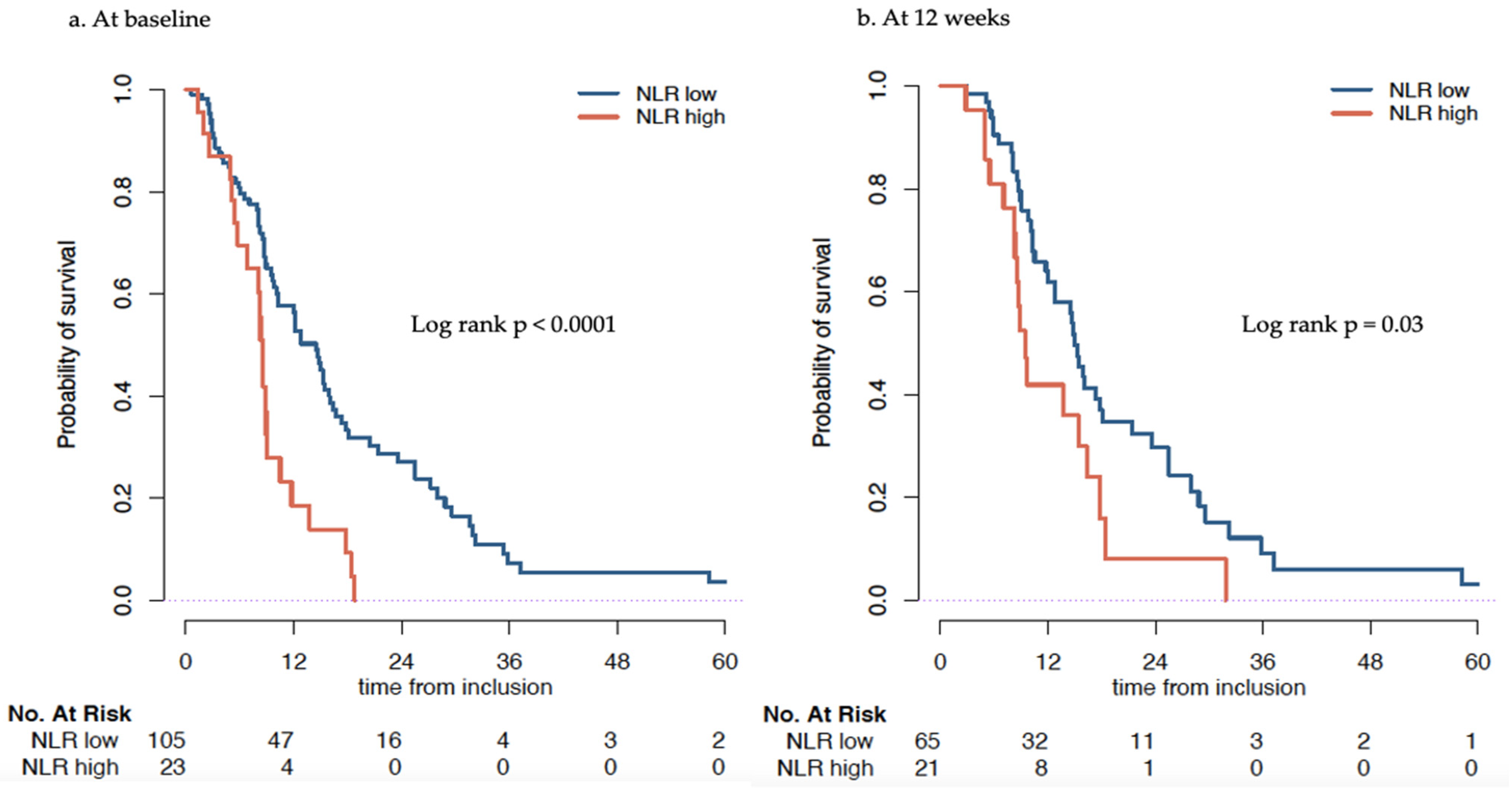
3.2.1. Association of NLR at Baseline with Overall Survival
3.2.2. Association of Change of NLR at 12 Weeks with OS
3.2.3. Evolution of ANC and ALC during Therapy
3.3. Primary Outcome within Docetaxel Treated Population
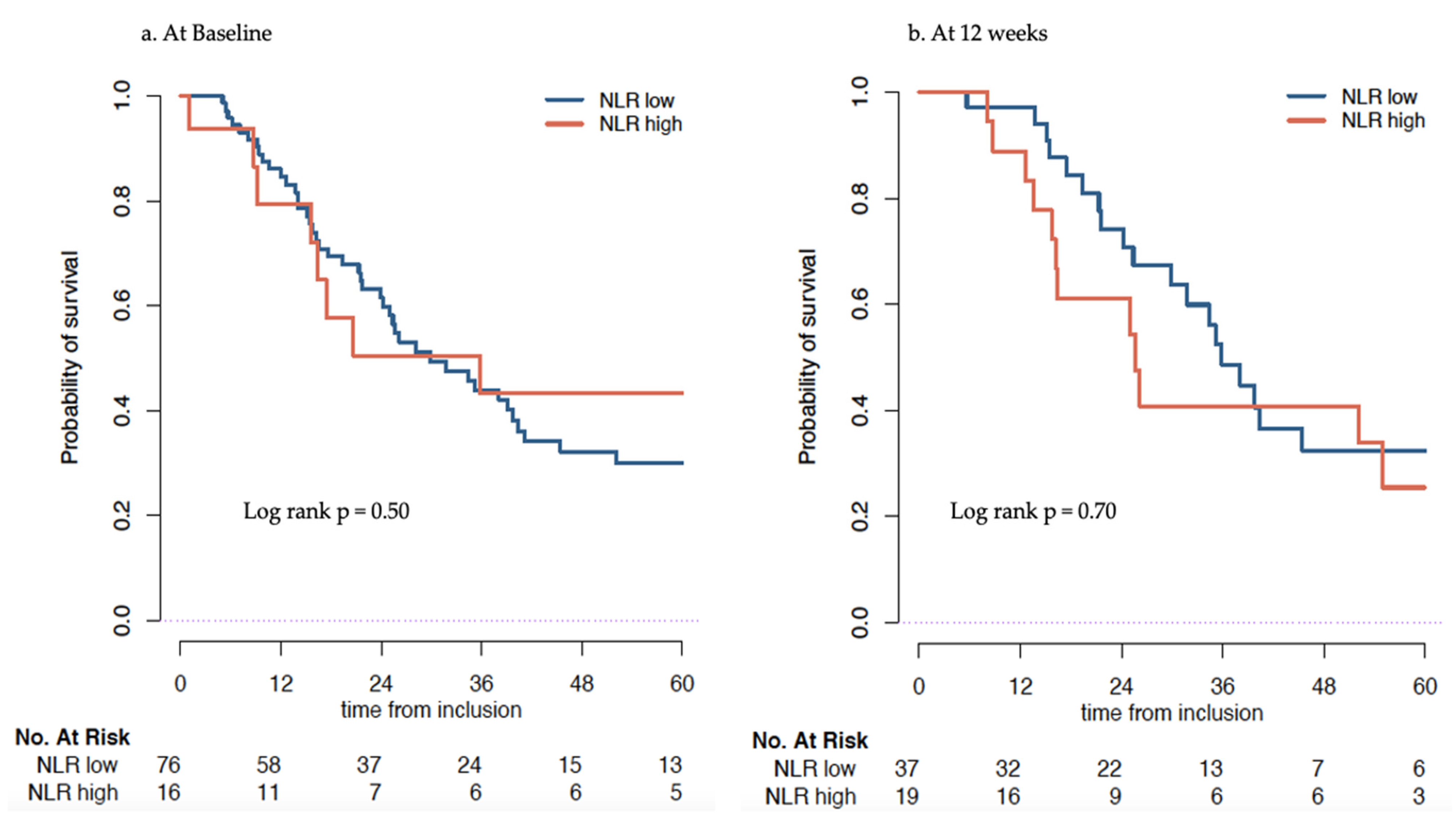
3.3.1. Association of NLR at Baseline with Overall Survival
3.3.2. Association of Change of NLR at 12 Weeks with OS
3.3.3. ANC and ALC Evolution during Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; Wülfing, C.; Castellano, D.; Kramer, G.; Eymard, J.-C.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Bamias, A.; Carles, J.; et al. Baseline Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Biomarker in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Cabazitaxel versus Abiraterone or Enzalutamide in the CARD Study. ESMO Open 2021, 6, 100241. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to Lymphocyte Ratio and Cancer Prognosis: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Mjaess, G.; Chebel, R.; Karam, A.; Moussa, I.; Pretot, D.; Abi Tayeh, G.; Sarkis, J.; Semaan, A.; Peltier, A.; Aoun, F.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR) in Urological Tumors: An Umbrella Review of Evidence from Systematic Reviews and Meta-Analyses. Acta Oncol. 2021, 60, 704–713. [Google Scholar] [CrossRef]
- Guo, J.; Fang, J.; Huang, X.; Liu, Y.; Yuan, Y.; Zhang, X.; Zou, C.; Xiao, K.; Wang, J. Prognostic Role of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Prostate Cancer: A Meta-Analysis of Results from Multivariate Analysis. Int. J. Surg. 2018, 60, 216–223. [Google Scholar] [CrossRef]
- Lalani, A.-K.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-Lymphocyte Ratio (NLR) in Response to Immune Checkpoint Blockade for Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef]
- Mezquita, L.; Preeshagul, I.; Auclin, E.; Saravia, D.; Hendriks, L.; Rizvi, H.; Park, W.; Nadal, E.; Martin-Romano, P.; Ruffinelli, J.C.; et al. Predicting Immunotherapy Outcomes under Therapy in Patients with Advanced NSCLC Using DNLR and Its Early Dynamics. Eur. J. Cancer 2021, 151, 211–220. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J. Preclinical Mechanisms of Action of Docetaxel and Docetaxel Combinations in Prostate Cancer. Semin. Oncol. 2001, 28, 3–7. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral Blood Markers Predictive of Outcome and Immune-Related Adverse Events in Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline Neutrophil-to-Lymphocyte Ratio (NLR) and Derived NLR Could Predict Overall Survival in Patients with Advanced Melanoma Treated with Nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Meisel, A.; Parker, C.; Kühne, R.; Sartor, O.; Stenner-Liewen, F. 637P The Prognostic Value of the Baseline Neutrophil-to-Lymphocyte Ratio (NLR) in Patients with Metastatic Castration-Resistant Prostate Cancer (MCRPC) Receiving Radium-223 (Ra-223): A Post-Hoc Analysis of the ALSYMPCA Phase-III Trial. Ann. Oncol. 2020, 31, S524–S525. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Signori, A.; Frantellizzi, V.; Murianni, V.; Lodi Rizzini, E.; Mascia, M.; Lavelli, V.; Donegani, M.I.; Ponzano, M.; et al. The Prognostic Power of Inflammatory Indices and Clinical Factors in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223 (BIO-Ra Study). Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1063–1074. [Google Scholar] [CrossRef]
- Bauckneht, M.; Rebuzzi, S.E.; Ponzano, M.; Borea, R.; Signori, A.; Frantellizzi, V.; Lodi Rizzini, E.; Mascia, M.; Lavelli, V.; Miceli, A.; et al. Prognostic Value of the BIO-Ra Score in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223 after the European Medicines Agency Restricted Use: Secondary Investigations of the Multicentric BIO-Ra Study. Cancers 2022, 14, 1744. [Google Scholar] [CrossRef]
- Lorente, D.; Mateo, J.; Templeton, A.J.; Zafeiriou, Z.; Bianchini, D.; Ferraldeschi, R.; Bahl, A.; Shen, L.; Su, Z.; Sartor, O.; et al. Baseline Neutrophil–Lymphocyte Ratio (NLR) Is Associated with Survival and Response to Treatment with Second-Line Chemotherapy for Advanced Prostate Cancer Independent of Baseline Steroid Use. Ann. Oncol. 2015, 26, 750–755. [Google Scholar] [CrossRef]
- Sümbül, A.T.; Sezer, A.; Abalı, H.; Köse, F.; Gültepe, I.; Mertsoylu, H.; Muallaoğlu, S.; Özyılkan, Ö. Neutrophil-to-Lymphocyte Ratio Predicts PSA Response, but Not Outcomes in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel. Int. Urol. Nephrol. 2014, 46, 1531–1535. [Google Scholar] [CrossRef]
- Linton, A.; Pond, G.; Clarke, S.; Vardy, J.; Galsky, M.; Sonpavde, G. Glasgow Prognostic Score as a Prognostic Factor in Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel-Based Chemotherapy. Clin. Genitourin. Cancer 2013, 11, 423–430. [Google Scholar] [CrossRef]
- Jiang, Z.-G.; Liao, S.-G. Baseline Neutrophil-Lymphocyte Ratio Is Associated with Outcomes in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel in South China. Medicine 2021, 100, e27361. [Google Scholar] [CrossRef] [PubMed]
- van Soest, R.J.; Templeton, A.J.; Vera-Badillo, F.E.; Mercier, F.; Sonpavde, G.; Amir, E.; Tombal, B.; Rosenthal, M.; Eisenberger, M.A.; Tannock, I.F.; et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Men with Metastatic Castration-Resistant Prostate Cancer Receiving First-Line Chemotherapy: Data from Two Randomized Phase III Trials. Ann. Oncol. 2015, 26, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Buttigliero, C.; Pisano, C.; Tucci, M.; Vignani, F.; Bertaglia, V.; Iaconis, D.; Guglielmini, P.; Numico, G.; Scagliotti, G.V.; Di Maio, M. Prognostic Impact of Pretreatment Neutrophil-to-Lymphocyte Ratio in Castration-Resistant Prostate Cancer Patients Treated with First-Line Docetaxel. Acta Oncol. 2017, 56, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Runcie, K.D.; Dallos, M.C. Prostate Cancer Immunotherapy—Finally in from the Cold? Curr. Oncol. Rep. 2021, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Formenti, S.C. Role of T Lymphocytes in Tumor Response to Radiotherapy. Front. Oncol. 2012, 2, 95. [Google Scholar] [CrossRef]
- McBride, W.H.; Chiang, C.-S.; Olson, J.L.; Wang, C.-C.; Hong, J.-H.; Pajonk, F.; Dougherty, G.J.; Iwamoto, K.S.; Pervan, M.; Liao, Y.-P. A Sense of Danger from Radiation. Radiat. Res. 2004, 162, 1–19. [Google Scholar] [CrossRef]
- Turner, P.G.; Jain, S.; Cole, A.; Grey, A.; Mitchell, D.; Prise, K.M.; Hounsell, A.R.; McGarry, C.K.; Biggart, S.; O’Sullivan, J.M. Toxicity and Efficacy of Concurrent Androgen Deprivation Therapy, Pelvic Radiotherapy, and Radium-223 in Patients with de Novo Metastatic Hormone-Sensitive Prostate Cancer. Clin. Cancer Res. 2021, 27, 4549–4556. [Google Scholar] [CrossRef]
- Pike, L.R.G.; Bang, A.; Mahal, B.A.; Taylor, A.; Krishnan, M.; Spektor, A.; Cagney, D.N.; Aizer, A.A.; Alexander, B.M.; Rahma, O.; et al. The Impact of Radiation Therapy on Lymphocyte Count and Survival in Metastatic Cancer Patients Receiving PD-1 Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 142–151. [Google Scholar] [CrossRef]
- Keisari, Y.; Kelson, I. The Potentiation of Anti-Tumor Immunity by Tumor Abolition with Alpha Particles, Protons, or Carbon Ion Radiation and Its Enforcement by Combination with Immunoadjuvants or Inhibitors of Immune Suppressor Cells and Checkpoint Molecules. Cells 2021, 10, 228. [Google Scholar] [CrossRef]
| (a) | ||||
| Whole Sample (n = 149) (%) | NLR Low (≤5) (n = 105) (%) | NLR High (>5) (n = 23) (%) | p-Value | |
| Prior Docetaxel treatment | 0.21 | |||
| No | 65 (43.6%) | 42 (40%) | 6 (26.1%) | |
| Yes | 84 (56.4%) | 63 (60%) | 17 (73.9%) | |
| Age (years) | 72 (65; 79) | 71 (64; 77) | 74 (66; 79.5) | 0.83 |
| Missing | 1 | 1 | 0 | |
| ECOG | 0.04 | |||
| 0 | 50 (36.8%) | 42 (43.8%) | 4 (17.4%) | |
| 1 | 68 (50%) | 45 (46.9%) | 15 (65.2%) | |
| 2 | 18 (13.2%) | 9 (9.4%) | 4 (17.4%) | |
| Missing | 13 | 9 | 0 | |
| PSA levels (ng/mL) | 53.2 (14.8; 182) | 38.3 (10.1; 122.5) | 86.2 (23.8; 307.6) | 0.79 |
| Missing | 7 | 1 | 0 | |
| tALP (IU/L) | 106 (69.5; 198.5) | 96 (67; 156) | 86.2 (23.8; 307.6) | 0.1 |
| Missing | 18 | 6 | 0 | |
| Hb (g/L) | 122 (113.8; 133) | 96 (67; 156) | 158 (92; 267) | 0.07 |
| Missing | 9 | 0 | 0 | |
| White blood count (109/L) | 6.5 (5.3; 7.9) | 6.2 (5.2; 7.9) | 7.7 (6.8; 9) | 0.01 |
| Neutrophils (109/L) | 4.4 (3.5; 5.6) | 4.1 (3.3; 5.1) | 5.7 (5.3; 6.6) | 0.001 |
| Lymphocytes (109/L) | 3.5 (2.5; 4.6) | 1.4 (1.1; 1.9) | 1 (0.7; 1.1) | <0.001 |
| NLR | 3.5 (2.5; 4.6) | 3.2 (2.3; 4) | 5.8 (5.4; 7) | |
| Missing | 21 | 0 | 0 | |
| Platelets (109/L) | 239 (186.8; 287) | 237 (186; 285) | 246 (209; 322) | 0.16 |
| Missing | 9 | 0 | 0 | |
| (b) | ||||
| Whole Sample (n = 170) (%) | NLR Low (≤5) (n = 76) (%) | NLR High (>5) (n = 16) (%) | p-Value | |
| Age | 67 (59; 74) | 69 (59.5; 75.5) | 66.5 (59.8; 69.2) | 0.15 |
| Missing | 1 | 1 | 0 | |
| Cancer Type | ||||
| Hormone-sensitive | 20 (12%) | 14 (18.7%) | 3 (18.8%) | 1 |
| Castration resistant | 147 (88%) | 61 (81.3%) | 13 (81.2%) | |
| Missing | 3 | 1 | 0 | |
| ECOG | 0.25 | |||
| 0 | 55 (43.7%) | 25 (58.1%) | 4 (36.4%) | |
| 1 | 57 (45.2%) | 12 (27.9%) | 6 (54.5%) | |
| 2 | 14 (11.1%) | 12 (27.9%) | 1 (9.1%) | |
| Missing | 44 | 33 | 5 | |
| PSA levels (ng/mL) | 29.4 (5.8; 115.7) | 20.4 (5.6; 94.3) | 121.4 (16.6; 567.8) | 0.09 |
| Missing | 34 | 0 | ||
| tALP (IU/L) | 84.5 (63.8; 168.2) | 86 (67; 167) | 90 (68.8; 292) | 0.1 |
| Missing | 74 | 7 | 0 | |
| Hb (g/L) | 130 (119; 143) | 132 (120.8; 143.2) | 122.5 (111.5; 134) | 0.14 |
| Missing | 77 | 0 | 0 | |
| White blood count (109/L) | 6.9 (5.5; 8.0) | 6.8 (5.5; 7.8) | 7.6 (5.8; 8.7) | 0.16 |
| Neutrophils (109/L) | 4.2 (3.4; 5.3) | 4.0 (3.2; 5) | 6.0 (4.6; 6.9) | 0.003 |
| Lymphocytes (109/L)) | 1.5 (1.1; 1.9) | 1.6 (1.3; 2) | 0.8 (0.7; 1) | <0.001 |
| NLR | 2.6 (2.1; 4.2) | 2.4 (1.9; 3) | 7.9 (5.6; 8; 7) | |
| Missing | 78 | 0 | 0 | |
| Platelets (109/L) | 227 (183.8; 264) | 230 (183; 261) | 200 (175.2; 270.8) | 0.29 |
| Missing | 78 | 0 | 0 | |
| NLR Low (≤5) | NLR High (>5) | HR (95% CI) * | p-Value | |
|---|---|---|---|---|
| baseline | 14.5 (10.2–16.4) | 8.5 (6.8–10.5) | 1.7 (1.00–2.90) | 0.05 |
| at 12 weeks | 15.0 (12.7–21.4) | 9.5 (8.3–18.4) | 1.88 (1.07–3.29) | 0.03 |
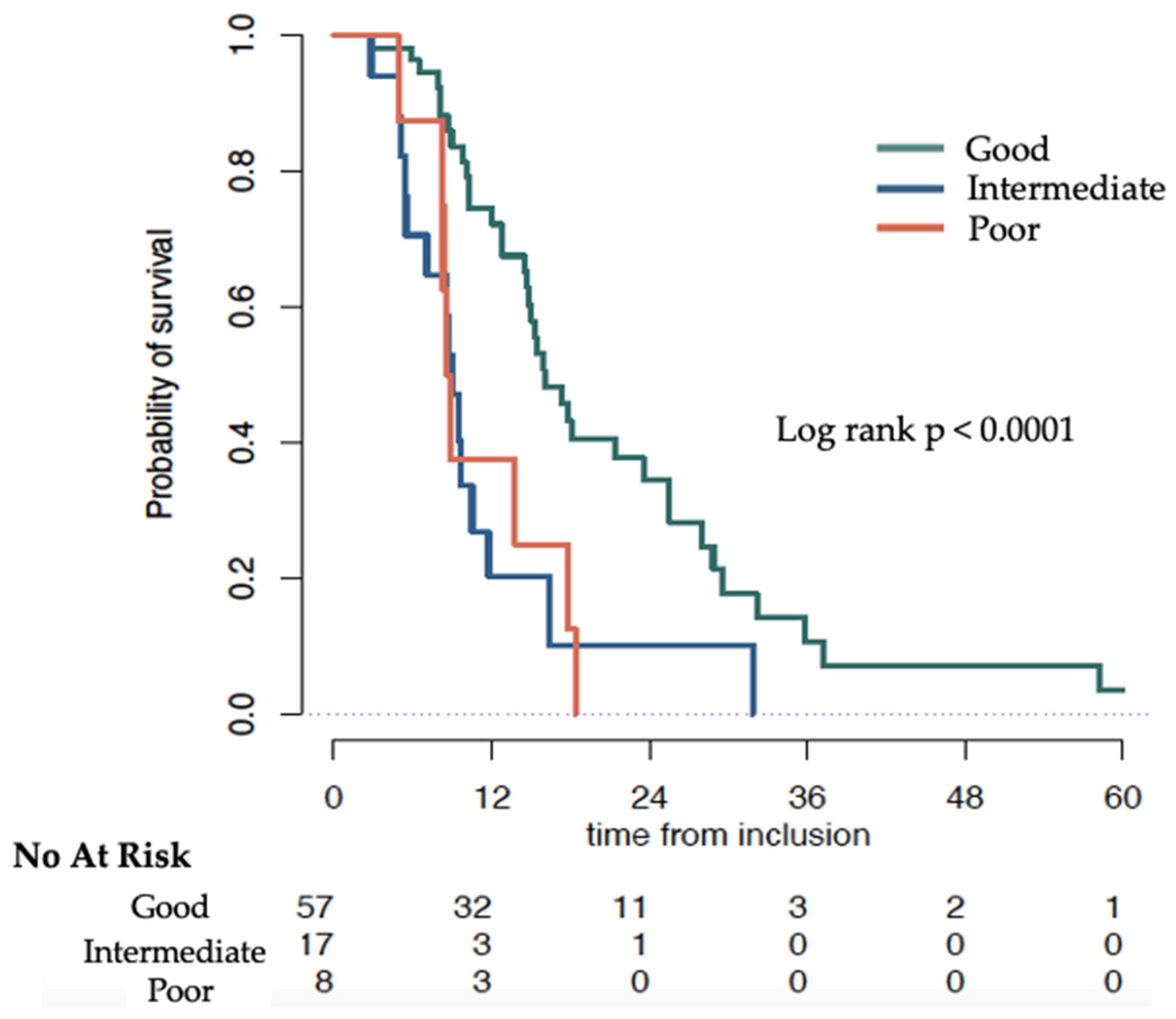
| Good | Intermediate | Poor | p-Value | |
|---|---|---|---|---|
| MedianOS Months (95% CI) | 16.0 (14.8–25.5) | 9.1 (7.1–NR) | 8.7 (8.3–NR) | Log rank 0.0001 |
| HR (95% CI) | Ref | 3.04 (1.62–5.68) | 2.88 (1.31–6.32) | Cox model 0.001 |
| NLR Low (≤5) | NLR High (>5) | HR (95% CI) * | p-Value | |
|---|---|---|---|---|
| Baseline | 29.9 (24.2–41.1) | 35.8 (16.8–NR) | 0.78 (0.37–1.6) | 0.50 |
| At 12 weeks | 35.8 (29.9–NR) | 25.6 (16.2–NR) | 1.27 (0.62–2.61) | 0.51 |
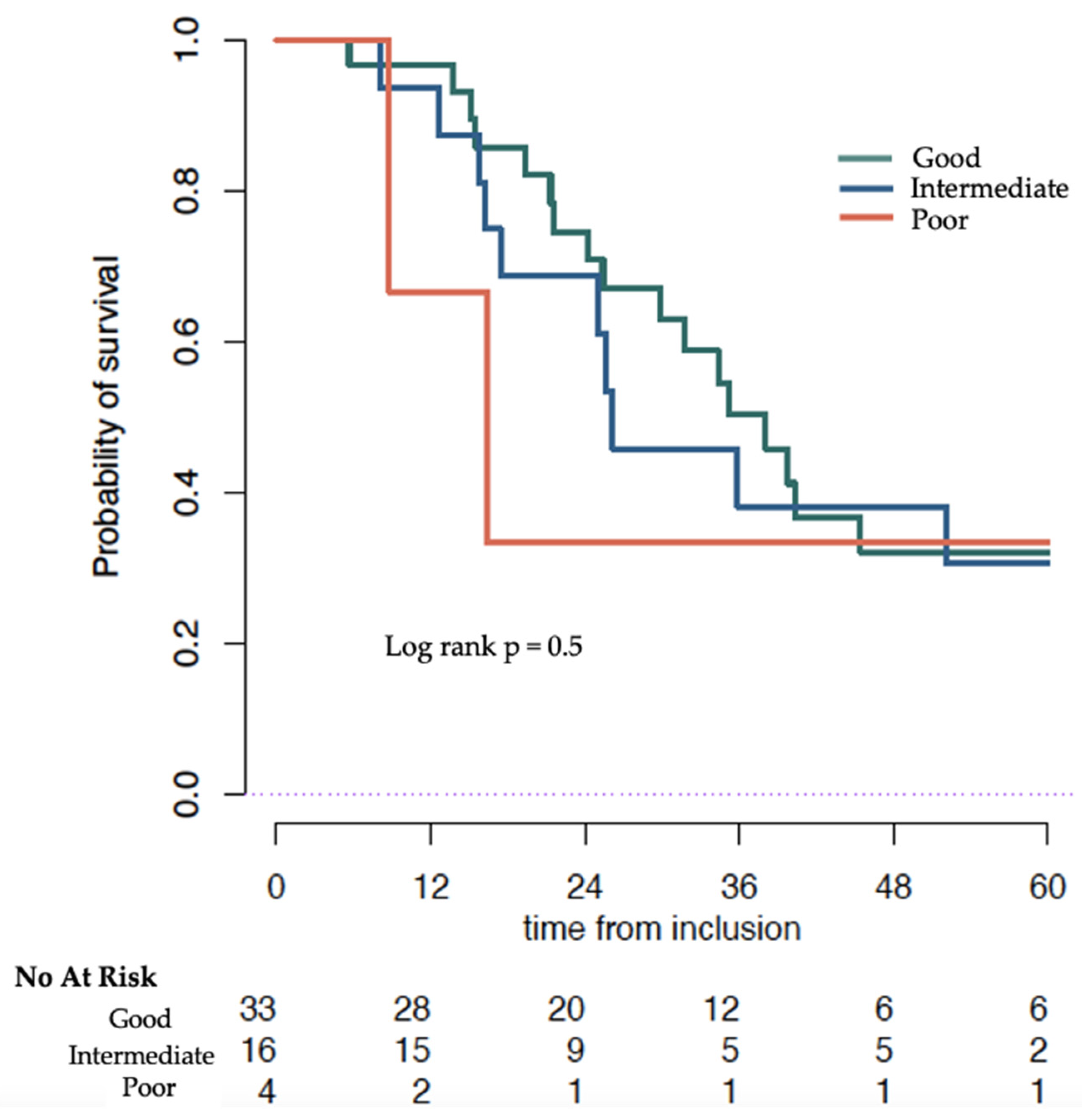
| Good | Intermediate | Poor | p-Value | |
|---|---|---|---|---|
| MedianOS months (95% CI) | 38.1 (30.0–NR) | 26.1 (17.4–NR) | 16.4 (8.8–NR) | Log rank 0.3 |
| HR (95% CI) | Ref | 1.11 (0.51–2.42) | 1.49 (0.34–6.47) | Cox model 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaulanjan, K.; Dahan, J.; Charrois-Durand, C.; Saad, F.; Brureau, L.; Delouya, G.; Taussky, D.; Auclin, E. Change of the Neutrophil-to-Lymphocyte Ratio during Treatment: A Potential Prognostic Biomarker in Metastatic Prostate Cancer Treated with Radium-223 Dichloride. Cancers 2022, 14, 4606. https://doi.org/10.3390/cancers14194606
Kaulanjan K, Dahan J, Charrois-Durand C, Saad F, Brureau L, Delouya G, Taussky D, Auclin E. Change of the Neutrophil-to-Lymphocyte Ratio during Treatment: A Potential Prognostic Biomarker in Metastatic Prostate Cancer Treated with Radium-223 Dichloride. Cancers. 2022; 14(19):4606. https://doi.org/10.3390/cancers14194606
Chicago/Turabian StyleKaulanjan, Kevin, Johanna Dahan, Cédric Charrois-Durand, Fred Saad, Laurent Brureau, Guila Delouya, Daniel Taussky, and Edouard Auclin. 2022. "Change of the Neutrophil-to-Lymphocyte Ratio during Treatment: A Potential Prognostic Biomarker in Metastatic Prostate Cancer Treated with Radium-223 Dichloride" Cancers 14, no. 19: 4606. https://doi.org/10.3390/cancers14194606
APA StyleKaulanjan, K., Dahan, J., Charrois-Durand, C., Saad, F., Brureau, L., Delouya, G., Taussky, D., & Auclin, E. (2022). Change of the Neutrophil-to-Lymphocyte Ratio during Treatment: A Potential Prognostic Biomarker in Metastatic Prostate Cancer Treated with Radium-223 Dichloride. Cancers, 14(19), 4606. https://doi.org/10.3390/cancers14194606







