Nanoparticles Design for Theranostic Approach in Cancer Disease
Abstract
Simple Summary
Abstract
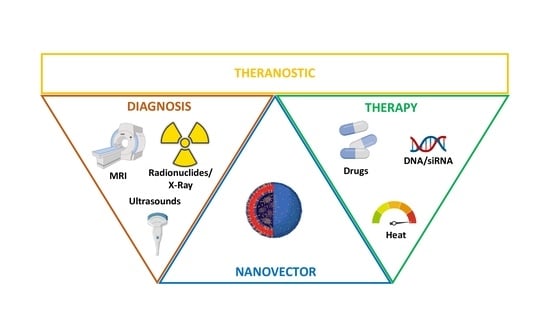
1. Introduction
2. NPs for Theranostic Applications
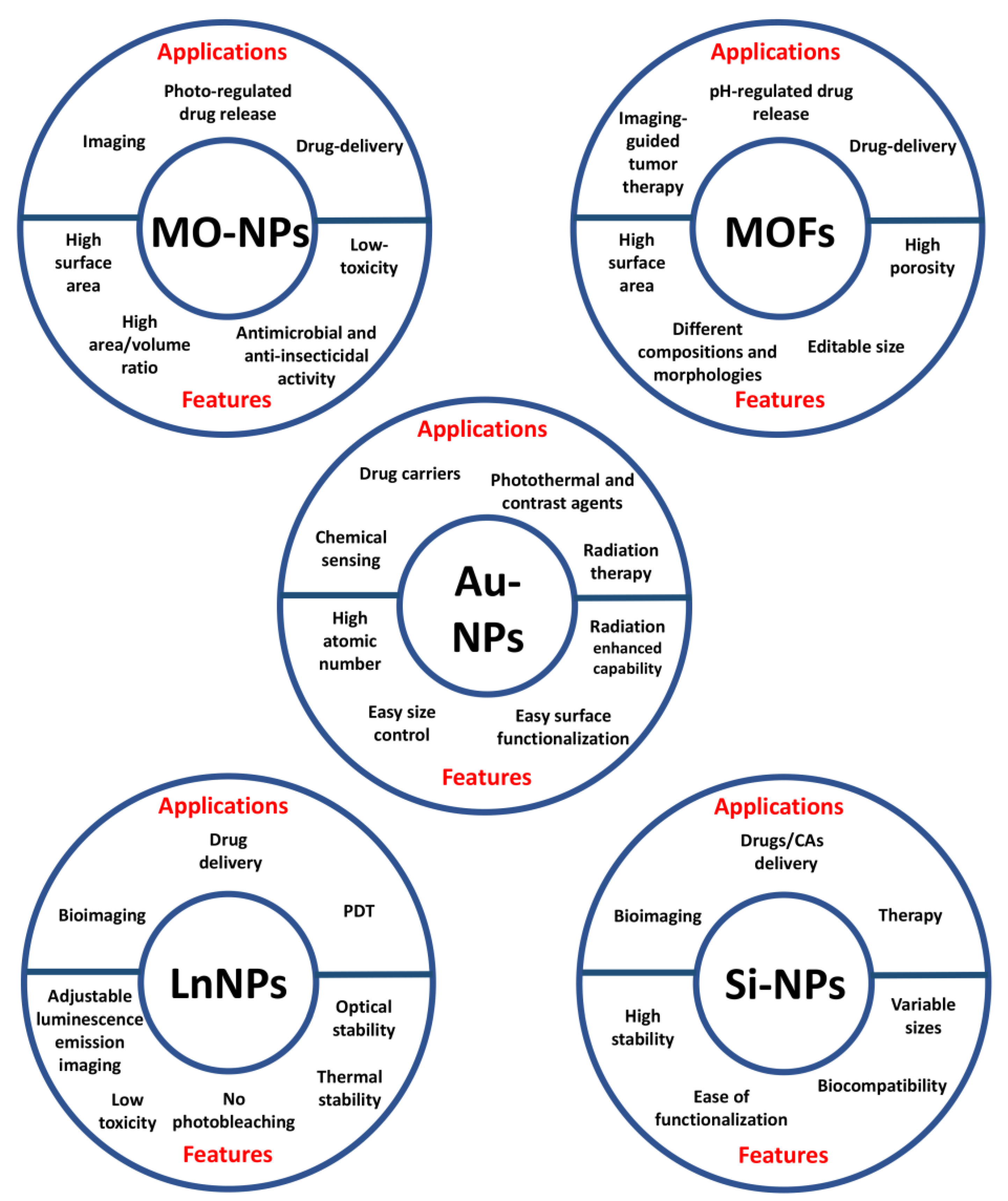
2.1. Inorganic Nanoparticles
2.1.1. Metal Oxide NPs
2.1.2. Metal Organic Frameworks
2.1.3. Gold NPs
2.1.4. Lanthanide-Doped NPs
2.1.5. Silicon Based NPs
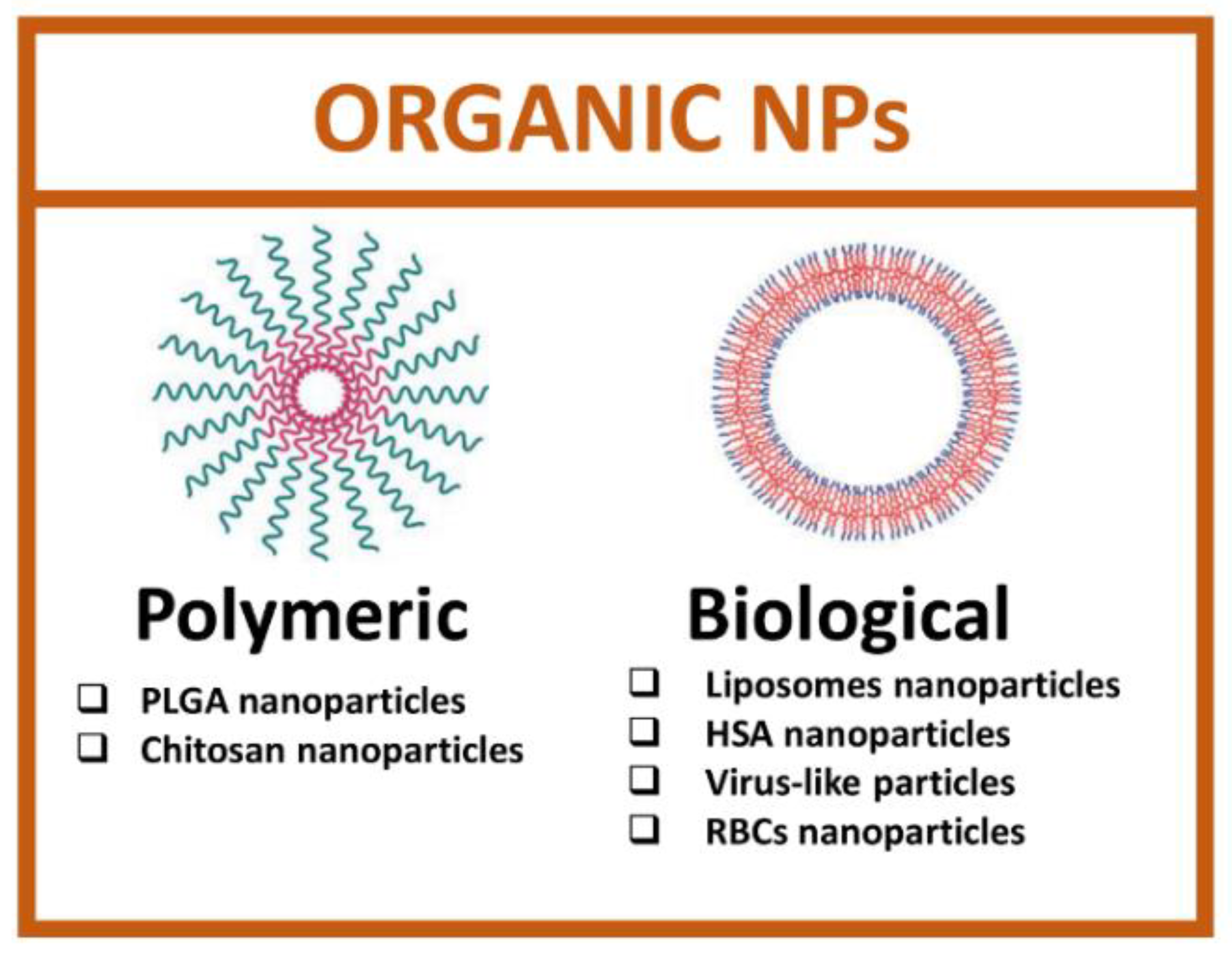
2.2. Organic Nanoparticles
2.2.1. Polymeric Nanoparticles
2.2.2. Biological Nanoparticles
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Ferlay, J. Global Cancer Statistics: 2011. CA Cancer J Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early Detection of Cancer: Past, Present, and Future. Am. Soc. Clin. Oncol. Educ. Book 2015, 30, 57–65. [Google Scholar] [CrossRef]
- Hiom, S.C. Diagnosing cancer earlier: Reviewing the evidence for improving cancer survival. Br. J. Cancer 2015, 112, S1–S5. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Muralidhar Reddy, P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. BioMedicine 2017, 7, 23. [Google Scholar] [CrossRef]
- Belkacemi, Y.; Hanna, N.E.; Besnard, C.; Majdoul, S.; Gligorov, J. Local and Regional Breast Cancer Recurrences: Salvage Therapy Options in the New Era of Molecular Subtypes. Front. Oncol. 2018, 8, 112. [Google Scholar] [CrossRef]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef]
- University of Iowa. What Is Theranostics? Available online: https://uihc.org/health-topics/what-theranostics (accessed on 3 September 2022).
- Kievit, F.M.; Zhang, M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Bin Emran, T.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2013, 32, 778–788. [Google Scholar] [CrossRef]
- Kingston, B.R.; Syed, A.M.; Ngai, J.; Sindhwani, S.; Chan, W.C.W. Assessing micrometastases as a target for nanoparticles using 3D microscopy and machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 14937–14946. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Sanità, G.; Armanetti, P.; Silvestri, B.; Carrese, B.; Calì, G.; Pota, G.; Pezzella, A.; D’Ischia, M.; Luciani, G.; Menichetti, L.; et al. Albumin-Modified Melanin-Silica Hybrid Nanoparticles Target Breast Cancer Cells via a SPARC-Dependent Mechanism. Front. Bioeng. Biotechnol. 2020, 8, 765. [Google Scholar] [CrossRef]
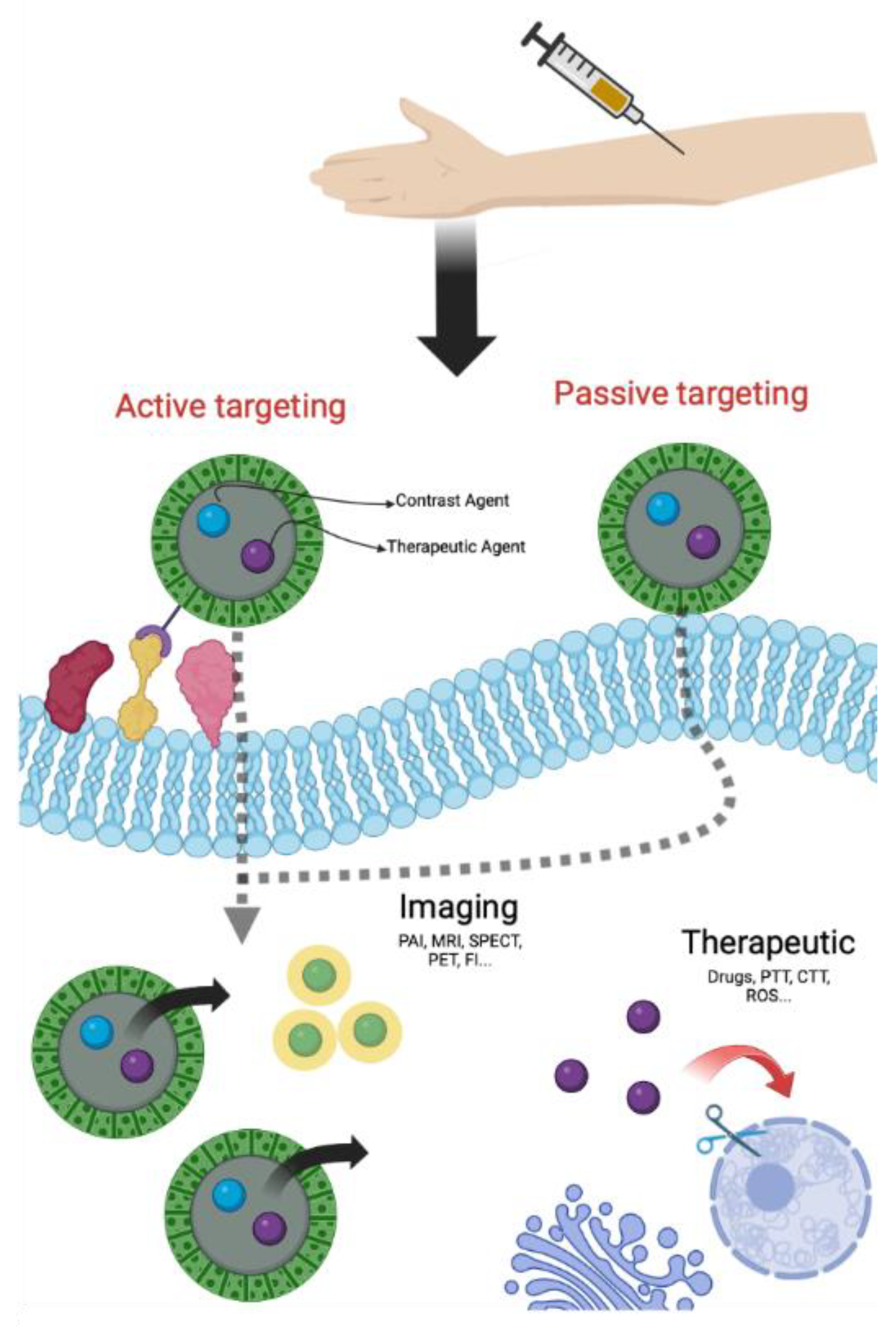
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef]
- Li, S.; Lui, K.-H.; Li, X.; Fang, X.; Lo, W.-S.; Gu, Y.-J.; Wong, W.-T. pH-Triggered Poly(ethylene glycol)–Poly(lactic acid/glycolic acid)/Croconaine Nanoparticles-Assisted Multiplexed Photoacoustic Imaging and Enhanced Photothermal Cancer Therapy. ACS Appl. Bio Mater. 2021, 4, 4152–4164. [Google Scholar] [CrossRef]
- Sonali, M.M.; Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, K.; Choudhary, C.; Mishra, P.K.; Vaidya, B. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif. Cells Nanomed. Biotechnol. 2016, 44, 672–679. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2019, 202, 111642. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA Coated Iron Oxide Nanoparticles as Drug Delivery Vehicles for Cancer Therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Zhong, H.; Niu, S.; Ding, C.; Lv, S. Iridium oxide nanoparticles-based theranostic probe for in vivo tumor imaging and synergistic chem/photothermal treatments of cancer cells. Chem. Eng. J. 2022, 430, 132675. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C. Nanoscale metal–organic frameworks as key players in the context of drug delivery: Evolution toward theranostic platforms. Anal. Bioanal. Chem. 2020, 412, 37–54. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y. Metal-organic framework-based cancer theranostic nanoplatforms. VIEW 2020, 1, e20. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 12, 114496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-X.; Zou, Q.; Sun, S.-K.; Yu, C.; Zhang, X.; Li, R.-J.; Fu, Y.-Y. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Kumar, A.; Bhamidipati, K.; Puvvada, N.; Sahu, S.K. Facile Strategy to Synthesize Magnetic Upconversion Nanoscale Metal–Organic Framework Composites for Theranostics Application. ACS Appl. Bio Mater. 2020, 3, 869–880. [Google Scholar] [CrossRef]
- Mostafavi, E.; Zarepour, A.; Barabadi, H.; Zarrabi, A.; Truong, L.B.; Medina-Cruz, D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: Beginning a new era in cancer theragnostic. Biotechnol. Rep. 2022, 34, e00714. [Google Scholar] [CrossRef]
- Toczek, J.; Sadłocha, M.; Major, K.; Stojko, R. Benefit of Silver and Gold Nanoparticles in Wound Healing Process after Endometrial Cancer Protocol. Biomedicines 2022, 10, 679. [Google Scholar] [CrossRef]
- Nosrati, H.; Seidi, F.; Hosseinmirzaei, A.; Mousazadeh, N.; Mohammadi, A.; Ghaffarlou, M.; Danafar, H.; Conde, J.; Sharafi, A. Prodrug Polymeric Nanoconjugates Encapsulating Gold Nanoparticles for Enhanced X-ray Radiation Therapy in Breast Cancer. Adv. Health Mater. 2022, 11, 2102321. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Jethva, P.; Momin, M.; Khan, T.; Omri, A. Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery. Materials 2022, 15, 2374. [Google Scholar] [CrossRef]
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical Applications of Lanthanide Nanomaterials, for Imaging, Sensing and Therapy. Nanotheranostics 2022, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C.; Su, Q. Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing. Biosensors 2022, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Francés-Soriano, L.; Ferrera-González, J.; González-Béjar, M.; Pérez-Prieto, J. Near-Infrared Excitation/Emission Microscopy with Lanthanide-Based Nanoparticles. Anal. Bioanal. Chem. 2022, 414, 4291–4310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, M.; Zeng, S.; Liu, H. Polydopamine coated multifunctional lanthanide theranostic agent for vascular malformation and tumor vessel imaging beyond 1500 nm and imaging-guided photothermal therapy. Theranostics 2019, 9, 3866–3878. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, Q.; Zhang, L.; Zhang, D.; Li, L.; Jiang, T.; Huang, L.; Xu, H.; Hu, M.; Jing, S. Ferrocene-functionalized core–shell lanthanide-doped upconversion nanoparticles: NIR light promoted chemodynamic therapy and luminescence imaging of solid tumors. Chem. Eng. J. 2022, 438, 135637. [Google Scholar] [CrossRef]
- Silvestri, B.; Armanetti, P.; Sanità, G.; Vitiello, G.; Lamberti, A.; Calì, G.; Pezzella, A.; Luciani, G.; Menichetti, L.; Luin, S.; et al. Silver-nanoparticles as plasmon-resonant enhancers for eumelanin′s photoacoustic signal in a self-structured hybrid nanoprobe. Mater. Sci. Eng. C 2019, 102, 788–797. [Google Scholar] [CrossRef]
- Carrese, B.; Cavallini, C.; Sanità, G.; Armanetti, P.; Silvestri, B.; Calì, G.; Pota, G.; Luciani, G.; Menichetti, L.; Lamberti, A. Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. Int. J. Mol. Sci. 2021, 22, 11228. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Goel, S.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett. 2021, 21, 4692–4699. [Google Scholar] [CrossRef]
- Nikdouz, A.; Namarvari, N.; Shayan, R.G.; Hosseini, A. Comprehensive Comparison of Theranostic Nanoparticles in Breast Cancer. Am. J. Clin. Exp. Immunol. 2022, 11, 1–27. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Hao, Y.; Niu, M.; Hu, Y.; Zhao, H.; Chang, J.; Zhang, Z.; Zhang, Y. Gold nanorod–based poly(lactic-co-glycolic acid) with manganese dioxide core–shell structured multifunctional nanoplatform for cancer theranostic applications. Int. J. Nanomed. 2017, 12, 3059–3075. [Google Scholar] [CrossRef] [PubMed]
- Asati, S.; Pandey, V.; Soni, V. RGD Peptide as a Targeting Moiety for Theranostic Purpose: An Update Study. Int. J. Pept. Res. Ther. 2019, 25, 49–65. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, H.; Wan, C.; Zheng, D.; Zhou, Z.; Xie, S.; Xu, L.; Du, J.; Li, F. Her2-Functionalized Gold-Nanoshelled Magnetic Hybrid Nanoparticles: A Theranostic Agent for Dual-Modal Imaging and Photothermal Therapy of Breast Cancer. Nanoscale Res. Lett. 2019, 14, 235. [Google Scholar] [CrossRef]
- Gholami, L.; Tafaghodi, M.; Abbasi, B.; Daroudi, M.; Kazemi Oskuee, R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell. Physiol. 2019, 234, 1547–1559. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Banerjee, S.; Ghosh, S.S.; Chattopadhyay, A. Simultaneous RGB Emitting Au Nanoclusters in Chitosan Nanoparticles for Anticancer Gene Theranostics. ACS Appl. Mater. Interfaces 2014, 6, 712–724. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid- and Polymer-Based Nanostructures for Cancer Theranostics. Theranostics 2012, 2, 1117–1126. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Yadav, A.S.; Gorain, M.; Chauhan, D.S.; Kundu, G.C.; Srivastava, R.; Selvaraj, K. Graphene Oxide Supported Liposomes as Red Emissive Theranostics for Phototriggered Tissue Visualization and Tumor Regression. ACS Appl. Bio Mater. 2019, 2, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Jo, J.H.; Song, M.G.; Park, J.Y.; Kim, Y.-H.; Youn, H.; Paek, S.H.; Chung, J.-K.; Jeong, J.M.; Lee, Y.-S.; et al. Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models. Theranostics 2019, 9, 7447–7457. [Google Scholar] [CrossRef]
- Zhao, W.; Li, T.; Long, Y.; Guo, R.; Sheng, Q.; Lu, Z.; Li, M.; Li, J.; Zang, S.; Zhang, Z.; et al. Self-promoted Albumin-Based Nanoparticles for Combination Therapy against Metastatic Breast Cancer via a Hyperthermia-Induced “Platelet Bridge”. ACS Appl. Mater. Interfaces 2021, 13, 25701–25714. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Z. Virus-Like Particles as Theranostic Platforms. Adv. Ther. 2020, 3, 1900194. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Pitek, A.S.; Hu, H.; Shukla, S.; Steinmetz, N.F. Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 39468–39477. [Google Scholar] [CrossRef]
- Yan, J.; Yu, J.; Wang, C.; Gu, Z. Red Blood Cells for Drug Delivery. Small Methods 2017, 1, 1700270. [Google Scholar] [CrossRef]
- Guido, C.; Maiorano, G.; Gutiérrez-Millán, C.; Cortese, B.; Trapani, A.; D’Amone, S.; Gigli, G.; Palamà, I. Erythrocytes and Nanoparticles: New Therapeutic Systems. Appl. Sci. 2021, 11, 2173. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional Theranostic Red Blood Cells for Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef]
- Burns, J.M.; Vankayala, R.; Mac, J.T.; Anvari, B. Erythrocyte-Derived Theranostic Nanoplatforms for Near Infrared Fluorescence Imaging and Photodestruction of Tumors. ACS Appl. Mater. Interfaces 2018, 10, 27621–27630. [Google Scholar] [CrossRef] [PubMed]

| Inorganic NPs | |||||
|---|---|---|---|---|---|
| Size and Superficial Charge | Diagnosis | Therapy | Model | Ref. | |
| Iron Oxide NPs | Size 45.7 nm ζ-potential n.r. | Magnetic Resonance Imaging | Magnetic resonance-guided focused ultrasound surgery | In vitro H460 cells In vivo H460 xenograft mice | [37] |
| Iron Oxide NPs | Size 50.8 nm ± 5.2 ζ-potential n.r. | Magnetic Resonance Imaging | Doxorubicin | In vitro 4T1 cells In vivo 4T1 xenograft mice | [38] |
| Iridium oxide NPs | Size 55.0 nm ζ-potential −0.40 mV | Fluorescence imaging | Doxorubicin Photothermal Therapy | In vitro HepG2 cells In vivo HepG2 xenograft mice | [40] |
| MOF Fe3O4@UiO-66 | Size 241.5 nm ± 28.5 ζ-potential −25.7 mV ± 5.2 | Magnetic Resonance Imaging | Doxorubicin | In vitro HeLa cells In vivo HeLa-tumor bearing mice | [44] |
| MOF NaGdF4:Yb/Er@MIL-53(Fe) | Size 245 nm ± 5.0 ζ-potential n.r. | Magnetic Resonance Imaging | Doxorubicin | In vitro B16−F10 and HEK293 cells | [45] |
| Gold NPs | Size 26.5 nm ± 1.1 ζ-potential n.r. | Fluorescence imaging | Photodynamic Therapy | In vitro PC-3 cells In vivo PC-3 xenograft mice | [49] |
| Gold NPs | Size 390.0 nm ζ-potential n.r. | Photoacoustic imaging | Photothermal Therapy | In vitro U-87MG cells In vivo U-87MG xenograft mice | [50] |
| Lanthanide-doped NPs NaYF4:Yb, Tm@NaYF4:Eu | Size 141.9 nm ζ-potential −20.2 mV | Upconversion luminescence imaging | Photodynamic Therapy | In vitro AGS cells In vivo AGS xenograft mice | [56] |
| Lanthanide-doped NPs NaLuF4 | Size 20 × 130 nm ζ-potential n.r. | NIR-II imaging | Photothermal therapy | In vitro HeLa cells In vivo HCT 116 xenograft mice and LLC | [55] |
| Silicon-based | Size 407.0 nm ± 29.0 ζ-potential −17.0 mV ± 2.16 | Photoacoustic Imaging | Photothermal therapy Doxorubicin | In vitro MCF10a and HS578T cells | [20,57,58] |
| Silicon-based | Size 13.5 nm ζ-potential n.r. | PET imaging | Radiotherapy | In vivo 4T1 tumor-bearing mice | [59] |
| Organic NPs | |||||
| PLGA-based NPs | Size 282.1 nm ± 6.2 ζ-potential −9.7 mV ± 1.4 | Magnetic Resonance Imaging | Radio frequency hyperthermia Docetaxel | In vitro MCF7 cells In vivo S180 xenograft mice | [65] |
| PLGA-based NPs | Size 185.1 nm ± 3.3 ζ-potential −1.2 mV ± 0.7 | Photoacoustic imaging | Photothermal therapy | In vitro MDA-MB-231 cells In vivo MDA-MB-231 xenograft mice | [26] |
| PLGA-based NPs | Size 248.3 nm ζ-potential −14.7 mV | Magnetic Resonance Imaging Dual-modal ultrasound | Photothermal therapy | In vitro SKBR3 and MDA-MB-231 cells | [67] |
| Chitosan-based NPs | Size 184.3 nm ± 4.4 ζ-potential + 17.33 mV ± 1.5 | Magnetic Resonance Imaging | Doxorubicin | In vitro C6 cells | [68] |
| Chitosan-based NPs | Size 92.2 nm ζ-potential + 24.0 mV | Fluorescence imaging | Nucleic acid | In vitro HeLa cells | [69] |
| Liposomes-based NPs | Size 95.0 nm ζ-potential n.r. | Positron Emission Tomography Fluorescence Photoacoustic imaging | Photodynamic therapy AQ4N | In vivo 4T1 Balb/c mice | [72] |
| Liposomes-based NPs | Size 150–300 nm ζ-potential + 13.2 mV | Fluorescence imaging | Photothermal therapy Doxorubicin | In vitro MDA-MB-231 and 4T1 cells In vivo 4T1 Balb/c mice | [73] |
| Albumin NPs | Size 142.2 nm ± 4.86 ζ-potential −30 mV | Fluorescence imaging | Photodynamic therapy Photothermal therapy Paclitaxel | In vitro 4T1 cells In vivo 4T1 Balb/c mice | [75] |
| Virus like-NPs | Size 212.0 nm ± 3.40 ζ-potential n.r. | Fluorescence imaging | Doxorubicin | In vitro 4T1and MDA-MB-231 cells In vivo 4T1 Balb/c mice MDA-MB-231 and PC-3 xenograft mice | [78] |
| Red Blood cells-based NPs | Size about 7 µm ζ-potential n.r. | Magnetic Resonance Imaging Fluorescence imaging | Photodynamic therapy Doxorubicin | In vitro 4T1 cells In vivo 4T1 Balb/c mice | [81] |
| Red Blood cells-based NPs | Size 79.0 nm ζ-potential n.r. | Fluorescence imaging | Photodestruction | In vitro SKBR3 cells In vivo SKBR3 xenograft mice | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrese, B.; Sanità, G.; Lamberti, A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers 2022, 14, 4654. https://doi.org/10.3390/cancers14194654
Carrese B, Sanità G, Lamberti A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers. 2022; 14(19):4654. https://doi.org/10.3390/cancers14194654
Chicago/Turabian StyleCarrese, Barbara, Gennaro Sanità, and Annalisa Lamberti. 2022. "Nanoparticles Design for Theranostic Approach in Cancer Disease" Cancers 14, no. 19: 4654. https://doi.org/10.3390/cancers14194654
APA StyleCarrese, B., Sanità, G., & Lamberti, A. (2022). Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers, 14(19), 4654. https://doi.org/10.3390/cancers14194654