Evaluation of Blood Soluble CD26 as a Complementary Biomarker for Colorectal Cancer Screening Programs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Colonoscopy
2.3. Blood Samples and Measurement of sCD26, DPP4 and Total Protein
2.4. Stool Samples and FIT
2.5. Data Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Serum sCD26 and DPP4 Enzymatic Levels in the Study Population
3.3. Serum sCD26 and DPP4 Levels in the Study Population According to the Colonoscopy Findings
3.4. Evaluation of Serum sCD26 and DPP4 Levels in the NN Group According to the Colonoscopy Findings
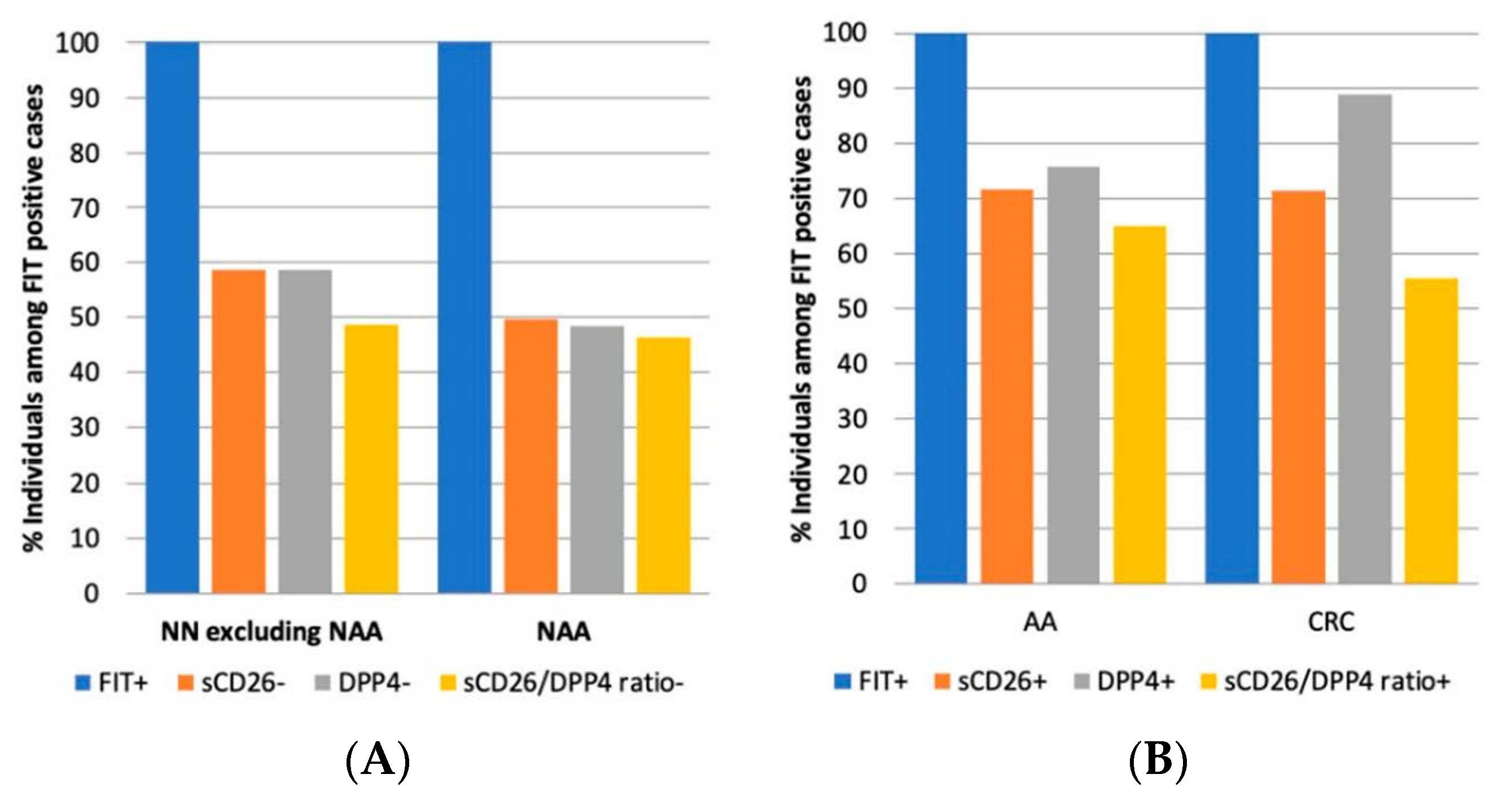
3.5. Preliminary Diagnostic Accuracy of sCD26, DPP4, and Their Ratio in the FIT Cohort
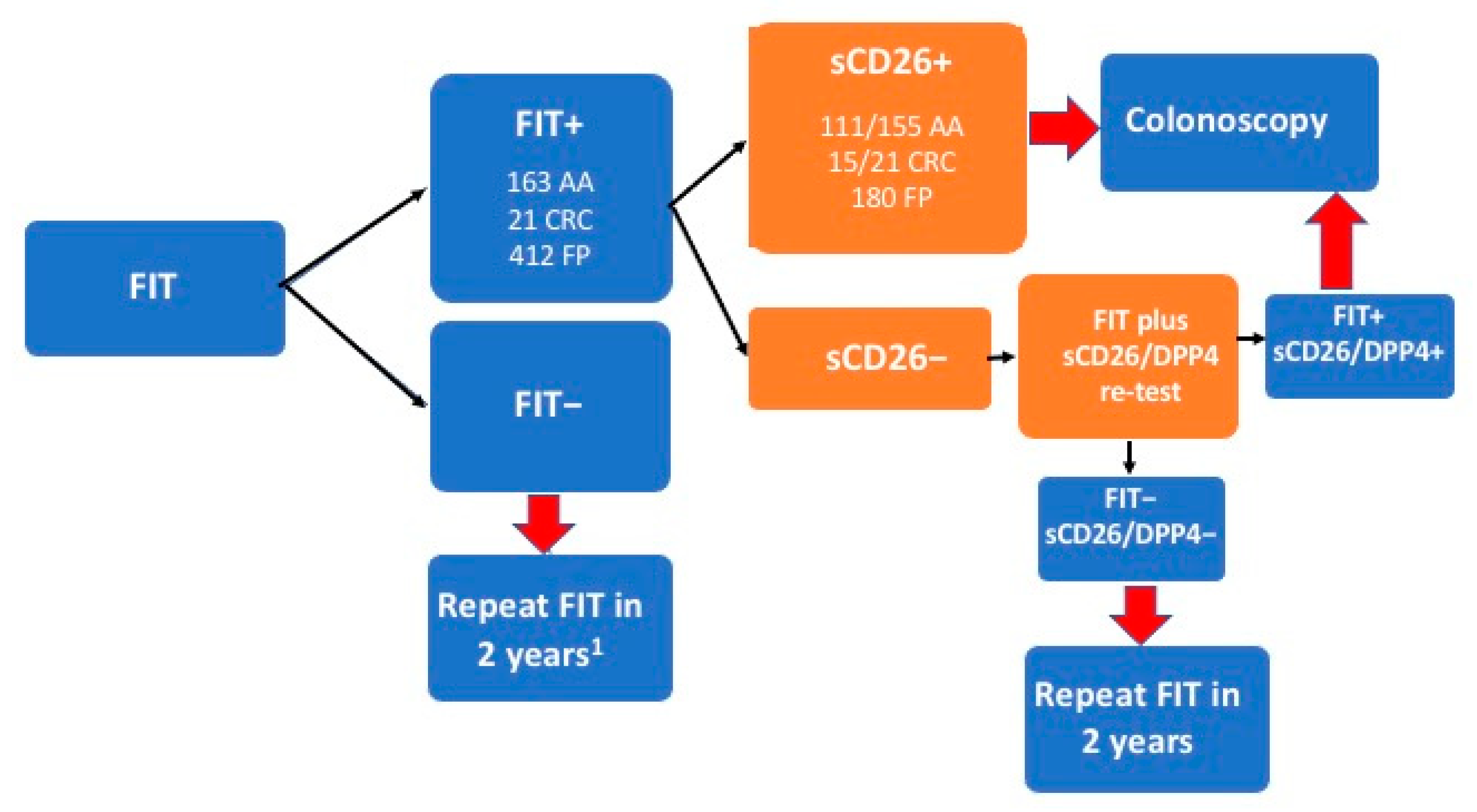
3.6. Proposal for the Integration of Our Biomarkers in FIT-Based Colorectal Cancer Screening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, H.; Kloor, M.; Pox, C. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Pellat, A.; Deyra, J.; Coriat, R.; Chaussade, S. Results of the national organised colorectal cancer screening program with FIT in Paris. Sci. Rep. 2018, 8, 4162. [Google Scholar] [CrossRef]
- Zauber, A.G. The Impact of Screening on Colorectal Cancer Mortality and Incidence: Has It Really Made a Difference? Dig. Dis. Sci. 2015, 60, 681–691. [Google Scholar] [CrossRef]
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: The UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef]
- Bretthauer, M.; Kaminski, M.F.; Løberg, M.; Zauber, A.G.; Regula, J.; Kuipers, E.J.; Hernán, M.A.; McFadden, E.; Sunde, A.; Kalager, M.; et al. Population-Based Colonoscopy Screening for Colorectal Cancer: A European randomized trial. JAMA Intern. Med. 2016, 176, 894–902. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef]
- Klabunde, C.; Blom, J.; Bulliard, J.-L.; Garcia, M.; Hagoel, L.; Mai, V.; Patnick, J.; Rozjabek, H.; Senore, C.; Törnberg, S. Participation rates for organized colorectal cancer screening programmes: An international comparison. J. Med. Screen. 2015, 22, 119–126. [Google Scholar] [CrossRef]
- Carethers, J.M. Fecal DNA Testing for Colorectal Cancer Screening. Annu. Rev. Med. 2020, 71, 59–69. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Weigl, K.; Tikk, K.; Holland-Letz, T.; Schrotz-King, P.; Borchers, C.H.; Brenner, H. Multiplex quantitation of 270 plasma protein markers to identify a signature for early detection of colorectal cancer. Eur. J. Cancer 2020, 127, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Gómez, M.; De Chiara, L.; Álvarez-Chaver, P.; Cubiella, J. Colorectal cancer screening and diagnosis: Omics-based technologies for development of a non-invasive blood-based method. Expert Rev. Anticancer Ther. 2021, 21, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Ladabaum, U. Opportunities and Challenges in Moving From Current Guidelines to Personalized Colorectal Cancer Screening. Gastroenterology 2019, 156, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Harlid, S.; Harbs, J.; Myte, R.; Brunius, C.; Gunter, M.J.; Palmqvist, R.; Liu, X.; Van Guelpen, B. A two-tiered targeted proteomics approach to identify pre-diagnostic biomarkers of colorectal cancer risk. Sci. Rep. 2021, 11, 5151. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Sasazuki, S.; Camargo, M.C.; Shimazu, T.; Charvat, H.; Yamaji, T.; Sawada, N.; Kemp, T.J.; Pfeiffer, R.M.; Hildesheim, A.; et al. Circulating inflammatory markers and colorectal cancer risk: A prospective case-cohort study in Japan. Int. J. Cancer 2018, 143, 2767–2776. [Google Scholar] [CrossRef]
- Cordero, O.J.; Varela-Calviño, R.; Graña-Suárez, B. Immunology and Immunotherapy of Colorectal Cancer. In Cancer Im-Munology; Rezaei, N., Ed.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 261–289. [Google Scholar] [CrossRef]
- Cordero, O.J.; Ayude, D.; Nogueira, M.; Rodríguez-Berrocal, F.J.; De La Cadena, M.P. Preoperative serum CD26 levels: Diagnostic efficiency and predictive value for colorectal cancer. Br. J. Cancer 2000, 83, 1139–1146. [Google Scholar] [CrossRef]
- Cordero, O.J.; Salgado, F.J.; Nogueira, M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol. Immunother. 2009, 58, 1723–1747. [Google Scholar] [CrossRef]
- Vázquez-Iglesias, L.; Barcia-Castro, L.; Rodríguez-Quiroga, M.; de la Cadena, M.P.; Rodríguez-Berrocal, J.; Cordero, O.J. Surface expression marker profile in colon cancer cell lines and sphere-derived cells suggests complexity in CD26+ cancer stem cells subsets. Biol. Open 2019, 8, bio041673. [Google Scholar] [CrossRef]
- De Chiara, L.; Rodriguez-Pineiro, A.M.; Cordero, O.J.; Vázquez-Tuñas, L.; Ayude, D.; Rodríguez-Berrocal, F.J.; De La Cadena, M.P. Postoperative Serum Levels of sCD26 for Surveillance in Colorectal Cancer Patients. PLoS ONE 2014, 9, e107470. [Google Scholar] [CrossRef]
- Larrinaga, G.; Perez, I.; Sanz, B.; Beitia, M.; Errarte, P.; Fernández, A.; Blanco, L.; Etxezarraga, M.C.; Gil, J.; López, J.I. Dipeptidyl-Peptidase IV Activity Is Correlated with Colorectal Cancer Prognosis. PLoS ONE 2015, 10, e0119436. [Google Scholar] [CrossRef]
- Cordero, O.J.; Imbernon, M.; De Chiara, L.; Martinez-Zorzano, V.S.; Ayude, D.; de la Cadena, M.P.; Rodriguez-Berrocal, F.J. Potential of soluble CD26 as a serum marker for colorectal cancer detection. World J. Clin. Oncol. 2011, 2, 245–261. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, L.; Rodríguez-Piñeiro, A.M.; Rodríguez-Berrocal, F.J.; Cordero, O.J.; Martínez-Ares, D.; De La Cadena, M.P. Serum CD26 is related to histopathological polyp traits and behaves as a marker for colorectal cancer and advanced adenomas. BMC Cancer 2010, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Grujic, M.; Matic, I.; Crnogorac, M.D.; Velickovic, A.D.; Kolundzija, B.; Cordero, O.J.; Juranic, Z.; Prodanovic, S.; Zlatanovic, M.; Babic, D.; et al. Activity and expression of dipeptidyl peptidase IV on peripheral blood mononuclear cells in patients with early steroid and disease modifying antirheumatic drugs naïve rheumatoid arthritis. Clin. Chem. Lab. Med. 2017, 55, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.R.; Nelson, M.H.; Majchrzak, K.; Bowers, J.S.; Wyatt, M.M.; Smith, A.S.; Neal, L.R.; Shirai, K.; Carpenito, C.; June, C.H.; et al. Human CD26high T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat. Commun. 2017, 8, 1961. [Google Scholar] [CrossRef]
- Casrouge, A.; Sauer, A.V.; da Silva, R.B.; Tejera-Alhambra, M.; Sánchez-Ramón, S.; ICARe, B.; Cancrini, C.; Ingersoll, M.A.; Aiuti, A.; Albert, M.L. Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4. Clin. Exp. Immunol. 2018, 194, 166–179. [Google Scholar] [CrossRef]
- Sánchez-Otero, N.; Rodríguez-Berrocal, F.J.; De La Cadena, M.P.; Botana-Rial, M.I.; Cordero, O.J. Evaluation of pleural effusion sCD26 and DPP-IV as diagnostic biomarkers in lung disease. Sci. Rep. 2014, 4, 3999. [Google Scholar] [CrossRef]
- De Chiara, L.; de la Cadena, M.P.; Rodríguez-Berrocal, J.; Alvarez-Pardiñas, M.C.; Pardiñas-Añón, M.C.; Varela-Calviño, R.; Cordero, O.J. CD26-Related Serum Biomarkers: sCD26 Protein, DPP4 Activity, and Anti-CD26 Isotype Levels in a Colorectal Cancer-Screening Context. Dis. Markers 2020, 2020, 4347936. [Google Scholar] [CrossRef]
- Varela-Calviño, R.; Imbernón, M.; Vázquez-Iglesias, L.; De La Cadena, M.P.; Bande-Rodríguez, M.; Piñeiro, A.; Pardo, M.; Cordero, O.J. Serum dipeptidyl peptidase IV activity and sCD26 concentration in patients with choroidal nevus or uveal melanoma. Clin. Chim. Acta 2015, 448, 193–194. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Li, X.; Tian, R.; Shang, K.; Dong, X.; Cao, B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2020, 497, 190–201. [Google Scholar] [CrossRef]
- Jover, R.; Herráiz, M.; Alarcón, O.; Brullet, E.; Bujanda, L.; Bustamante, M.; Campo, R.; Carreño, R.; Castells, A.; Cubiella, J.; et al. Clinical practice Guidelines: Quality of colonoscopy in colorectal cancer screening. Endoscopy 2012, 44, 444–451. [Google Scholar] [CrossRef]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Berrocal, F.J.; De La Cadena, M.P.; Cubiella, J.; Castro, I.; Gonzalez-Mao, C.; Hernandez, V.; Martínez-Zorzano, V.S. Serum sCD26 for colorectal cancer screening in family-risk individuals: Comparison with faecal immunochemical test. Br. J. Cancer 2014, 112, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Haug, U.; Kuntz, K.M.; Knudsen, A.B.; Hundt, S.; Brenner, H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br. J. Cancer 2011, 104, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Cock, C.; Anwar, S.; Byrne, S.E.; Meng, R.; Pedersen, S.; Fraser, R.J.L.; Young, G.P.; Symonds, E.L. Low Sensitivity of Fecal Immunochemical Tests and Blood-Based Markers of DNA Hypermethylation for Detection of Sessile Serrated Adenomas/Polyps. Dig. Dis. Sci. 2019, 64, 2555–2562. [Google Scholar] [CrossRef]
- Shimwell, N.J.; Wei, W.; Wilson, S.; Wakelam, M.J.; Ismail, T.; Iqbal, T.; Johnson, P.J.; Martin, A.; Ward, D.G. Assessment of novel combinations of biomarkers for the detection of colorectal cancer. Cancer Biomarkers 2011, 7, 123–132. [Google Scholar] [CrossRef]
- Sin, R.W.; Foo, D.C.; Iyer, D.N.; Fan, M.S.; Li, X.; Lo, O.S.; Law, W.L.; Ng, L. A Pilot Study Investigating the Expression Levels of Pluripotency-Associated Genes in Rectal Swab Samples for Colorectal Polyp and Cancer Diagnosis and Prognosis. Stem Cells Int. 2021, 2021, 4139528. [Google Scholar] [CrossRef]
- Tejera-Alhambra, M.; Casrouge, A.; de Andrés, C.; Ramos-Medina, R.; Alonso, B.; Vega, J.; Albert, M.L.; Sánchez-Ramón, S. Low DPP4 expression and activity in multiple sclerosis. Clin. Immunol. 2014, 150, 170–183. [Google Scholar] [CrossRef]
- Cordero, O.J.; Varela-Calvino, R.; López-González, T.; Calviño-Sampedro, C.; Viñuela, J.E.; Mouriño, C.; Hernández-Rodríguez, Í.; Rodríguez-López, M.; De La Iglesia, B.A.; Pego-Reigosa, J.M. CD26 Expression on T Helper Populations and sCD26 Serum Levels in Patients with Rheumatoid Arthritis. PLoS ONE 2015, 10, e0131992. [Google Scholar] [CrossRef]
- De Zutter, A.; Van Damme, J.; Struyf, S. The Role of Post-Translational Modifications of Chemokines by CD26 in Cancer. Cancers 2021, 13, 4247. [Google Scholar] [CrossRef] [PubMed]
- Busek, P.; Duke-Cohan, J.S.; Sedo, A. Does DPP-IV Inhibition Offer New Avenues for Therapeutic Intervention in Malignant Disease? Cancers 2022, 14, 2072. [Google Scholar] [CrossRef]
- Yatabe, S.; Eto, K.; Haruki, K.; Shiba, H.; Kosuge, M.; Ohkuma, M.; Ito, D.; Takeda, Y.; Sugano, H.; Sasaki, S.; et al. Signification of Systemic Immune-Inflammation Index for prediction of prognosis after resecting in patients with colorectal cancer. Int. J. Color. Dis. 2020, 35, 1549–1555. [Google Scholar] [CrossRef]
- Pinto-Lopes, P.; Melo, F.; Afonso, J.; Pinto-Lopes, R.; Rocha, C.; Melo, D.; Macedo, G.; Dias, C.C.; Carneiro, F.; Magro, F.; et al. Fecal Dipeptidyl Peptidase-4: An Emergent Biomarker in Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2021, 12, e00320. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome–like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef]
- Wang, D.D.; Qian, X.K.; Li, H.X.; Jia, G.H.; Jin, Q.; Luan, X.; Zhu, Y.D.; Wang, Y.N.; Huang, J.; Zou, L.W.; et al. Sensing and imaging of exosomal CD26 secreted from cancer cells and 3D colorectal tumor model using a novel near-infrared fluorogenic probe. Mater. Sci. Eng. C 2021, 130, 112472. [Google Scholar] [CrossRef]
- Coto-Llerena, M.; Ercan, C.; Kancherla, V.; Taha-Mehlitz, S.; Eppenberger-Castori, S.; Soysal, S.D.; Ng, C.K.Y.; Bolli, M.; Von Flüe, M.; Nicolas, G.P.; et al. High Expression of FAP in Colorectal Cancer Is Associated With Angiogenesis and Immunoregulation Processes. Front. Oncol. 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Margamuljana, L.; Joseph, C.; Schouteden, S.; Buckley, S.M.; Verfaillie, C.M. Glypican-3–mediated inhibition of CD26 by TFPI: A novel mechanism in hematopoietic stem cell homing and maintenance. Blood 2013, 121, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, L.R.; Bilandzic, M.; Wilson, A.L.; Chen, Y.; Gorrell, M.D.; Oehler, M.K.; Plebanski, M.; Stephens, A.N. Hypoxia Regulates DPP4 Expression, Proteolytic Inactivation, and Shedding from Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8110. [Google Scholar] [CrossRef]
- Pérez-Durillo, F.T.; Segarra, A.B.; Villarejo, A.B.; Ramírez-Sánchez, M.; Prieto, I. Influence of Diet and Gender on Plasma DPP4 Activity and GLP-1 in Patients with Metabolic Syndrome: An Experimental Pilot Study. Molecules 2018, 23, 1564. [Google Scholar] [CrossRef]
- Stubbe, H.C.; Dahlke, C.; Rotheneder, K.; Stirner, R.; Roider, J.; Conca, R.; Seybold, U.; Bogner, J.; Addo, M.M.; Draenert, R. Integration of microarray data and literature mining identifies a sex bias in DPP4+CD4+ T cells in HIV-1 infection. PLoS ONE 2020, 15, e0239399. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Rainczuk, A.; Oehler, M.K.; Jobling, T.W.; Plebanski, M.; Stephens, A.N. Active Ratio Test (ART) as a Novel Diagnostic for Ovarian Cancer. Diagnostics 2021, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
| CENTER | Complexo Hospitalario Universitario de Ourense | Hospital Donostia | Hospital Clínic de Barcelona | Hospital General Universitario de Alicante | Total Patients Recruited |
|---|---|---|---|---|---|
| Number of patients recruited | 436 | 601 | 480 | 186 | 1703 |
| Sex (% men) | 47.7% | 52.2% | 46.3% | 58.6% | 50.1% |
| Age range and mean | 29–87 (58.2) | 41–92 (64.5) | 49–70 (59.7) | 48–85 (63.6) | 29–92 (61.5) |
| Ne neoplasia | 333 | 334 | 373 | 42 | 1082 |
| NCF | 116 | 148 | 73 | 7 | 344 |
| hemorrhoids | 58 | 7 | 46 | 8 | 119 |
| diverticula | 43 | 6 | 43 | 5 | 97 |
| polyps 1 | 33 | 2 | 9 | 2 | 46 |
| others 2 | 6 | 1 | 16 | 1 | 24 |
| NAA | 77 | 170 | 186 | 19 | 452 |
| Advanced adenomas | 72 | 131 | 82 | 87 | 372 |
| distal | 47 | 83 | 39 | 68 | 237 |
| proximal | 25 | 48 | 43 | 19 | 135 |
| Colorectal cancer | 31 | 136 | 25 | 57 | 249 |
| Stage I | 4 | 32 | 18 | 19 | 73 |
| Stage II | 4 | 52 | 7 | 12 | 75 |
| Stage III | 19 | 39 | 19 | 77 | |
| Stage IV | 4 | 13 | 4 | 21 | |
| Unknown stage | 3 | 3 |
| Variable | sCD26 Mean ± SD (ng/mL) N p-Value | DPP4 Mean ± SD (mU/mL) N p-Value | Total Protein Mean ± SD (mg/mL) N p-Value | Sp. Act (DPP4/Prot) Mean ± SD (mU/mg) N p-Value | sCD26/DPP4 Ratio Mean ± SD (ng/mU) N p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex Women Men | 840 826 | 498.30 ± 189.29 440.96 ± 192.04 | <0.0011 | 573 601 | 42.77 ± 12.39 36.30 ± 12.51 | <0.0011 | 573 601 | 76.56 ± 13.06 76.50 ± 14.16 | 0.939 1 | 573 601 | 0.57 ± 0.18 0.51 ± 0.73 | 0.076 1 | 563 574 | 11.79 ± 5.37 12.18 ± 5.39 | 0.219 1 |
| Age (years) | |||||||||||||||
| ≤49 | 126 | 503.68 ± 187.20 | <0.0012 | 23 | 39.64 ± 9.97 | <0.0012 | 23 | 77.40 ± 14.54 | 0.128 2 | 23 | 0.52 ± 0.15 | 0.306 2 | 21 | 9.95 ± 4.13 | 0.205 2 |
| 50–59 | 570 | 507.49. ± 188.91 | 0.837 3 | 399 | 43.48 ± 12.79 | 0.089 3 | 399 | 77.61 ± 13.70 | 0.946 3 | 399 | 0.58 ± 0.19 | 0.122 3 | 393 | 11.97 ± 4.01 | 0.0413 |
| ≥60 | 970 | 443.38 ± 191.50 | 0.0014 | 752 | 37.28 ± 12.47 | 0.279 4 | 752 | 75.92 ± 13.54 | 0.635 4 | 752 | 0.52 ± 0.66 | 0.999 4 | 723 | 12.07 ± 6.02 | 0.0334 |
| Age in women | |||||||||||||||
| ≤49 | 65 | 469.58 ± 176.10 | 0.0012 | 9 | 38.96 ± 10.68 | <0.0012 | 9 | 80.92 ± 16.46 | 0.0072 | 9 | 0.50 ± 0.16 | <0.0012 | 9 | 8.15 ± 3.61 | 0.105 2 |
| 50–59 | 307 | 529.61 ± 179.76 | 0.0153 | 211 | 46.50 ± 11.77 | 0.069 3 | 211 | 78.59 ± 15.02 | 0.687 3 | 211 | 0.61 ± 0.18 | 0.082 3 | 209 | 11.69 ± 3.85 | 0.0193 |
| ≥60 | 468 | 481.75 ± 194.71 | 0.608 4 | 353 | 40.63 ± 12.28 | 0.656 4 | 353 | 75.23 ± 11.46 | 0.331 4 | 353 | 0.55 ± 0.18 | 0.402 4 | 345 | 11.95 ± 6.12 | 0.0144 |
| Age in men | |||||||||||||||
| ≤49 | 61 | 540.02 ± 193.21 | <0.0012 | 14 | 40.07 ± 9.88 | <0.0012 | 14 | 75.13 ± 13.29 | 0.936 2 | 14 | 0.54 ± 0.14 | 0.862 2 | 12 | 11.30 ± 4.11 | 0.834 2 |
| 50–59 | 263 | 481.67 ± 196.27 | 0.0373 | 188 | 40.10 ± 13.07 | 0.995 3 | 188 | 76.51 ± 11.98 | 0.712 3 | 188 | 0.54 ± 0.20 | 0.944 3 | 184 | 12.27 ± 4.17 | 0.446 3 |
| ≥60 | 502 | 407.60 ± 181.48 | <0.0014 | 399 | 34.31 ± 11.88 | 0.0514 | 399 | 76.54 ± 15.13 | 0.705 4 | 399 | 0.50 ± 0.89 | 0.521 4 | 378 | 12.17 ± 5.94 | 0.491 4 |
| Pathology | sCD26 N Mean ± SD (ng/mL) p-Value | DPP4 N Mean ± SD (ng/mL) p-Value | Total Protein N Mean ± SD (ng/mL) p-Value | Sp. Act (DPP4/Prot) N Mean ± SD (ng/mL) p-Value | sCD26/DPP4 Ratio N Mean ± SD (ng/mL) p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No neoplasia | 1072 | 518.65 ± 183.93 | 0.0331 | 671 | 43.45 ± 11.37 | 0.0141 | 671 | 76.30 ± 12.60 | 0.469 1 | 671 | 0.61 ± 0.69 | 0.338 1 | 661 | 12.34 ± 5.09 | 0.190 1 |
| NCF | 341 | 543.70 ± 186.88 | - | 188 | 45.36 ± 11.57 | - | 188 | 75.50 ± 11.81 | - | 188 | 0.71 ± 1.27 | - | 185 | 12.85 ± 4.72 | - |
| hemorrhoids | 116 | 496.55 ± 176.98 | 0.0182 | 55 | 42.31 ± 12.69 | 0.094 2 | 55 | 75.99 ± 11.21 | 0.783 2 | 55 | 0.56 ± 0.17 | 0.391 2 | 52 | 11.43 ± 4.11 | 0.050 2 |
| diverticula | 96 | 496.76 ± 181.62 | 0.0292 | 52 | 42.91 ± 9.63 | 0.165 2 | 52 | 73.92 ± 9.98 | 0.380 2 | 52 | 0.59 ± 0.14 | 0.488 2 | 51 | 10.99 ± 3.41 | 0.0092 |
| polyps * | 45 | 481.22 ± 140.90 | 0.0312 | 14 | 37.17 ± 9.04 | 0.0102 | 14 | 74.79 ± 7.81 | 0.823 2 | 14 | 0.51 ± 0.15 | 0.554 2 | 13 | 11.68 ± 2.64 | 0.377 2 |
| other † | 24 | 541.30 ± 190.95 | 0.952 2 | 19 | 47.60 ± 12.93 | 0.426 2 | 19 | 77.07 ± 11.95 | 0.581 2 | 19 | 0.64 ± 0.24 | 0.811 2 | 19 | 12.55 ± 5.75 | 0.793 2 |
| NAA | 450 | 512.57 ± 185.82 | 0.0202 | 343 | 42.69 ± 11.11 | 0.0102 | 343 | 77.18 ± 13.71 | 0.157 2 | 343 | 0.57 ± 0.17 | 0.0472 | 341 | 12.41 ± 5.61 | 0.369 2 |
| Advanced neoplasia | 594 | 381.85 ± 176.48 | <0.0013 | 503 | 34.08 ± 12.80 | <0.0013 | 503 | 76.82 ± 14.90 | 0.530 3 | 503 | 0.45 ± 0.17 | <0.0013 | 476 | 11.51 ± 5.73 | 0.0103 |
| AA | 345 | 419.95 ± 169.05 | <0.0014 | 261 | 38.13. ± 11.55 | <0.0014 | 261 | 77.91 ± 12.01 | 0.077 4 | 261 | 0.50 ± 0.16 | 0.0104 | 234 | 11.60 ± 4.53 | 0.0494 |
| CRC | 249 | 329.05 ± 173.25 | <0.0015 | 242 | 29.72 ± 12.66 | <0.0015 | 242 | 75.65 ± 17.43 | 0.591 5 | 242 | 0.40 ± 0.17 | <0.0015 | 242 | 11.42 ± 6.70 | 0.0295 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Chiara, L.; Barcia-Castro, L.; Gallardo-Gómez, M.; Páez de la Cadena, M.; Martínez-Zorzano, V.S.; Rodríguez-Berrocal, F.J.; Bujanda, L.; Etxart, A.; Castells, A.; Balaguer, F.; et al. Evaluation of Blood Soluble CD26 as a Complementary Biomarker for Colorectal Cancer Screening Programs. Cancers 2022, 14, 4563. https://doi.org/10.3390/cancers14194563
De Chiara L, Barcia-Castro L, Gallardo-Gómez M, Páez de la Cadena M, Martínez-Zorzano VS, Rodríguez-Berrocal FJ, Bujanda L, Etxart A, Castells A, Balaguer F, et al. Evaluation of Blood Soluble CD26 as a Complementary Biomarker for Colorectal Cancer Screening Programs. Cancers. 2022; 14(19):4563. https://doi.org/10.3390/cancers14194563
Chicago/Turabian StyleDe Chiara, Loretta, Leticia Barcia-Castro, María Gallardo-Gómez, María Páez de la Cadena, Vicenta S. Martínez-Zorzano, Francisco J. Rodríguez-Berrocal, Luis Bujanda, Ane Etxart, Antoni Castells, Francesc Balaguer, and et al. 2022. "Evaluation of Blood Soluble CD26 as a Complementary Biomarker for Colorectal Cancer Screening Programs" Cancers 14, no. 19: 4563. https://doi.org/10.3390/cancers14194563
APA StyleDe Chiara, L., Barcia-Castro, L., Gallardo-Gómez, M., Páez de la Cadena, M., Martínez-Zorzano, V. S., Rodríguez-Berrocal, F. J., Bujanda, L., Etxart, A., Castells, A., Balaguer, F., Jover, R., Cubiella, J., & Cordero, O. J. (2022). Evaluation of Blood Soluble CD26 as a Complementary Biomarker for Colorectal Cancer Screening Programs. Cancers, 14(19), 4563. https://doi.org/10.3390/cancers14194563









