Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery
Abstract
Simple Summary
Abstract
1. Introduction
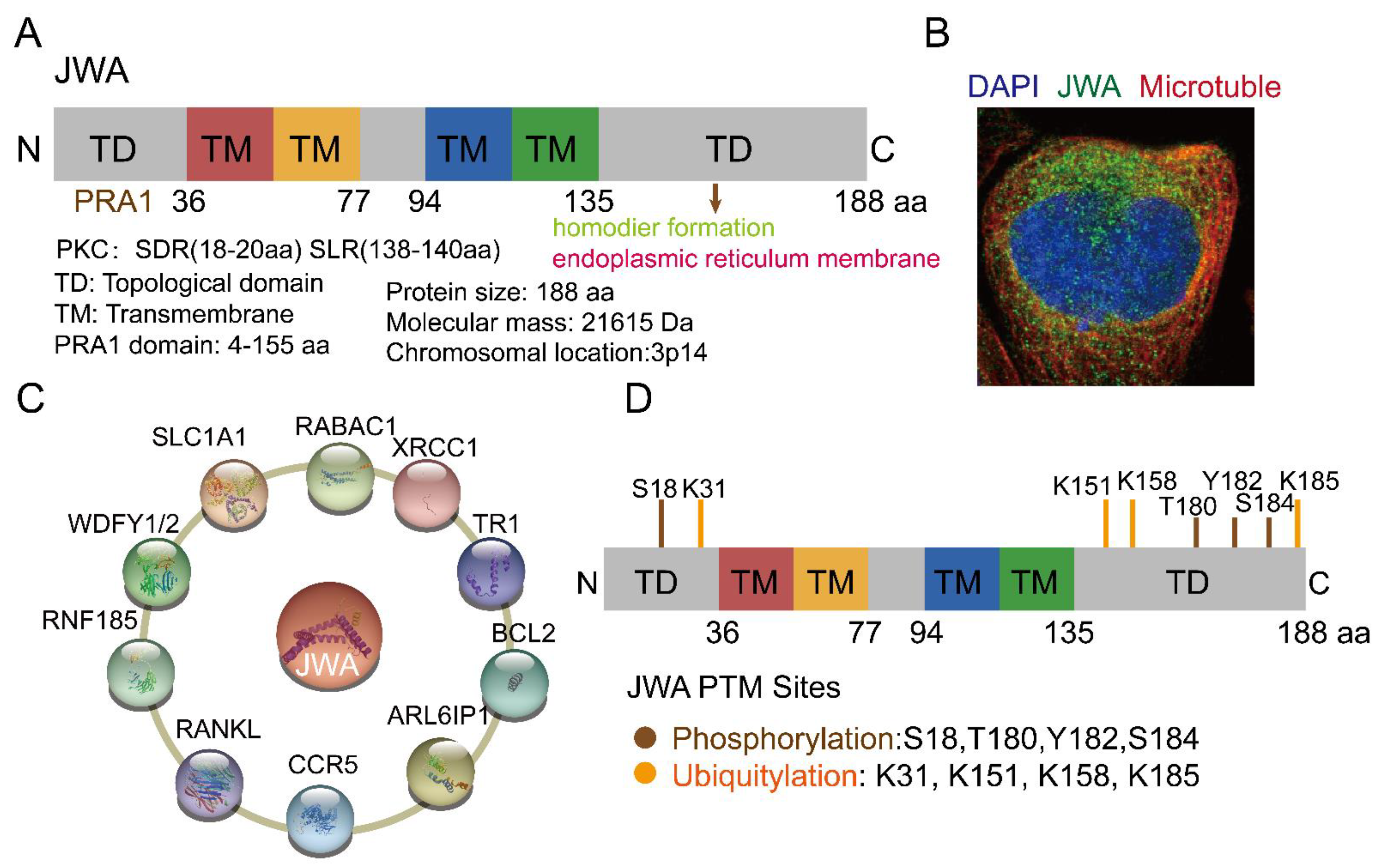
2. The Structure and Functions of JWA
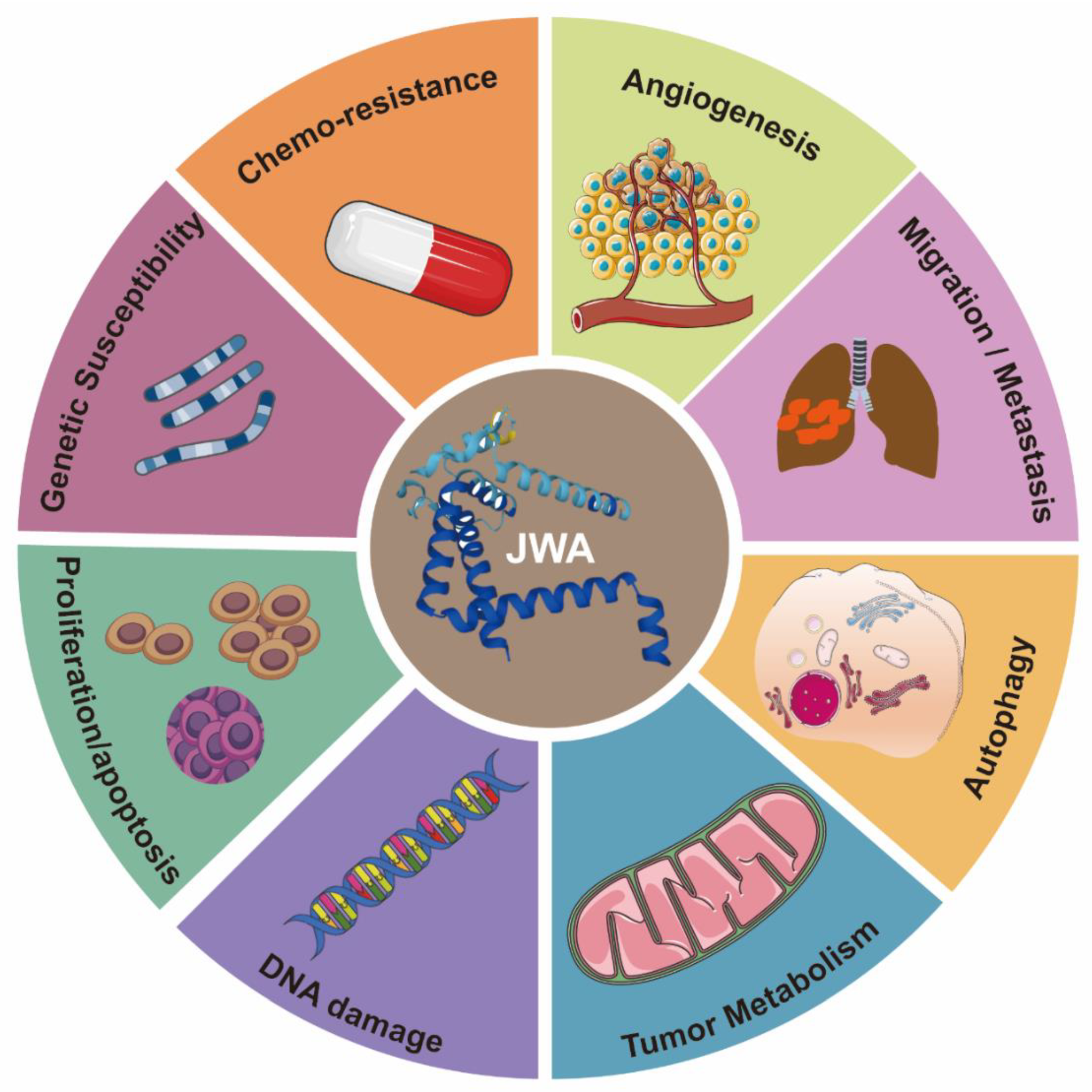
3. The Functions of JWA as a Tumor Suppressor Gene
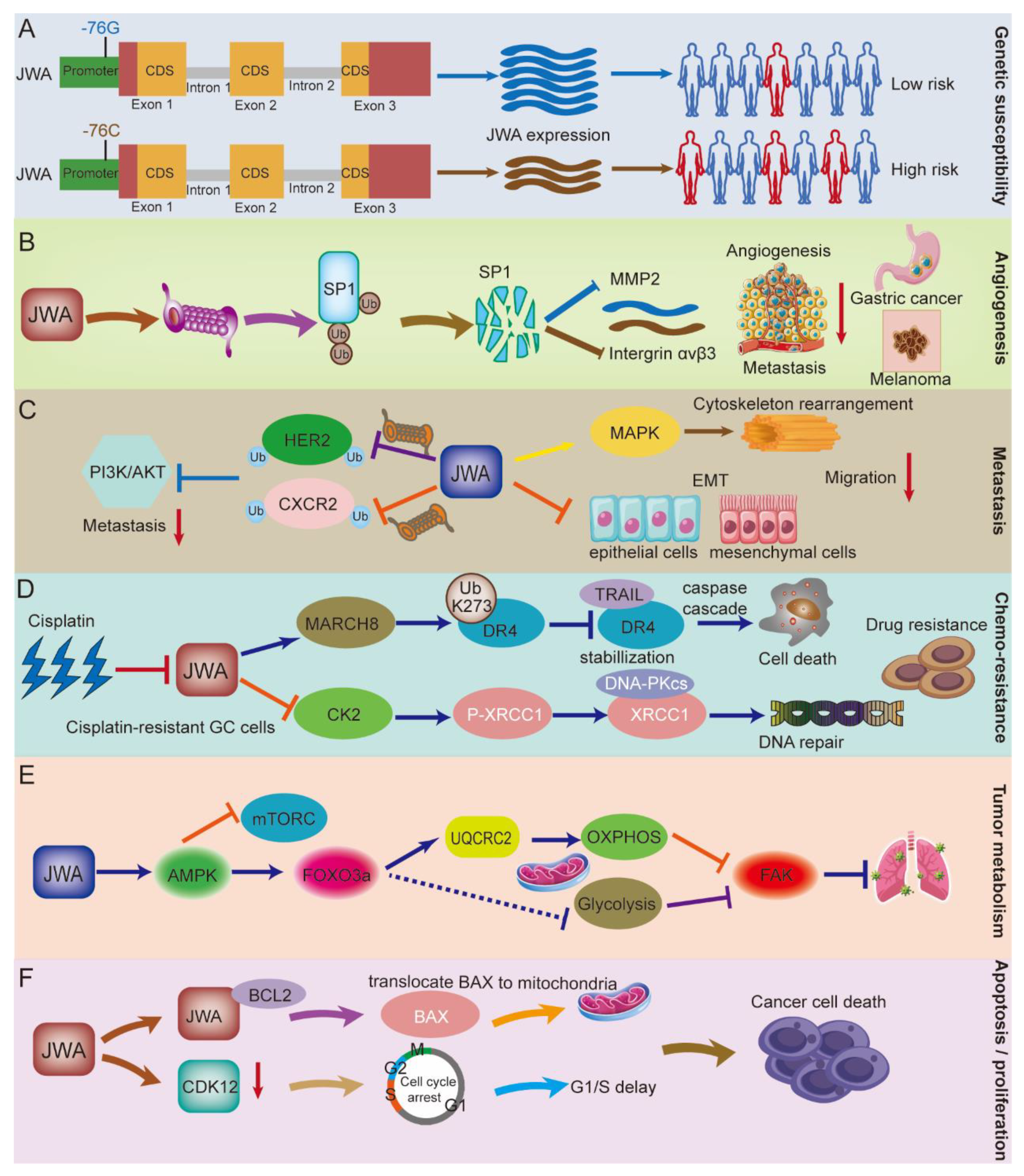
3.1. JWA Gene Polymorphisms Increase the Risk of Cancer Occurrence
3.2. The Role of JWA in Angiogenesis
3.3. The Role of JWA in the Migration, Invasion, and Metastasis of Cancer
3.4. The Role of JWA in Chemotherapy Resistance
3.5. The Role of JWA in Tumor Metabolism
3.6. The Role of JWA in Cell Proliferation and Apoptosis
3.7. The Role of JWA in DNA Damage Repair
3.8. The Role of JWA in Autophagy
3.9. JWA Exists as a Valid Biomarker
4. Anticancer Strategies Targeting JWA
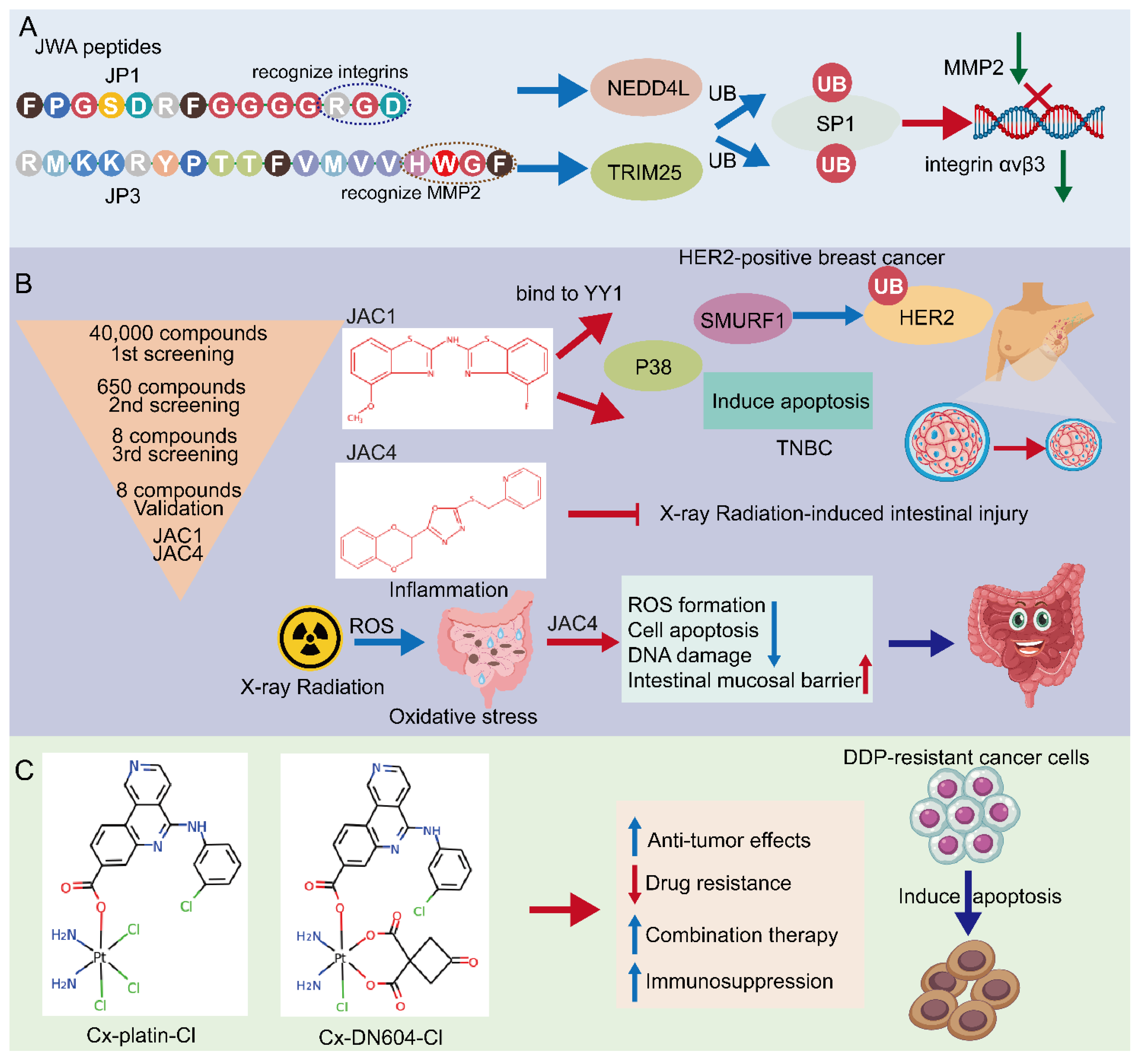
4.1. JWA Peptide—JP1 and JP3
4.2. Small Molecular JWA Agonists
4.3. Emerging JWA-Targeted Pt (IV) Prodrugs Conjugated with CX-4945
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARL6IP1 | ADP-ribosylation factor-like 6 interacting protein 1 |
| ARL6IP5/JWA | ADP-ribosylation-related factor 6-linked protein 5 |
| ATP | Adenosine triphosphate |
| CDK12 | Cyclin-dependent kinase 12 |
| CK2 | Casein-kinase 2 |
| CXCR4 | C-X-C chemokine receptor 4 |
| DDR | DNA damage response |
| DMBA | 7,12-dimethylbenz(a)anthracene |
| DR4 | Death receptor 4 |
| EAAC1 | Excitatory amino acid carrier 1 |
| EGCG | (-)-epigallocatechin-3-gallate |
| EMT | Epithelial-mesenchymal transition |
| E2F1 | E2 promoter binding factor 1 |
| FAK | Focal adhesion kinase |
| FOXO3 | Forkhead box O3 |
| GC | Gastric cancer |
| GTRAP3-18 | Glutamate transport-associated protein 3-18 |
| HCC | Hepatocellular cancer |
| HER2 | Human epidermal growth factor receptor 2 |
| ILK | Integrin-linked kinase |
| IL6 | Interleukin 6 |
| LC3 | Light chain 3 |
| MARCH 8 | Membrane-associated Ring-CH-8 |
| MMP2 | Matrix metalloproteinases 2 |
| MRP | Multidrug resistance-associated protein |
| NEDD4L | Neural precursor cell expressed developmentally downregulated 4-like |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| P-GP | P-glycoprotein |
| PKC | Protein kinase C |
| RA | Retinoic acid |
| RANKL | Receptor activator of NF-kB ligand |
| RNF185 | RING Finger Protein 185 |
| SNPs | Single nucleotide polymorphisms |
| SP1 | Specificity protein 1 |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| TRIM25 | Tripartite motif-containing 25 |
| VEGF | Vascular endothelial-derived growth factor |
| XRCC1 | X-ray repair cross-complementing group 1 |
| YY1 | Zinc-finger Yin Yang 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.H.; Devarakonda, S.; Govindan, R. Overcoming chemotherapy resistance in sclc. J. Thorac. Oncol. 2021, 16, 2002–2015. [Google Scholar] [CrossRef]
- Cytlak, U.M.; Dyer, D.P.; Honeychurch, J.; Williams, K.J.; Travis, M.A.; Illidge, T.M. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat. Rev. Immunol. 2022, 22, 124–138. [Google Scholar] [CrossRef]
- Petroni, G.; Cantley, L.C.; Santambrogio, L.; Formenti, S.C.; Galluzzi, L. Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer. Nat. Rev. Clin. Oncol. 2022, 19, 114–131. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, M.; Ma, T.; Zhang, C.; Huang, S.; Karimzadeh, M.R.; Momtazi-Borojeni, A.A.; Chen, S. Mechanisms underlying low-clinical responses to pd-1/pd-l1 blocking antibodies in immunotherapy of cancer: A key role of exosomal pd-l1. J. Immunother Cancer 2021, 9, e001698. [Google Scholar] [CrossRef]
- Blange, D.; Stroes, C.I.; Derks, S.; Bijlsma, M.F.; van Laarhoven, H. Resistance mechanisms to her2-targeted therapy in gastroesophageal adenocarcinoma: A systematic review. Cancer Treat. Rev. 2022, 108, 102418. [Google Scholar] [CrossRef] [PubMed]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting mutations in cancer. J. Clin. Investig. 2022, 132, e154943. [Google Scholar] [CrossRef]
- Zhou, J. Structure and Function of the Novel Environmental Response Gene Jwa; Nanjing Medical University: Nanjing, China, 2003. [Google Scholar]
- Xia, W.; Zhou, J.; Cao, H.; Zou, C.; Wang, C.; Shen, Q.; Lu, H. Relationship between structure and function of jwa in the modulation of cell differentiation. China Sci. Bull. Engl. Version 2001, 24, 2063–2067. [Google Scholar]
- Mao, W.G.; Li, A.P.; Ye, J.; Huang, S.; Li, A.Q.; Zhou, J.W. Effect of differentiation inducer and heat stress on the expression of jwa protein and hsp70 of k562 cells. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2003, 21, 253–256. [Google Scholar]
- Mao, W.G.; Li, A.P.; Ye, J.; Huang, S.; Li, A.Q.; Zhou, J.W. Expressions of jwa protein and heat stress protein 70 induced by cell differentiation inducers combined with heat stress in k562 cells. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2004, 22, 60–63. [Google Scholar] [PubMed]
- Wang, R.; Zhao, X.; Xu, J.; Wen, Y.; Li, A.; Lu, M.; Zhou, J. Astrocytic jwa deletion exacerbates dopaminergic neurodegeneration by decreasing glutamate transporters in mice. Cell Death Dis. 2018, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.H.; Sun, H.B.; Ye, Y.; Yang, J.J.; Shi, Y.W.; Lu, M.; Hu, G.; Zhou, J.W. Astrocytic jwa expression is essential to dopaminergic neuron survival in the pathogenesis of parkinson’s disease. CNS Neurosci. Ther. 2014, 20, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, J.; Wu, J.; Shen, L.; Zeng, J.; Ding, J.; Wu, Y.; Gong, Z.; Li, A.; Xu, S.; et al. Jwa regulates melanoma metastasis by integrin alphavbeta3 signaling. Oncogene 2010, 29, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Huang, Y.; Xia, X.; Zhang, J.; Zhou, Y.; Tan, Y.; He, S.; Qiang, F.; Li, A.; et al. Jwa suppresses tumor angiogenesis via sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis 2014, 35, 442–451. [Google Scholar] [CrossRef]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. Dna repair, genome stability and cancer: A historical perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef]
- Tang, W.Y.; Wang, L.; Li, C.; Hu, Z.B.; Chen, R.; Zhu, Y.J.; Shen, H.B.; Wei, Q.Y.; Zhou, J.W. Identification and functional characterization of jwa polymorphisms and their association with risk of gastric cancer and esophageal squamous cell carcinoma in a chinese population. J. Toxicol. Environ. Health A 2007, 70, 885–894. [Google Scholar] [CrossRef]
- Wu, W.; Li, C.P.; Chen, R.; Cao, X.J.; Li, A.P.; Wang, Y.; Yang, K.H.; Qian, L.X.; Liu, Q.Z.; Li, Z.L.; et al. A case-control study on jwa promoter -76G-->C polymorphism and the susceptibility of bladder cancer. Zhonghua Yixue Yichuanxue Zazhi 2005, 22, 648–652. [Google Scholar]
- Lu, J.; Tang, Y.; Cheng, Y.; Zhang, G.; Yip, A.; Martinka, M.; Dong, Z.; Zhou, J.; Li, G. Ing4 regulates jwa in angiogenesis and their prognostic value in melanoma patients. Br. J. Cancer 2013, 109, 2842–2852. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Q.; Wang, Q.; Sun, Y.; Wang, S.; Li, A.; Xu, S.; Roe, O.D.; Wang, M.; Zhang, R.; et al. Jwa reverses cisplatin resistance via the ck2-xrcc1 pathway in human gastric cancer cells. Cell Death Dis. 2014, 5, e1551. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Zhu, L.; Chen, M.; Xu, W.; Panday, S.; Wang, Z.; Li, A.; Roe, O.D.; Chen, R.; et al. Jwa regulates trail-induced apoptosis via march8-mediated dr4 ubiquitination in cisplatin-resistant gastric cancer cells. Oncogenesis 2017, 6, e353. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Qian, C.; Xie, Y.; Huang, X.; Chen, J.; Ren, Y.; Fu, Z.; Li, Y.; Zeng, T.; Yang, F.; et al. Jwa suppresses proliferation in trastuzumab-resistant breast cancer by downregulating cdk12. Cell Death Discov. 2021, 7, 306. [Google Scholar] [CrossRef]
- Chang, X.; Liu, X.; Wang, H.; Yang, X.; Gu, Y. Glycolysis in the progression of pancreatic cancer. Am. J. Cancer Res. 2022, 12, 861–872. [Google Scholar] [PubMed]
- Cui, J.; Shu, C.; Xu, J.; Chen, D.; Li, J.; Ding, K.; Chen, M.; Li, A.; He, J.; Shu, Y.; et al. Jp1 suppresses proliferation and metastasis of melanoma through mek1/2 mediated nedd4l-sp1-integrin alphavbeta3 signaling. Theranostics 2020, 10, 8036–8050. [Google Scholar] [CrossRef]
- Chen, J.J.; Ren, Y.L.; Shu, C.J.; Zhang, Y.; Chen, M.J.; Xu, J.; Li, J.; Li, A.P.; Chen, D.Y.; He, J.D.; et al. Jp3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through trim25/sp1/mmp2 axis. J. Exp. Clin. Cancer Res. 2020, 39, 118. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Che, Z.; Shu, C.; Chen, D.; Ding, K.; Li, A.; Zhou, J. Jp3 enhances the toxicity of cisplatin on drug-resistant gastric cancer cells while reducing the damage to normal cells. J. Cancer 2021, 12, 1894–1906. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Chen, D.; Zhai, Z.; Chen, J.; Li, A.; Liang, Y.; Zhou, J. Jac1 suppresses proliferation of breast cancer through the jwa/p38/smurf1/her2 signaling. Cell Death Discov. 2021, 7, 85. [Google Scholar] [CrossRef]
- Zhai, Z.; Ren, Y.; Shu, C.; Chen, D.; Liu, X.; Liang, Y.; Li, A.; Zhou, J. Jac1 targets yy1 mediated jwa/p38 mapk signaling to inhibit proliferation and induce apoptosis in tnbc. Cell Death Discov. 2022, 8, 169. [Google Scholar] [CrossRef]
- Li, A.; Li, A.; Mao, W.; Chen, H.; Huang, S.; Qi, H.; Ye, J.; Zhang, Z.; Wang, X.; Sun, F.; et al. Jwa, a novel microtubule-associated protein, regulates homeostasis of intracellular amino acids in pc12 cells. Chin. Sci. Bull. 2003, 48, 1828–1834. [Google Scholar] [CrossRef]
- Chen, H.; Bai, J.; Ye, J.; Liu, Z.; Chen, R.; Mao, W.; Li, A.; Zhou, J. Jwa as a functional molecule to regulate cancer cells migration via mapk cascades and f-actin cytoskeleton. Cell. Signal. 2007, 19, 1315–1327. [Google Scholar] [CrossRef]
- Chen, R.; Li, A.; Zhu, T.; Li, C.; Liu, Q.; Chang, H.C.; Zhou, J. Jwa—A novel environmental-responsive gene, involved in estrogen receptor-associated signal pathway in mcf-7 and mda-mb-231 breast carcinoma cells. J. Toxicol. Environ. Health A 2005, 68, 445–456. [Google Scholar] [CrossRef]
- Lin, C.I.; Orlov, I.; Ruggiero, A.M.; Dykes-Hoberg, M.; Lee, A.; Jackson, M.; Rothstein, J.D. Modulation of the neuronal glutamate transporter eaac1 by the interacting protein gtrap3-18. Nature 2001, 410, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Schweneker, M.; Bachmann, A.S.; Moelling, K. Jm4 is a four-transmembrane protein binding to the ccr5 receptor. FEBS Lett. 2005, 579, 1751–1758. [Google Scholar] [CrossRef]
- Ikemoto, M.J.; Inoue, K.; Akiduki, S.; Osugi, T.; Imamura, T.; Ishida, N.; Ohtomi, M. Identification of addicsin/gtrap3-18 as a chronic morphine-augmented gene in amygdala. Neuroreport 2002, 13, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Fo, C.S.; Coleman, C.S.; Wallick, C.J.; Vine, A.L.; Bachmann, A.S. Genomic organization, expression profile, and characterization of the new protein pra1 domain family, member 2 (praf2). Gene 2006, 371, 154–165. [Google Scholar] [CrossRef]
- Koomoa, D.L.; Go, R.C.; Wester, K.; Bachmann, A.S. Expression profile of praf2 in the human brain and enrichment in synaptic vesicles. Neurosci. Lett. 2008, 436, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Peng, Y.; Ding, Y.; Deng, L.; Fu, Q. Overexpression of arl6ip5 in osteoblast regulates rankl subcellualr localization. Biochem. Biophys. Res. Commun. 2015, 464, 1275–1281. [Google Scholar] [CrossRef]
- Akiduki, S.; Ikemoto, M.J. Modulation of the neural glutamate transporter eaac1 by the addicsin-interacting protein arl6ip1. J. Biol. Chem. 2008, 283, 31323–31332. [Google Scholar] [CrossRef] [PubMed]
- Arano, T.; Fujisaki, S.; Ikemoto, M.J. Identification of tomoregulin-1 as a novel addicsin-associated factor. Neurochem. Int. 2014, 71, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Watabe, M.; Nakaki, T. Modulation of neuronal glutathione synthesis by eaac1 and its interacting protein gtrap3-18. Amino Acids 2012, 42, 163–169. [Google Scholar] [CrossRef]
- Watabe, M.; Aoyama, K.; Nakaki, T. Regulation of glutathione synthesis via interaction between glutamate transport-associated protein 3-18 (gtrap3-18) and excitatory amino acid carrier-1 (eaac1) at plasma membrane. Mol. Pharmacol. 2007, 72, 1103–1110. [Google Scholar] [CrossRef]
- Huang, S.; Shen, Q.; Mao, W.G.; Li, A.P.; Ye, J.; Liu, Q.Z.; Zou, C.P.; Zhou, J.W. Jwa, a novel signaling molecule, involved in all-trans retinoic acid induced differentiation of hl-60 cells. J. Biomed. Sci. 2006, 13, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, A.; Qiu, W.; Zhou, J.; Zhou, J. The effect of pkc phosphorylation sites mutation in jwa coding region on tpa-induced mcf-7 cell differentiation. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2007, 25, 398–401. [Google Scholar] [PubMed]
- Qiu, D.; Wang, Q.; Wang, Z.; Chen, J.; Yan, D.; Zhou, Y.; Li, A.; Zhang, R.; Wang, S.; Zhou, J. Rnf185 modulates jwa ubiquitination and promotes gastric cancer metastasis. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Vento, M.T.; Zazzu, V.; Loffreda, A.; Cross, J.R.; Downward, J.; Stoppelli, M.P.; Iaccarino, I. Praf2 is a novel bcl-xl/bcl-2 interacting protein with the ability to modulate survival of cancer cells. PLoS ONE 2010, 5, e15636. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The string database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Chabra, I.; Kornhauser, J.M.; Skrzypek, E.; Zhang, B. Phosphosite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 2004, 4, 1551–1561. [Google Scholar] [CrossRef]
- Lin, J.; Ma, T.; Jiang, X.; Ge, Z.; Ding, W.; Wu, Y.; Jiang, G.; Feng, J.; Cui, G.; Tan, Y. Jwa regulates human esophageal squamous cell carcinoma and human esophageal cells through different mitogen-activated protein kinase signaling pathways. Exp. Ther. Med. 2014, 7, 1767–1771. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Ma, T.L.; Ge, Z.J.; Lin, J.; Ding, W.L.; Feng, J.K.; Zhou, S.J.; Chen, G.C.; Tan, Y.F.; Cui, G.X. Jwa gene regulates panc-1 pancreatic cancer cell behaviors through mek-erk1/2 of the mapk signaling pathway. Oncol. Lett. 2014, 8, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, J.; Ge, Z.; Chen, H.; Ding, W.; Zhu, W.; Tang, X.; Chen, Y.; Tan, Y.; Ma, T. Effects of the jwa gene in the regulation of human breast cancer cells. Mol. Med. Rep. 2015, 11, 3848–3853. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhou, J.W.; Sheng, R.L.; Zhu, G.R.; Cao, H.X.; Lu, H. Jwa gene in regulating committed differentiation of hl-60 cells induced by atra, ara-c and tpa. Zhongguo Shiyan Xueyexue Zazhi 2005, 13, 804–808. [Google Scholar]
- Huang, S.; Shen, Q.; Mao, W.G.; Li, A.P.; Ye, J.; Liu, Q.Z.; Zou, C.P.; Zhou, J.W. Jwa, a novel signaling molecule, involved in the induction of differentiation of human myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2006, 341, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tang, Y.; Farshidpour, M.; Cheng, Y.; Zhang, G.; Jafarnejad, S.M.; Yip, A.; Martinka, M.; Dong, Z.; Zhou, J.; et al. Jwa inhibits melanoma angiogenesis by suppressing ilk signaling and is an independent prognostic biomarker for melanoma. Carcinogenesis 2013, 34, 2778–2788. [Google Scholar] [CrossRef]
- Gu, D.A.; Li, A.P.; Zhu, T.; Ye, J.; Zhou, J.W. Relationship between jwa expression and dna damage-repair in human embryonic lung cells by benzo(a) pyrene. Zhonghua Yufang Yixue Zazhi 2005, 39, 187–190. [Google Scholar]
- Zhao, M.; Chen, R.; Li, A.P.; Zhou, J.W. Effects of hemin and thermal stress exposure on jwa expression. Zhonghua Laodong Weisheng Zhiyebing Zazhi 2006, 24, 209–213. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, R.; Xiong, J.; Yan, D.; Li, A.; Wang, S.; Xu, J.; Zhou, J. Jwa antagonizes paraquat-induced neurotoxicity via activation of nrf2. Toxicol. Lett. 2017, 277, 32–40. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, W.Y.; Li, C.P.; Chen, R.; Zhu, Y.J.; Huang, S.; Li, A.P.; Zhou, J.W. Functional variations in the jwa gene are associated with increased odds of leukemias. Leuk. Res. 2007, 31, 783–790. [Google Scholar] [CrossRef]
- Li, C.P.; Zhu, Y.J.; Chen, R.; Wu, W.; Li, A.P.; Liu, J.; Liu, Q.Z.; Wei, Q.Y.; Zhang, Z.D.; Zhou, J.W. Functional polymorphisms of jwa gene are associated with risk of bladder cancer. J. Toxicol. Environ. Health A 2007, 70, 876–884. [Google Scholar] [CrossRef]
- Zheng, R.; Li, F.; Li, F.; Gong, A. Targeting tumor vascularization: Promising strategies for vascular normalization. J. Cancer Res. Clin. Oncol. 2021, 147, 2489–2505. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.R.; Cereda, V.; Coppola, L.; Giordano, G.; Remo, A.; De Santis, E. Propensity for early metastatic spread in breast cancer: Role of tumor vascularization features and tumor immune infiltrate. Cancers 2021, 13, 5917. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.U.; Varricchi, G.; Galdiero, M.R. Neutrophil extracellular traps in cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors—Clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Li, L.; Zhao, G.; Liu, J.X. Copper stress impairs angiogenesis and lymphangiogenesis during zebrafish embryogenesis by down-regulating perk1/2-foxm1-mmp2/9 axis and epigenetically regulating ccbe1 expression. Angiogenesis 2022, 25, 241–257. [Google Scholar] [CrossRef]
- Rao, N.; Lee, Y.F.; Ge, R. Novel endogenous angiogenesis inhibitors and their therapeutic potential. Acta Pharmacol. Sin. 2015, 36, 1177–1190. [Google Scholar] [CrossRef]
- Lyden, D.; Ghajar, C.M.; Correia, A.L.; Aguirre-Ghiso, J.A.; Cai, S.; Rescigno, M.; Zhang, P.; Hu, G.; Fendt, S.M.; Boire, A.; et al. Metastasis. Cancer Cell 2022, 40, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Reticker-Flynn, N.E.; Zhang, W.; Belk, J.A.; Basto, P.A.; Escalante, N.K.; Pilarowski, G.; Bejnood, A.; Martins, M.M.; Kenkel, J.A.; Linde, I.L.; et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 2022, 185, 1924–1942. [Google Scholar] [CrossRef]
- Liu, S.J.; Dang, H.X.; Lim, D.A.; Feng, F.Y.; Maher, C.A. Long noncoding rnas in cancer metastasis. Nat. Rev. Cancer 2021, 21, 446–460. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L.; Yang, F.; Pei, B.; Liu, X.; Zhou, J.; Zhu, Y.; Wang, S. Jwa suppresses the invasion of human breast carcinoma cells by downregulating the expression of cxcr4. Mol. Med. Rep. 2018, 17, 8137–8144. [Google Scholar] [CrossRef]
- Wu, X.; Chen, H.; Gao, Q.; Bai, J.; Wang, X.; Zhou, J.; Qiu, S.; Xu, Y.; Shi, Y.; Wang, X.; et al. Downregulation of jwa promotes tumor invasion and predicts poor prognosis in human hepatocellular carcinoma. Mol. Carcinog. 2014, 53, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Liu, W.; Huang, L.; Wang, Y.; Li, D.; Wang, G.; Zhao, Z.; Chi, X.; Xue, Y.; et al. Long noncoding rna vestar regulates lymphangiogenesis and lymph node metastasis of esophageal squamous cell carcinoma by enhancing vegfc mrna stability. Cancer Res. 2021, 81, 3187–3199. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Y.; Zhao, Y.; Kong, Y.; Zheng, H.; Li, Y.; Gao, B.; Ai, L.; Huang, H.; Huang, J.; et al. Circehbp1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via mir-130a-3p/tgfbetar1/vegf-d signaling. Mol. Ther. 2021, 29, 1838–1852. [Google Scholar] [CrossRef]
- Zhou, J.; Ge, Z.; Tan, Y.; Jiang, G.; Feng, J.; Wang, H.; Shi, G. Downregulation of jwa expression in human esophageal squamous cell carcinoma and its clinical significance. Oncol. Res. 2012, 20, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, W.; Wang, K.; Ma, L.; Xu, J.; Xu, T.; Roe, O.D.; Li, A.; Zhou, J.; Shu, Y. Jwa loss promotes cell migration and cytoskeletal rearrangement by affecting her2 expression and identifies a high-risk subgroup of her2-positive gastric carcinoma patients. Oncotarget 2016, 7, 36865–36884. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, X.; Wang, S.; Wu, X.; Zhang, J.; Zhou, Y.; Tan, Y.; He, S.; Qiang, F.; Li, A.; et al. High fak combined with low jwa expression: Clinical prognostic and predictive role for adjuvant fluorouracil-leucovorin-oxaliplatin treatment in resectable gastric cancer patients. J. Gastroenterol. 2013, 48, 1034–1044. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic emt: A multi-tool for tumor progression. Embo. J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. Emt transition states during tumor progression and metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. Emt, met, plasticity, and tumor metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef]
- Qi, H.; Li, A. Jwa deficiency induces malignant transformation of murine embryonic fibroblast cells. Exp. Ther. Med. 2018, 15, 3509–3515. [Google Scholar] [PubMed]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and overcoming resistance to parp inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Hao, L.; Han, X.X.; Wu, Z.X.; Pang, K.; Dong, Y.; Qin, J.X.; Wang, G.Y.; Zhang, X.M.; Xia, T.; et al. Targeting hnrnpu to overcome cisplatin resistance in bladder cancer. Mol. Cancer 2022, 21, 37. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of abc transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Tian, T.; Tian, T.; Li, A.; Zhou, J.; Xu, S. Effect of Jwa genes on p-glycoprotein expression and its function in tumor cells. Chin. J. Tumor Biother. 2010, 17, 292–296. [Google Scholar]
- Zhang, Y.; Zhou, J.; Xu, W.; Li, A.; Zhou, J.; Xu, S. Jwa sensitizes p-glycoprotein-mediated drug-resistant choriocarcinoma cells to etoposide via jnk and mitochondrial-associated signal pathway. J. Toxicol. Environ. Health Part. A 2009, 72, 774–781. [Google Scholar] [CrossRef]
- Wang, S.; Wu, X.; Chen, Y.; Zhang, J.; Ding, J.; Zhou, Y.; He, S.; Tan, Y.; Qiang, F.; Bai, J.; et al. Prognostic and predictive role of jwa and xrcc1 expressions in gastric cancer. Clin. Cancer Res. 2012, 18, 2987–2996. [Google Scholar] [CrossRef]
- Wei, B.; Han, Q.; Xu, L.; Zhang, X.; Zhu, J.; Wan, L.; Jin, Y.; Qian, Z.; Wu, J.; Gao, Y.; et al. Effects of jwa, xrcc1 and brca1 mrna expression on molecular staging for personalized therapy in patients with advanced esophageal squamous cell carcinoma. BMC Cancer 2015, 15, 331. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Yu, Y.; Deng, J.; Zhang, H.; Yao, Q.; Fan, Y.; Zhou, Y. Expression of jwa and xrcc1 as prognostic markers for gastric cancer recurrence. Int. J. Clin. Exp. Pathol. 2020, 13, 3120–3127. [Google Scholar]
- Liu, X.; Wang, S.; Xia, X.; Chen, Y.; Zhou, Y.; Wu, X.; Zhang, J.; He, S.; Tan, Y.; Qiang, F.; et al. Synergistic role between p53 and jwa: Prognostic and predictive biomarkers in gastric cancer. PLoS ONE 2012, 7, e52348. [Google Scholar] [CrossRef]
- Ye, Y.; Li, X.; Yang, J.; Miao, S.; Wang, S.; Chen, Y.; Xia, X.; Wu, X.; Zhang, J.; Zhou, Y.; et al. Mdm2 is a useful prognostic biomarker for resectable gastric cancer. Cancer Sci. 2013, 104, 590–598. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; Deberardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yu, M.; Ma, X.; Sun, J.; Liu, C.; Wang, C.; Wu, S.; Fu, P.; Yang, Z.; He, Y.; et al. Ifnalpha potentiates anti-pd-1 efficacy by remodeling glucose metabolism in the hepatocellular carcinoma microenvironment. Cancer Discov. 2022, 12, 1718–1741. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. Ros/pi3k/akt and wnt/beta-catenin signalings activate hif-1alpha-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Gimple, R.C.; Kidwell, R.L.; Kim, L.; Sun, T.; Gromovsky, A.D.; Wu, Q.; Wolf, M.; Lv, D.; Bhargava, S.; Jiang, L.; et al. Glioma stem cell-specific superenhancer promotes polyunsaturated fatty-acid synthesis to support egfr signaling. Cancer Discov. 2019, 9, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef]
- An, Y.; Duan, H. The role of m6a rna methylation in cancer metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef]
- Kim, J.; Deberardinis, R.J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Wang, X.; Ding, K.; Wang, Z.; Li, A.; Chen, D.; Zhou, J. Jwa regulates energy metabolism reprogramming to inhibit pancreatic cancer cell migration. J. Nanjing Med. Univ. Nat. Sci. Ed. 2019, 39, 1728–1736. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef]
- Shen, L.; Xu, W.; Li, A.; Ye, J.; Zhou, J. Jwa enhances As2O3induced tubulin polymerization and apoptosis via p38 in hela and mcf-7 cells. Apoptosis 2011, 16, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ye, J.; Zhao, X.; Li, A.; Zhou, J. Jwa is required for arsenic trioxide induced apoptosis in hela and mcf-7 cells via reactive oxygen species and mitochondria linked signal pathway. Toxicol. Appl. Pharmacol. 2008, 230, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. Dna damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal. Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Reuvers, T.; Kanaar, R.; Nonnekens, J. Dna damage-inducing anticancer therapies: From global to precision damage. Cancers 2020, 12, 2098. [Google Scholar] [CrossRef]
- Cheng, B.; Pan, W.; Xing, Y.; Xiao, Y.; Chen, J.; Xu, Z. Recent advances in ddr (dna damage response) inhibitors for cancer therapy. Eur. J. Med. Chem. 2022, 230, 114109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gong, Z.; Chen, R.; Liu, Y.; Li, A.; Li, G.; Zhou, J. Jwa regulates xrcc1 and functions as a novel base excision repair protein in oxidative-stress-induced dna single-strand breaks. Nucleic Acids Res. 2009, 37, 1936–1950. [Google Scholar] [CrossRef]
- Gong, Z.; Shi, Y.; Zhu, Z.; Li, X.; Ye, Y.; Zhang, J.; Li, A.; Li, G.; Zhou, J. Jwa deficiency suppresses dimethylbenz[a]anthracene-phorbol ester induced skin papillomas via inactivation of mapk pathway in mice. PLoS ONE 2012, 7, e34154. [Google Scholar] [CrossRef]
- Huang, D.; Kraus, W.L. The expanding universe of parp1-mediated molecular and therapeutic mechanisms. Mol. Cell 2022, 82, 2315–2334. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, C.; Yang, G.; Zhao, B.; Wang, W. The role of autophagy in pancreatic cancer progression. Biochim. Biophys. Acta. Rev. Cancer 2021, 1876, 188592. [Google Scholar] [CrossRef] [PubMed]
- Raudenska, M.; Balvan, J.; Masarik, M. Crosstalk between autophagy inhibitors and endosome-related secretory pathways: A challenge for autophagy-based treatment of solid cancers. Mol. Cancer 2021, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Y.; Wei, B.; Wang, J.R.; Wang, Q.L.; Gao, Y.; Chen, X.F. Autophagy regulated by jwa influenced sensitivity of esophageal cancer to cisplatin. Zhonghua Yixue Zazhi 2017, 97, 2141–2144. [Google Scholar] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal. Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Tang, K.; Lv, J. Peptide-drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol. Sci. 2021, 42, 857–869. [Google Scholar] [CrossRef]
- Wilkes, G.M. Targeted therapy: Attacking cancer with molecular and immunological targeted agents. Asia Pac. J. Oncol. Nurs. 2018, 5, 137–155. [Google Scholar] [CrossRef]
- Casali, B.C.; Gozzer, L.T.; Baptista, M.P.; Altei, W.F.; Selistre-De-Araujo, H.S. The effects of alphavbeta3 integrin blockage in breast tumor and endothelial cells under hypoxia in vitro. Int. J. Mol. Sci. 2022, 23, 1745. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.; Kadri, M.; Arosio, D.; Dal Corso, A.; Coll, J.L.; Gennari, C.; Boturyn, D. Multimeric presentation of rgd peptidomimetics enhances integrin binding and tumor cell uptake. Chemistry 2020, 26, 7492–7496. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin signaling in cancer: Mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.; Roper, J.A.; Jenkins, R.G.; Hatley, R. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkila, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; et al. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774. [Google Scholar] [CrossRef]
- Turunen, M.P.; Puhakka, H.L.; Koponen, J.K.; Hiltunen, M.O.; Rutanen, J.; Leppanen, O.; Turunen, A.M.; Narvanen, A.; Newby, A.C.; Baker, A.H.; et al. Peptide-retargeted adenovirus encoding a tissue inhibitor of metalloproteinase-1 decreases restenosis after intravascular gene transfer. Mol. Ther. 2002, 6, 306–312. [Google Scholar] [CrossRef]
- Lu, B.; Atala, A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov. Today 2014, 19, 801–808. [Google Scholar] [CrossRef] [PubMed]
- An, G. Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J. Clin. Pharmacol. 2020, 60, 149–163. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, W.; Xiang, L.; Wu, X.; Zhang, P.; Wang, J.; Liu, G.; Zhang, W.; Peng, Y.; Huang, X.; et al. The p300/yy1/mir-500a-5p/hdac2 signalling axis regulates cell proliferation in human colorectal cancer. Nat. Commun. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Fang, E.; Mei, H.; Chen, Y.; Li, H.; Li, D.; Song, H.; Wang, J.; Hong, M.; Xiao, W.; et al. Cis-acting circ-ctnnb1 promotes beta-catenin signaling and cancer progression via ddx3-mediated transactivation of yy1. Cancer Res. 2019, 79, 557–571. [Google Scholar] [CrossRef]
- Pan, G.; Diamanti, K.; Cavalli, M.; Lara, G.A.; Komorowski, J.; Wadelius, C. Multifaceted regulation of hepatic lipid metabolism by yy1. Life Sci. Alliance 2021, 4, e202000928. [Google Scholar] [CrossRef] [PubMed]
- Khachigian, L.M. The yin and yang of yy1 in tumor growth and suppression. Int. J. Cancer 2018, 143, 460–465. [Google Scholar] [CrossRef]
- Wang, K.X.; Cui, W.W.; Yang, X.; Tao, A.B.; Lan, T.; Li, T.S.; Luo, L. Mesenchymal stem cells for mitigating radiotherapy side effects. Cells 2021, 10, 294. [Google Scholar] [CrossRef]
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Li, X.; Wang, L.; Hu, L.; Li, A.; Zhou, J. Jac4 protects from x-ray radiation-induced intestinal injury by jwa-mediated anti-oxidation/inflammation signaling. Antioxidants 2022, 11, 1067. [Google Scholar] [CrossRef]
- Zhao, J.; Blayney, A.; Liu, X.; Gandy, L.; Jin, W.; Yan, L.; Ha, J.H.; Canning, A.J.; Connelly, M.; Yang, C.; et al. Egcg binds intrinsically disordered n-terminal domain of p53 and disrupts p53-mdm2 interaction. Nat. Commun. 2021, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin gallate (egcg) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (egcg): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef]
- Li, Y.; Shen, X.; Wang, X.; Li, A.; Wang, P.; Jiang, P.; Zhou, J.; Feng, Q. Egcg regulates the cross-talk between jwa and topoisomerase iiα in non-small-cell lung cancer (nsclc) cells. Sci. Rep. 2015, 5, 11009. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Chen, F.; Huang, X.; Wu, M.; Gou, S.; Hu, W. A ck2-targeted pt(iv) prodrug to disrupt dna damage response. Cancer Lett. 2017, 385, 168–178. [Google Scholar] [CrossRef]
- Gibson, D. Platinum(iv) anticancer agents; Are we en route to the holy grail or to a dead end? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Pei, S.; Wang, X.; Zhu, Q.; Gou, S. Emerging jwa-targeted pt(iv) prodrugs conjugated with cx-4945 to overcome chemo-immune-resistance. Biochem. Biophys. Res. Commun. 2020, 521, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Pei, S.; Zhu, Q.; Chen, F. Disruption of ssbs repair to combat platinum resistance via the jwa-targeted pt(iv) prodrug conjugated with a wogonin derivative. Die Pharm. 2020, 75, 94–101. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, K.; Liu, X.; Wang, L.; Zou, L.; Jiang, X.; Li, A.; Zhou, J. Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery. Cancers 2022, 14, 4655. https://doi.org/10.3390/cancers14194655
Ding K, Liu X, Wang L, Zou L, Jiang X, Li A, Zhou J. Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery. Cancers. 2022; 14(19):4655. https://doi.org/10.3390/cancers14194655
Chicago/Turabian StyleDing, Kun, Xia Liu, Luman Wang, Lu Zou, Xuqian Jiang, Aiping Li, and Jianwei Zhou. 2022. "Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery" Cancers 14, no. 19: 4655. https://doi.org/10.3390/cancers14194655
APA StyleDing, K., Liu, X., Wang, L., Zou, L., Jiang, X., Li, A., & Zhou, J. (2022). Targeting JWA for Cancer Therapy: Functions, Mechanisms and Drug Discovery. Cancers, 14(19), 4655. https://doi.org/10.3390/cancers14194655




