Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants
Abstract
Simple Summary
Abstract
1. Introduction
2. Standard Immunotherapy for HR-NB Patients
3. Paving the Road for Allogeneic Hematopoietic Stem Cell Transplants in NB: A Lesson from Pediatric Hematological Malignancies
4. Haploidentical Hematopoietic Cell Transplantation in HR-NB Patients: Where We Are
5. Exploring the NK/NB Molecular Interactions to Optimize Haploidentical Transplant
6. Unveiling the Role of NB and NK-Derived Exosomes: How Can They Improve Immune Cell-Based Treatment Strategies
7. Additional Immunotherapeutic Strategies and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lundberg, K.I.; Treis, D.; Johnsen, J.I. Neuroblastoma Heterogeneity, Plasticity, and Emerging Therapies. Curr. Oncol. Rep. 2022, 24, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Prim. 2016, 2, 16078. [Google Scholar] [CrossRef]
- Braoudaki, M.; Hatziagapiou, K.; Zaravinos, A.; Lambrou, G.I. MYCN in Neuroblastoma: “Old Wine into New Wineskins”. Diseases 2021, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Wulf, A.M.; Moreno, M.M.; Paka, C.; Rampasekova, A.; Liu, K.J. Defining pathological activities of alk in neuroblastoma, a neural crest-derived cancer. Int. J. Mol. Sci. 2021, 22, 11718. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E. Revised neuroblastoma risk classification system: A report from the children’s oncology group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Zage, P.E. Novel therapies for relapsed and refractory neuroblastoma. Children 2018, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–3. [Google Scholar] [CrossRef]
- Salemi, F.; Alam, W.; Sadat, M.; Zohreh, S.; Mohammad, S.; Mortazavizadeh, R.; Khan, H. Neuroblastoma: Essential genetic pathways and current therapeutic options. Eur. J. Pharmacol. 2022, 926, 175030. [Google Scholar] [CrossRef]
- George, S.L.; Parmar, V.; Lorenzi, F.; Marshall, L.V.; Jamin, Y.; Poon, E.; Angelini, P.; Chesler, L. Novel therapeutic strategies targeting telomere maintenance mechanisms in high-risk neuroblastoma. J. Exp. Clin. Cancer Res. 2020, 39, 39. [Google Scholar] [CrossRef]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef]
- Bellora, F.; Castriconi, R.; Dondero, A.; Carrega, P.; Mantovani, A.; Ferlazzo, G.; Moretta, A.; Bottino, C. Human NK cells and NK receptors. Immunol. Lett. 2014, 161, 168–173. [Google Scholar] [CrossRef]
- Falco, M.; Moretta, L.; Moretta, A.; Bottino, C. KIR and KIR ligand polymorphism: A new area for clinical applications? Tissue Antigens 2013, 82, 363–373. [Google Scholar] [CrossRef]
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK cell development: One road or many? Front. Immunol. 2019, 10, 2078. [Google Scholar] [CrossRef]
- De Maria, A.; Bozzano, F.; Cantoni, C.; Moretta, L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-γ on activation. Proc. Natl. Acad. Sci. USA 2011, 108, 728–732. [Google Scholar] [CrossRef]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Aspects Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Meazza, R.; Falco, M.; Loiacono, F.; Canevali, P.; Della Chiesa, M.; Bertaina, A.; Pagliara, D.; Merli, P.; Indio, V.; Galaverna, F.; et al. Phenotypic and functional characterization of nk cells in αβt-cell and b-cell depleted haplo-hsct to cure pediatric patients with acute leukemia. Cancers 2020, 12, 2187. [Google Scholar] [CrossRef]
- Bottino, C.; Dondero, A.; Castriconi, R. Inhibitory axes impacting on the activity and fate of Innate Lymphoid Cells. Mol. Aspects Med. 2021, 80, 100985. [Google Scholar] [CrossRef]
- Bottino, C.; Dondero, A.; Bellora, F.; Moretta, L.; Locatelli, F.; Pistoia, V.; Moretta, A.; Castriconi, R. Natural killer cells and neuroblastoma: Tumor recognition, escape mechanisms, and possible novel immunotherapeutic approaches. Front. Immunol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Dondero, A.; Pastorino, F.; Della Chiesa, M.; Corrias, M.V.; Morandi, F.; Pistoia, V.; Olive, D.; Bellora, F.; Locatelli, F.; Castellano, A.; et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology 2016, 5, e1064578. [Google Scholar] [CrossRef]
- Ehlert, K.; Hansjuergens, I.; Zinke, A.; Otto, S.; Siebert, N.; Henze, G.; Lode, H. Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma. J. Immunother. Cancer 2020, 8, e000540. [Google Scholar] [CrossRef]
- Marrella, A.; Dondero, A.; Aiello, M.; Casu, B.; Olive, D.; Regis, S.; Bottino, C.; Pende, D.; Meazza, R.; Caluori, G. Cell-laden hydrogel as a clinical-relevant 3D model for analyzing neuroblastoma growth, immunophenotype, and susceptibility to therapies. Front. Immunol. 2019, 10, 1876. [Google Scholar] [CrossRef]
- Quintarelli, C.; Orlando, D.; Boffa, I.; Guercio, M.; Polito, V.A.; Petretto, A.; Lavarello, C.; Sinibaldi, M.; Weber, G.; Del Bufalo, F.; et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 2018, 7, e1433518. [Google Scholar] [CrossRef]
- Quamine, A.E.; Olsen, M.R.; Cho, M.M.; Capitini, C.M. Approaches to enhance natural killer cell-based immunotherapy for pediatric solid tumors. Cancers 2021, 13, 2796. [Google Scholar] [CrossRef]
- Du, H.; Hirabayashi, K.; Ahn, S.; Kren, N.P.; Montgomery, S.A.; Wang, X.; Tiruthani, K.; Mirlekar, B.; Michaud, D.; Greene, K.; et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell 2019, 35, 221–237. [Google Scholar] [CrossRef]
- Blavier, L.; Yang, R.M.; Declerck, Y.A. The tumor microenvironment in neuroblastoma: New players, new mechanisms of interaction and new perspectives. Cancers 2020, 12, 2912. [Google Scholar] [CrossRef]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-β-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef]
- Castriconi, R.; Dondero, A.; Corrias, M.V.; Lanino, E.; Pende, D.; Moretta, L.; Bottino, C.; Moretta, A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: Critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004, 64, 9180–9184. [Google Scholar] [CrossRef]
- Marimpietri, D.; Airoldi, I.; Faini, A.C.; Malavasi, F.; Morandi, F. The role of extracellular vesicles in the progression of human neuroblastoma. Int. J. Mol. Sci. 2021, 22, 3964. [Google Scholar] [CrossRef]
- Illhardt, T.; Toporski, J.; Feuchtinger, T.; Turkiewicz, D.; Teltschik, H.M.; Ebinger, M.; Schwarze, C.P.; Holzer, U.; Lode, H.N.; Albert, M.H.; et al. Haploidentical Stem Cell Transplantation for Refractory/Relapsed Neuroblastoma. Biol. Blood Marrow Transplant. 2018, 24, 1005–1012. [Google Scholar] [CrossRef]
- Delgado, D.C.; Hank, J.A.; Kolesar, J.; Lorentzen, D.; Gan, J.; Seo, S.; Kim, K.M.; Shusterman, S.; Gillies, S.D.; Reisfeld, R.A.; et al. Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010, 70, 9554–9561. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.; Cheresh, D.A.; Varki, N.M.; Staffilano, L.K.; Reisfeld, R.A.; Yu, A. Detection of Ganglioside GD2 in Tumor Tissues and Sera of Neuroblastoma Patients. Cancer Res. 1984, 44, 5914–5920. [Google Scholar] [PubMed]
- Saito, M.; Robert, K.Y.; Cheung, N.K.V. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochem. Biophys. Res. Commun. 1985, 127, 1–7. [Google Scholar] [CrossRef]
- Cheung, N.K.V.; Cheung, I.Y.; Canete, A.; Yeh, S.J.; Kushner, B.; Bonilla, M.A.; Heller, G.; Larson, S.M. Antibody Response to Murine Anti-GD2 Monoclonal Antibodies: Correlation with Patient Survival. Cancer Res. 1994, 54, 2228–2233. [Google Scholar]
- Siebert, N.; Eger, C.; Seidel, D.; Jüttner, M.; Lode, H.N. Validated detection of human anti-chimeric immune responses in serum of neuroblastoma patients treated with ch14.18/CHO. J. Immunol. Methods 2014, 407, 108–115. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Kushner, B.H.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.K.V. Phase I trial of anti-GD2 monoclonal antibody hu3F8 plus GM-CSF: Impact of body weight, immunogenicity and anti-GD2 response on pharmacokinetics and survival. Oncoimmunology 2017, 6, e1358331. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Gazitt, Y.; Rosenberger, M.; Grippo, J.F.; Lucas, D.A.; Prankerd, R.J. Aromatic retinoid Acid-derivatives are potent inducers of differentiation of neuroblastoma-cells-structure-function relationship and the involvement of the nuclear retinoic Acid receptors. Int. J. Oncol. 1994, 5, 243–251. [Google Scholar] [CrossRef]
- Villablanca, J.G.; Khan, A.A.; Avramis, V.I.; Seeger, R.C.; Matthay, K.K.; Ramsay, N.K.; Reynolds, C.P. Phase I trial of 13-cis-retinoic acid in children with neuroblastoma following bone marrow transplantation. J. Clin. Oncol. 1995, 13, 894–901. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C. Long-Term Follow-up of a Phase III Study of ch14. 18 (Dinutuximab)+ Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032Long-Term Follow-up of Immunotherapy for Neuroblastoma. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef]
- Tarek, N.; Le Luduec, J.B.; Gallagher, M.M.; Zheng, J.; Venstrom, J.M.; Chamberlain, E.; Modak, S.; Heller, G.; Dupont, B.; Cheung, N.K.V.; et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Investig. 2012, 122, 3260–3270. [Google Scholar] [CrossRef]
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Modak, S.; Kramer, K.; Roberts, S.S.; Basu, E.M.; Yataghene, K.; Cheung, N.K.V. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget 2016, 7, 4155–4166. [Google Scholar] [CrossRef]
- Simon, T.; Hero, B.; Faldum, A.; Handgretinger, R.; Schrappe, M.; Klingebiel, T.; Berthold, F. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer 2011, 11, 21. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the role of dinutuximab beta-based immunotherapy in the siopen high-risk neuroblastoma 1 trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef]
- Siebert, N.; Jensen, C.; Troschke-Meurer, S.; Zumpe, M.; Jüttner, M.; Ehlert, K.; Kietz, S.; Müller, I.; Lode, H.N. Neuroblastoma patients with high-affinity FCGR2A, -3A and stimulatory KIR 2DS2 treated by long-term infusion of anti-GD2 antibody ch14.18/CHO show higher ADCC levels and improved event-free survival. Oncoimmunology 2016, 5, e1235108. [Google Scholar] [CrossRef]
- Bellora, F.; Castriconi, R.; Dondero, A.; Reggiardo, G.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc. Natl. Acad. Sci. USA 2010, 107, 21659–21664. [Google Scholar] [CrossRef]
- Lapaque, N.; Walzer, T.; Méresse, S.; Vivier, E.; Trowsdale, J. Interactions between Human NK Cells and Macrophages in Response to Salmonella Infection. J. Immunol. 2009, 182, 4339–4348. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Romagnani, C.; Thiel, A.; Moretta, L.; Moretta, A. Multidirectional interactions are bridging human NK cells with plasmacytoid and monocyte-derived dendritic cells during innate immune responses. Blood 2006, 108, 3851–3858. [Google Scholar] [CrossRef]
- Nassin, M.L.; Nicolaou, E.; Gurbuxani, S.; Cohn, S.L.; Cunningham, J.M.; LaBelle, J.L. Immune Reconstitution Following Autologous Stem Cell Transplantation in Patients with High-Risk Neuroblastoma at the Time of Immunotherapy. Biol. Blood Marrow Transplant. 2018, 24, 452–459. [Google Scholar] [CrossRef]
- Copelan, E.A. Hematopoietic Stem-Cell Transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef]
- Rocha, V.; Locatelli, F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant. 2008, 41, 207–214. [Google Scholar] [CrossRef]
- Aversa, F.; Tabilio, A.; Velardi, A.; Cunningham, I.; Terenzi, A.; Falzetti, F.; Ruggeri, L.; Barbabietola, G.; Aristei, C.; Latini, P.; et al. Treatment of High-Risk Acute Leukemia with T-Cell–Depleted Stem Cells from Related Donors with One Fully Mismatched HLA Haplotype. N. Engl. J. Med. 1998, 339, 1186–1193. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell aloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef]
- Locatelli, F.; Merli, P.; Pagliara, D.; Li Pira, G.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after ab T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef]
- Locatelli, F.; Pende, D.; Falco, M.; Della Chiesa, M.; Moretta, A.; Moretta, L. NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol. 2018, 39, 577–590. [Google Scholar] [CrossRef]
- Locatelli, F.; Pende, D.; Mingari, M.C.; Bertaina, A.; Falco, M.; Moretta, A.; Moretta, L. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: Role of alloreactive NK cells. Front. Immunol. 2013, 4, 15. [Google Scholar] [CrossRef]
- Mancusi, A.; Ruggeri, L.; Urbani, E.; Pierini, A.; Massei, M.S.; Carotti, A.; Terenzi, A.; Falzetti, F.; Tosti, A.; Topini, F.; et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces nonrelapse mortality. Blood 2015, 125, 3173–3182. [Google Scholar] [CrossRef]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-like receptors (KIRs): Their role in NK cell modulation and developments leading to their clinical exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef]
- Blunt, M.D.; Khakoo, S.I. Activating killer cell immunoglobulin-like receptors: Detection, function and therapeutic use. Int. J. Immunogenet. 2020, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Marcenaro, S.; Falco, M.; Martini, S.; Bernardo, M.E.; Montagna, D.; Romeo, E.; Cognet, C.; Martinetti, M.; Maccario, R.; et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009, 113, 3119–3129. [Google Scholar] [CrossRef]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.E.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J. CD56dimCD57+ NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Taras, E.; Chiuppesi, F.; Wagner, J.E.; Blazar, B.R.; Brunstein, C.; Luo, X.; Diamond, D.J.; Cooley, S.; Weisdorf, D.J.; et al. Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight 2019, 4, e125553. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S.; et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 2018, 131, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.R.; Chakraborty, S.; Lakhchaura, R.; Shashi, P.; Mehta, A.; Soni, M.; Chakrabarti, S. Early and Sustained Expansion of Adaptive Natural Killer Cells Following Haploidentical Transplantation and CTLA4Ig-Primed Donor Lymphocyte Infusions Dissociate Graft-versus-Leukemia and Graft-versus-Host Effects. Transplant. Cell. Ther. 2021, 27, 144–151. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.J.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef]
- Capuano, C.; Battella, S.; Pighi, C.; Franchitti, L.; Turriziani, O.; Morrone, S.; Santoni, A.; Galandrini, R.; Palmieri, G. Tumor-Targeting Anti-CD20 antibodies mediate in vitro expansion of memory natural killer cells: Impact of CD16 affinity ligation conditions and in vivo priming. Front. Immunol. 2018, 9, 1031. [Google Scholar] [CrossRef]
- Chaleff, S.; Otto, M.; Barfield, R.C.; Leimig, T.; Iyengar, R.; Martin, J.; Holiday, M.; Houston, J.; Geiger, T.; Huppert, V.; et al. A large-scale method for the selective depletion of αβ T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy 2007, 9, 746–754. [Google Scholar] [CrossRef]
- Li Pira, G.; Malaspina, D.; Girolami, E.; Biagini, S.; Cicchetti, E.; Conflitti, G.; Broglia, M.; Ceccarelli, S.; Lazzaro, S.; Pagliara, D.; et al. Selective Depletion of αβ T Cells and B Cells for Human Leukocyte Antigen–Haploidentical Hematopoietic Stem Cell Transplantation. A Three-Year Follow-Up of Procedure Efficiency. Biol. Blood Marrow Transplant. 2016, 22, 2056–2064. [Google Scholar] [CrossRef]
- Bertaina, A.; Roncarolo, M.G. Graft engineering and adoptive immunotherapy: New approaches to promote immune tolerance after hematopoietic stem cell transplantation. Front. Immunol. 2019, 10, 1342. [Google Scholar] [CrossRef]
- Stern, M.; Ruggeri, L.; Mancusi, A.; Bernardo, M.E.; De Angelis, C.; Bucher, C.; Locatelli, F.; Aversa, F.; Velardi, A. Survival after T cell depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 2008, 112, 2990–2995. [Google Scholar] [CrossRef]
- Sivori, S.; Meazza, R.; Quintarelli, C.; Carlomagno, S.; Della Chiesa, M.; Falco, M.; Moretta, L.; Locatelli, F.; Pende, D. NK Cell-Based Immunotherapy for Hematological Malignancies. J. Clin. Med. 2019, 8, 1702. [Google Scholar] [CrossRef]
- Tumino, N.; Besi, F.; Di Pace, A.L.; Mariotti, F.R.; Merli, P.; Li Pira, G.; Galaverna, F.; Pitisci, A.; Ingegnere, T.; Pelosi, A.; et al. PMN-MDSC are a new target to rescue graft-versus-leukemia activity of NK cells in haplo-HSC transplantation. Leukemia 2020, 34, 932–937. [Google Scholar] [CrossRef]
- O’Donnell, P.V.; Luznik, L.; Jones, R.J.; Vogelsang, G.B.; Leffell, M.S.; Phelps, M.; Rhubart, P.; Cowan, K.; Piantadosi, S.; Fuchs, E.J. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2002, 8, 377–386. [Google Scholar] [CrossRef]
- Ruggeri, A.; Galimard, J.E.; Paina, O.; Fagioli, F.; Tbakhi, A.; Yesilipek, A.; Navarro, J.M.F.; Faraci, M.; Hamladji, R.M.; Skorobogatova, E.; et al. Outcomes of Unmanipulated Haploidentical Transplantation Using Post-Transplant Cyclophosphamide (PT-Cy) in Pediatric Patients With Acute Lymphoblastic Leukemia. Transplant. Cell. Ther. 2021, 27, 424.e1–424.e9. [Google Scholar] [CrossRef]
- Hong, K.T.; Kang, H.J.; Choi, J.Y.; Hong, C.R.; Cheon, J.E.; Park, J.D.; Park, K.D.; Song, S.H.; Yu, K.S.; Jang, I.J.; et al. Favorable Outcome of Post-Transplantation Cyclophosphamide Haploidentical Peripheral Blood Stem Cell Transplantation with Targeted Busulfan-Based Myeloablative Conditioning Using Intensive Pharmacokinetic Monitoring in Pediatric Patients. Biol. Blood Marrow Transplant. 2018, 24, 2239–2244. [Google Scholar] [CrossRef]
- Melchionda, F.; Oncology, P.; Spreafico, F.; Unit, P.O.; Hemato-oncology, P.; Ciceri, S.; Unit, G.T.; Medicine, P.; Lima, M.; Unit, P.S.; et al. KIR–HLA Receptor-Ligand Mismatch Associated With a Graft-Versus-Tumor. Pediatr. Blood Cancer 2009, 53, 120–124. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, E.S.; Sung, K.W.; Yi, E.; Lee, S.H.; Yoo, K.H.; Koo, H.H. Incorporation of high-dose 131I-metaiodobenzylguanidine treatment into killer immunoglobulin-like receptor/HLA-ligand mismatched haploidentical stem cell transplantation for children with neuroblastoma who failed tandem autologous stem cell transplantatio. Pediatr. Blood Cancer 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Kanold, J.; Paillard, C.; Tchirkov, A.; Lang, P.; Kelly, A.; Halle, P.; Isfan, F.; Merlin, E.; Marabelle, A.; Rochette, E. NK Cell immunotherapy for high-risk neuroblastoma relapse after haploidentical HSCT. Pediatr. Blood Cancer 2012, 59, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Sondel, P.M.; Hank, J.A.; Hutson, P.; Meagher, M.; Shafer, A.; Ng, C.Y.; Leung, W.; et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin. Cancer Res. 2017, 23, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Le Luduec, J.B.; Cheung, I.Y.; Goldman, D.A.; Ostrovnaya, I.; Doubrovina, E.; Basu, E.; Kushner, B.H.; Kramer, K.; Roberts, S.S.; et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology 2018, 7, e1461305. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.M.; Feuchtinger, T.; Teltschik, H.M.; Schumm, M.; Müller, I.; Handgretinger, R.; Lang, P. Reconstitution of natural killer cell receptors influences natural killer activity and relapse rate after haploidentical transplantation of T- and B-cell depleted grafts in children. Haematologica 2010, 95, 1381–1388. [Google Scholar] [CrossRef]
- Khandelwal, P.; Teusink-Cross, A.; Davies, S.M.; Nelson, A.S.; Dandoy, C.E.; El-Bietar, J.; Marsh, R.A.; Kumar, A.R.; Grimley, M.S.; Jodele, S.; et al. Ruxolitinib as Salvage Therapy in Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric Hematopoietic Stem Cell Transplant Patients. Biol. Blood Marrow Transplant. 2017, 23, 1122–1127. [Google Scholar] [CrossRef]
- Seitz, C.M.; Flaadt, T.; Mezger, M.; Lang, A.M.; Michaelis, S.; Katz, M.; Syring, D.; Joechner, A.; Rabsteyn, A.; Siebert, N.; et al. Immunomonitoring of Stage IV Relapsed Neuroblastoma Patients Undergoing Haploidentical Hematopoietic Stem Cell Transplantation and Subsequent GD2 (ch14.18/CHO) Antibody Treatment. Front. Immunol. 2021, 12, 690467. [Google Scholar] [CrossRef]
- Dondero, A.; Morini, M.; Cangelosi, D.; Mazzocco, K.; Serra, M.; Spaggiari, G.M.; Rotta, G.; Tondo, A.; Locatelli, F.; Castellano, A.; et al. Multiparametric flow cytometry highlights B7-H3 as a novel diagnostic/therapeutic target in GD2neg/low neuroblastoma variants. J. Immunother. Cancer 2021, 9, e002293. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef]
- Raffaghello, L.; Prigione, I.; Bocca, P.; Morandi, F.; Camoriano, M.; Gambini, C.; Wang, X.; Ferrone, S.; Pistoia, V. Multiple defects of the antigen-processing machinery components in human neuroblastoma: Immunotherapeutic implications. Oncogene 2005, 24, 4634–4644. [Google Scholar] [CrossRef]
- Gregorio, A.; Corrias, M.V.; Castriconi, R.; Dondero, A.; Mosconi, M.; Gambini, C.; Moretta, A.; Moretta, L.; Bottino, C. Small round blue cell tumours: Diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008, 53, 73–80. [Google Scholar] [CrossRef]
- Prasad, D.V.R.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y.; Dong, C. Murine B7-H3 Is a Negative Regulator of T Cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Melaiu, O.; Mina, M.; Chierici, M.; Boldrini, R.; Jurman, G.; Romania, P.; D’Alicandro, V.; Benedetti, M.C.; Castellano, A.; Liu, T.; et al. PD-L1 is a therapeutic target of the bromodomain inhibitor JQ1 and, combined with HLA class I, a promising prognostic biomarker in neuroblastoma. Clin. Cancer Res. 2017, 23, 4462–4472. [Google Scholar] [CrossRef]
- Siebert, N.; Zumpe, M.; Jüttner, M.; Troschke-Meurer, S.; Lode, H.N. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology 2017, 6, e1343775. [Google Scholar] [CrossRef]
- Maas, R.J.; Hoogstad-van Evert, J.S.; Van der Meer, J.M.; Mekers, V.; Rezaeifard, S.; Korman, A.J.; de Jonge, P.K.; Cany, J.; Woestenenk, R.; Schaap, N.P.; et al. TIGIT blockade enhances functionality of peritoneal NK cells with altered expression of DNAM-1/TIGIT/CD96 checkpoint molecules in ovarian cancer. Oncoimmunology 2020, 9, 1843247. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Open Peer Review Targeting the “PVR-TIGIT axis” with immune checkpoint therapies [version 1; peer review: 2 approved]. F1000Researh 2020, 9, 354. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef]
- Stanietsky, N.; Mandelboim, O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett. 2010, 584, 4895–4900. [Google Scholar] [CrossRef]
- Blake, S.J.; Dougall, W.C.; Miles, J.J.; Teng, M.W.L.; Smyth, M.J. Molecular pathways: Targeting CD96 and TIGIT for cancer immunotherapy. Clin. Cancer Res. 2016, 22, 5183–5188. [Google Scholar] [CrossRef]
- Muntasell, A.; Ochoa, M.C.; Cordeiro, L.; Berraondo, P.; López-Díaz de Cerio, A.; Cabo, M.; López-Botet, M.; Melero, I. Targeting NK-cell checkpoints for cancer immunotherapy. Curr. Opin. Immunol. 2017, 45, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Correa, B.; Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Bañas, H.; Casas-Avilés, I.; Durán, E.; et al. DNAM-1 and the TIGIT/PVRIG/TACTILE axis: Novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers 2019, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A.; Sahasranaman, S.; Budha, N. Targeting tigit for immunotherapy of cancer: Update on clinical development. Biomedicines 2021, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Matta, J.; Baratin, M.; Chiche, L.; Forel, J.M.; Cognet, C.; Thomas, G.; Farnarier, C.; Piperoglou, C.; Papazian, L.; Chaussabel, D.; et al. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 2013, 122, 394–404. [Google Scholar] [CrossRef]
- Vieillard, V.; Baychelier, F.; Debré, P. NKp44L: A new tool for fighting cancer. Oncoimmunology 2014, 3, 20–22. [Google Scholar] [CrossRef]
- Nausch, N.; Cerwenka, A. NKG2D ligands in tumor immunity. Oncogene 2008, 27, 5944–5958. [Google Scholar] [CrossRef]
- Stabile, H.; Nisti, P.; Peruzzi, G.; Fionda, C.; Pagliara, D.; Brescia, P.L.; Merli, P.; Locatelli, F.; Santoni, A.; Gismondi, A. Reconstitution of multifunctional CD56lowCD16low natural killer cell subset in children with acute leukemia given α/β T cell-depleted HLA-haploidentical haematopoietic stem cell transplantation. Oncoimmunology 2017, 6, e1342024. [Google Scholar] [CrossRef]
- Crinier, A.; Dumas, P.Y.; Escalière, B.; Piperoglou, C.; Gil, L.; Villacreces, A.; Vély, F.; Ivanovic, Z.; Milpied, P.; Narni-Mancinelli, É.; et al. Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell. Mol. Immunol. 2021, 18, 1290–1304. [Google Scholar] [CrossRef]
- Castriconi, R.; Dondero, A.; Bellora, F.; Moretta, L.; Castellano, A.; Locatelli, F.; Corrias, M.V.; Moretta, A.; Bottino, C. Neuroblastoma-Derived TGF-β1 Modulates the Chemokine Receptor Repertoire of Human Resting NK Cells. J. Immunol. 2013, 190, 5321–5328. [Google Scholar] [CrossRef]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef]
- Vitale, M.; Della Chiesa, M.; Carlomagno, S.; Pende, D.; Aricò, M.; Moretta, L.; Moretta, A. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood 2005, 106, 566–571. [Google Scholar] [CrossRef]
- Pallandre, J.R.; Krzewski, K.; Bedel, R.; Ryffel, B.; Caignard, A.; Rohrlich, P.S.; Pivot, X.; Tiberghien, P.; Zitvogel, L.; Strominger, J.L.; et al. Dendritic cell and natural killer cell cross-talk: A pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood 2008, 112, 4420–4424. [Google Scholar] [CrossRef][Green Version]
- Walzer, T.; Dalod, M.; Robbins, S.H.; Zitvogel, L.; Vivier, E. Natural-killer cells and dendritic cells: “L’union fait la force”. Blood 2005, 106, 2252–2258. [Google Scholar] [CrossRef]
- Ali, A.K.; Oh, J.S.; Vivier, E.; Busslinger, M.; Lee, S.-H. NK Cell–Specific Gata3 Ablation Identifies the Maturation Program Required for Bone Marrow Exit and Control of Proliferation. J. Immunol. 2016, 196, 1753–1767. [Google Scholar] [CrossRef]
- Tang, P.M.K.; Zhou, S.; Meng, X.M.; Wang, Q.M.; Li, C.J.; Lian, G.Y.; Huang, X.R.; Tang, Y.J.; Guan, X.Y.; Yan, B.P.Y.; et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat. Commun. 2017, 8, 14677. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56 bright Perforin low Noncytotoxic Human NK Cells Are Abundant in Both Healthy and Neoplastic Solid Tissues and Recirculate to Secondary Lymphoid Organs via Afferent Lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef]
- Casu, B.; Dondero, A.; Regis, S.; Caliendo, F.; Petretto, A.; Bartolucci, M.; Bellora, F.; Bottino, C.; Castriconi, R. Novel immunoregulatory functions of IL-18, an accomplice of TGF-β1. Cancers 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.C.; Wan, Z.; Sheard, M.A.; Sun, J.; Jackson, J.R.; Malvar, J.; Xu, Y.; Wang, L.; Sposto, R.; Kim, E.S.; et al. TGFβR1 blockade with galunisertib (LY2157299) enhances anti-neuroblastoma activity of the anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin. Cancer Res. 2017, 23, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Borg, C.; Terme, M.; Taïeb, J.; Ménard, C.; Flament, C.; Robert, C.; Maruyama, K.; Wakasugi, H.; Angevin, E.; Thielemans, K.; et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Investig. 2004, 114, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Rusakiewicz, S.; Routy, B.; Ayyoub, M.; Kroemer, G. Immunological off-target effects of imatinib. Nat. Rev. Clin. Oncol. 2016, 13, 431–446. [Google Scholar] [CrossRef]
- Bellora, F.; Dondero, A.; Corrias, M.V.; Casu, B.; Regis, S.; Caliendo, F.; Moretta, A.; Cazzola, M.; Elena, C.; Vinti, L.; et al. Imatinib and Nilotinib Off-Target Effects on Human NK Cells, Monocytes, and M2 Macrophages. J. Immunol. 2017, 199, 1516–1525. [Google Scholar] [CrossRef]
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W.; et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Varahram, M.; Movassaghi, M.; Kraneveld, A.D.; Garssen, J.; Adcock, I.M. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front. Immunol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Marimpietri, D.; Petretto, A.; Raffaghello, L.; Pezzolo, A.; Gagliani, C.; Tacchetti, C.; Mauri, P.; Melioli, G.; Pistoia, V. Proteome Profiling of Neuroblastoma-Derived Exosomes Reveal the Expression of Proteins Potentially Involved in Tumor Progression. PLoS ONE 2013, 8, e75054. [Google Scholar] [CrossRef]
- Nakata, R.; Shimada, H.; Fernandez, G.E.; Fanter, R.; Fabbri, M.; Malvar, J.; Zimmermann, P.; DeClerck, Y.A. Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J. Extracell. Vesicles 2017, 6, 1332941. [Google Scholar] [CrossRef]
- Colletti, M.; Petretto, A.; Galardi, A.; Di Paolo, V.; Tomao, L.; Lavarello, C.; Inglese, E.; Bruschi, M.; Lopez, A.A.; Pascucci, L.; et al. Proteomic Analysis of Neuroblastoma-Derived Exosomes: New Insights into a Metastatic Signature. Proteomics 2017, 17, 1600430. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef]
- Morini, M.; Cangelosi, D.; Segalerba, D.; Marimpietri, D.; Raggi, F.; Castellano, A.; Fruci, D.; De Mora, J.F.; Cañete, A.; Yáñez, Y.; et al. Exosomal microRNAs from longitudinal liquid biopsies for the prediction of response to induction chemotherapy in high-risk neuroblastoma patients: A proof of concept SIOPEN study. Cancers 2019, 11, 1476. [Google Scholar] [CrossRef]
- Clayton, A.; Mitchell, J.P.; Court, J.; Linnane, S.; Mason, M.D.; Tabi, Z. Human Tumor-Derived Exosomes Down-Modulate NKG2D Expression. J. Immunol. 2008, 180, 7249–7258. [Google Scholar] [CrossRef]
- Hosseini, R.; Sarvnaz, H.; Arabpour, M.; Ramshe, S.M.; Asef-Kabiri, L.; Yousefi, H.; Akbari, M.E.; Eskandari, N. Cancer exosomes and natural killer cells dysfunction: Biological roles, clinical significance and implications for immunotherapy. Mol. Cancer 2022, 21, 15. [Google Scholar] [CrossRef]
- Battke, C.; Ruiss, R.; Welsch, U.; Wimberger, P.; Lang, S.; Jochum, S.; Zeidler, R. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol. Immunother. 2011, 60, 639–648. [Google Scholar] [CrossRef]
- Hong, C.S.; Sharma, P.; Yerneni, S.S.; Simms, P.; Jackson, E.K.; Whiteside, T.L.; Boyiadzis, M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017, 7, 14684. [Google Scholar] [CrossRef]
- Liu, X.; Wills, C.A.; Chen, L.; Zhang, J.; Zhao, Y.; Zhou, M.; Sundstrom, J.M.; Schell, T.; Spiegelman, V.S.; Young, M.M.; et al. Small extracellular vesicles induce resistance to anti-GD2 immunotherapy unveiling tipifarnib as an adjunct to neuroblastoma immunotherapy. J. Immunother. Cancer 2022, 10, e004399. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, X.; Wang, X.; Wang, Y.; Cao, W.; Zhang, J.; Chen, W. Oral cancer cell-derived exosomes modulate natural killer cell activity by regulating the receptors on these cells. Int. J. Mol. Med. 2020, 46, 2115–2125. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Hamidieh, A.A.; Behfar, M.; Mohseni, R.; Mortazavi-Tabatabaei, S.A.; Asgharzadeh, S. NK Cell-derived Exosomes from NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-Activated NK Cells. J. Immunother. 2017, 40, 265–276. [Google Scholar] [CrossRef]
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019, 79, 1151–1164. [Google Scholar] [CrossRef]
- Seo, E.S.; Shin, M.; Lim, H.; Cho, H.W.; Ju, H.Y.; Cho, Y.S.; Yoo, K.H.; Koo, H.H.; Lee, J.W.; Sung, K.W. Clinical implication of residual MIBG-positive disease in the follow-up of high-risk neuroblastoma treated with tandem high-dose chemotherapy and autologous stem cell transplantation. Pediatr. Blood Cancer 2022, 69, e29502. [Google Scholar] [CrossRef]
- Druy, A.E.; Shorikov, E.V.; Tsaur, G.A.; Popov, A.M.; Zaychikov, A.N.; Tuponogov, S.N.; Saveliev, L.I.; Tytgat, G.A.M.; Fechina, L.G. Prospective investigation of applicability and the prognostic significance of bone marrow involvement in patients with neuroblastoma detected by quantitative reverse transcription PCR. Pediatr. Blood Cancer 2018, 65, e27354. [Google Scholar] [CrossRef]
- Furman, W.L.; Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Davidoff, A.M.; Krasin, M.J.; Sahr, N.; Sykes, A.; Wu, J.; Brennan, R.C.; et al. A Phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin. Cancer Res. 2019, 25, 6320–6328. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef]
- Jing, Y.; Ni, Z.; Wu, J.; Higgins, L.A.; Markowski, T.W.; Kaufman, D.S.; Walcheck, B. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS ONE 2015, 10, e0121788. [Google Scholar] [CrossRef]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16×33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef]
- Vincent, M.; Bessard, A.; Cochonneau, D.; Teppaz, G.; Solé, V.; Maillasson, M.; Birklé, S.; Garrigue-Antar, L.; Quéméner, A.; Jacques, Y. Tumor targeting of the IL-15 superagonist RLI by an anti-GD2 antibody strongly enhances its antitumor potency. Int. J. Cancer 2013, 133, 757–765. [Google Scholar] [CrossRef]
- Chu, Y.; Nayyar, G.; Jiang, S.; Rosenblum, J.M.; Soon-Shiong, P.; Safrit, J.T.; Lee, D.A.; Cairo, M.S. Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2 + pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG. J. Immunother. Cancer 2021, 9, e002267. [Google Scholar] [CrossRef] [PubMed]
- Ptacin, J.L.; Caffaro, C.E.; Ma, L.; San Jose Gall, K.M.; Aerni, H.R.; Acuff, N.V.; Herman, R.W.; Pavlova, Y.; Pena, M.J.; Chen, D.B.; et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat. Commun. 2021, 12, 4785. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.V.; Kim, H.; Bjordahl, R.; Davis, Z.B.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 2021, 28, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Huntington, N.D.; Legrand, N.; Alves, N.L.; Jaron, B.; Weijer, K.; Plet, A.; Corcuff, E.; Mortier, E.; Jacques, Y.; Spits, H.; et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 2009, 206, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Bao, G.; Zhang, Y.; Lin, D.; Wu, Y.; Wu, D.; Liu, H. Donor NK Cells and IL-15 Promoted Engraftment in Nonmyeloablative Allogeneic Bone Marrow Transplantation. J. Immunol. 2012, 189, 1661–1670. [Google Scholar] [CrossRef]
- Alpdogan, O.; Eng, J.M.; Muriglan, S.J.; Willis, L.M.; Hubbard, V.M.; Tjoe, K.H.; Terwey, T.H.; Kochman, A.; Van Den Brink, M.R.M. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood 2005, 105, 865–873. [Google Scholar] [CrossRef]
- Shan, L.; Flavell, R.A.; Herndler-Brandstetter, D. Development of Humanized Mouse Models for Studying Human NK Cells in Health and Disease. In Natural Killer (NK) Cells; Springer: Berlin/Heidelberg, Germany, 2022; pp. 53–66. [Google Scholar]
- Orrantia, A.; Terrén, I.; Astarloa-Pando, G.; González, C.; Uranga, A.; Mateos-Mazón, J.J.; García-Ruiz, J.C.; Riñón, M.; Rey, M.; Pérez-Fernandez, S.; et al. NK Cell Reconstitution After Autologous Hematopoietic Stem Cell Transplantation: Association Between NK Cell Maturation Stage and Outcome in Multiple Myeloma. Front. Immunol. 2021, 12, 748207. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Desai, S.; Pence, P.; Cooley, S. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood J. Am. Soc. Hematol. 2022, 139, 1177–1183. [Google Scholar] [CrossRef]
- Delconte, R.B.; Kolesnik, T.B.; Dagley, L.F.; Rautela, J.; Shi, W.; Putz, E.M.; Stannard, K.; Zhang, J.G.; Teh, C.; Firth, M.; et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 2016, 17, 816–824. [Google Scholar] [CrossRef]
- Delconte, R.B.; Guittard, G.; Goh, W.; Hediyeh-Zadeh, S.; Hennessy, R.J.; Rautela, J.; Davis, M.J.; Souza-Fonseca-Guimaraes, F.; Nunès, J.A.; Huntington, N.D. NK Cell Priming From Endogenous Homeostatic Signals Is Modulated by CIS. Front. Immunol. 2020, 11, 75. [Google Scholar] [CrossRef]
- Daher, M.; Basar, R.; Gokdemir, E.; Baran, N.; Uprety, N.; Nunez Cortes, A.K.; Mendt, M.; Kerbauy, L.N.; Banerjee, P.P.; Shanley, M.; et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 2021, 137, 624–636. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Rossi, G.R.; Dagley, L.F.; Foroutan, M.; McCulloch, T.R.; Yousef, J.; Park, H.-Y.; Gunter, J.H.; Beavis, P.A.; Lin, C.-Y. TGFβ and CIS Inhibition Overcomes NK-cell Suppression to Restore Antitumor Immunity. Cancer Immunol. Res. 2022, 10, 1047–1054. [Google Scholar] [CrossRef]
- Chiossone, L.; Vienne, M.; Kerdiles, Y.M.; Vivier, E. Natural killer cell immunotherapies against cancer: Checkpoint inhibitors and more. Semin. Immunol. 2017, 31, 55–63. [Google Scholar] [CrossRef]
- Van Hall, T.; André, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; Van Der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the novel immune checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 2018, 175, 1731–1743. [Google Scholar] [CrossRef]
- Borst, L.; van der Burg, S.H.; van Hall, T. The NKG2A-HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin. Cancer Res. 2021, 26, 5549–5556. [Google Scholar] [CrossRef]
- Choi, Y.B.; Son, M.H.; Cho, H.W.; Ma, Y.; Lee, J.W.; Kang, E.S.; Yoo, K.H.; Her, J.H.; Lim, O.; Jung, M.; et al. Safety and immune cell kinetics after donor natural killer cell infusion following haploidentical stem cell transplantation in children with recurrent neuroblastoma. PLoS ONE 2019, 14, e0225998. [Google Scholar] [CrossRef]
- Toporski, J.; Garkavij, M.; Tennvall, J.; Øra, I.; Gleisner, K.S.; Dykes, J.H.; Lenhoff, S.; Juliusson, G.; Scheding, S.; Turkiewicz, D.; et al. High-Dose Iodine-131-Metaiodobenzylguanidine with Haploidentical Stem Cell Transplantation and Posttransplant Immunotherapy in Children with Relapsed/Refractory Neuroblastoma. Biol. Blood Marrow Transplant. 2009, 15, 1077–1085. [Google Scholar] [CrossRef]
- Dong, H.; Dongjoo, J.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H. Memory-like NK cells armed with a neoepitope-speci fi c CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Birch, G.C.; Hu, G.; Vergara Cadavid, J.; Nikiforow, S.; Baginska, J.; Ali, A.K.; Tarannum, M.; Sheffer, M.; Abdulhamid, Y.Z.; et al. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J. Clin. Investig. 2022, 132, e154334. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Astarloa-Pando, G.; Amarilla-Irusta, A.; Zenarruzabeitia, O.; Borrego, F. Cytokine-Induced Memory-Like NK Cells: From the Basics to Clinical Applications. Front. Immunol. 2022, 13, 4648. [Google Scholar] [CrossRef]
- Valteau-Couanet, D.; Leboulaire, C.; Maincent, K.; Tournier, M.; Hartmann, O.; Bénard, J.; Beaujean, F.; Boccaccio, C.; Zitvogel, L.; Angevin, E. Dendritic cells for NK/LAK activation: Rationale for multicellular immunotherapy in neuroblastoma patients. Blood 2002, 100, 2554–2561. [Google Scholar] [CrossRef]
- Caforio, M.; Sorino, C.; Caruana, I.; Weber, G.; Camera, A.; Cifaldi, L.; De Angelis, B.; Del Bufalo, F.; Vitale, A.; Goffredo, B.M.; et al. GD2 redirected CAR T and activated NK-cell-mediated secretion of IFNγovercomes MYCN-dependent IDO1 inhibition, contributing to neuroblastoma cell immune escape. J. Immunother. Cancer 2021, 9, e001502. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Talbot, L.J.; Chabot, A.; Funk, A.; Nguyen, P.; Wagner, J.; Ross, A.; Tillman, H.; Davidoff, A.; Gottschalk, S.; DeRenzo, C. A Novel Orthotopic Implantation Technique for Osteosarcoma Produces Spontaneous Metastases and Illustrates Dose-Dependent Efficacy of B7-H3-CAR T Cells. Front. Immunol. 2021, 12, 2209. [Google Scholar] [CrossRef]
- Tian, M.; Ho, M.; Khan, J. An optimized bicistronic chimeric antigen receptor against GPC2 or CD276 overcomes heterogeneous expression in neuroblastoma. J. Clin. Investig. 2022, 132, e155621. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Bosse, K.R.; Zhu, Z.; Zhelev, D.; Majzner, R.G.; Radosevich, M.T.; Dhingra, S.; Sotillo, E.; Buongervino, S.; Pascual-Pasto, G.; et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell 2022, 40, 53–69.e9. [Google Scholar] [CrossRef]
- Künkele, A.; Taraseviciute, A.; Finn, L.S.; Johnson, A.J.; Berger, C.; Finney, O.; Chang, C.A.; Rolczynski, L.S.; Brown, C.; Mgebroff, S. Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing FeasibilityPreclinical Study of CE7-CAR T-cell Therapy in Neuroblastoma. Clin. Cancer Res. 2017, 23, 466–477. [Google Scholar] [CrossRef]
- Qin, V.M.; D’souza, C.; Neeson, P.J.; Zhu, J.J. Chimeric antigen receptor beyond CAR-T cells. Cancers 2021, 13, 404. [Google Scholar] [CrossRef]
- Laskowski, T.J.; Biederstädt, A. Natural Killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022. [CrossRef]
- Marofi, F.; Abdul-Rasheed, O.F.; Rahman, H.S.; Budi, H.S.; Jalil, A.T.; Yumashev, A.V.; Hassanzadeh, A.; Yazdanifar, M.; Motavalli, R.; Chartrand, M.S.; et al. CAR-NK cell in cancer immunotherapy; A promising frontier. Cancer Sci. 2021, 112, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jayasinghe, R.; Devenport, J.M.; Ritchey, J.K.; Rettig, M.P.; O’Neal, J.; Staser, K.W.; Kennerly, K.M.; Carter, A.J.; Gao, F.; et al. A long-acting interleukin-7, rhIL-7-hyFc, enhances CAR T cell expansion, persistence, and anti-tumor activity. Nat. Commun. 2022, 13, 3296. [Google Scholar] [CrossRef] [PubMed]
- López-Cantillo, G.; Urueña, C.; Camacho, B.A.; Ramírez-Segura, C. CAR-T Cell Performance: How to Improve Their Persistence? Front. Immunol. 2022, 13, 1757. [Google Scholar] [CrossRef] [PubMed]
- Caruana, I.; Savoldo, B.; Hoyos, V.; Weber, G.; Liu, H.; Kim, E.S.; Ittmann, M.M.; Marchetti, D.; Dotti, G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015, 21, 524–529. [Google Scholar] [CrossRef]
- Abbasi, J. Mixed Findings in Pediatric Neuroblastoma CAR-T Therapy Trial. JAMA 2021, 325, 121. [Google Scholar] [CrossRef]
- Bates, P.D.; Rakhmilevich, A.L.; Cho, M.M.; Bouchlaka, M.N.; Rao, S.L.; Hales, J.M.; Orentas, R.J.; Fry, T.J.; Gilles, S.D.; Sondel, P.M.; et al. Combining Immunocytokine and Ex Vivo Activated NK Cells as a Platform for Enhancing Graft-Versus-Tumor Effects Against GD2+ Murine Neuroblastoma. Front. Immunol. 2021, 12, 3392. [Google Scholar] [CrossRef]
- Guercio, M.; Manni, S.; Boffa, I.; Caruso, S.; Di Cecca, S.; Sinibaldi, M.; Abbaszadeh, Z.; Camera, A.; Ciccone, R.; Polito, V.A.; et al. Inclusion of the Inducible Caspase 9 Suicide Gene in CAR Construct Increases Safety of CAR.CD19 T Cell Therapy in B-Cell Malignancies. Front. Immunol. 2021, 12, 4269. [Google Scholar] [CrossRef]
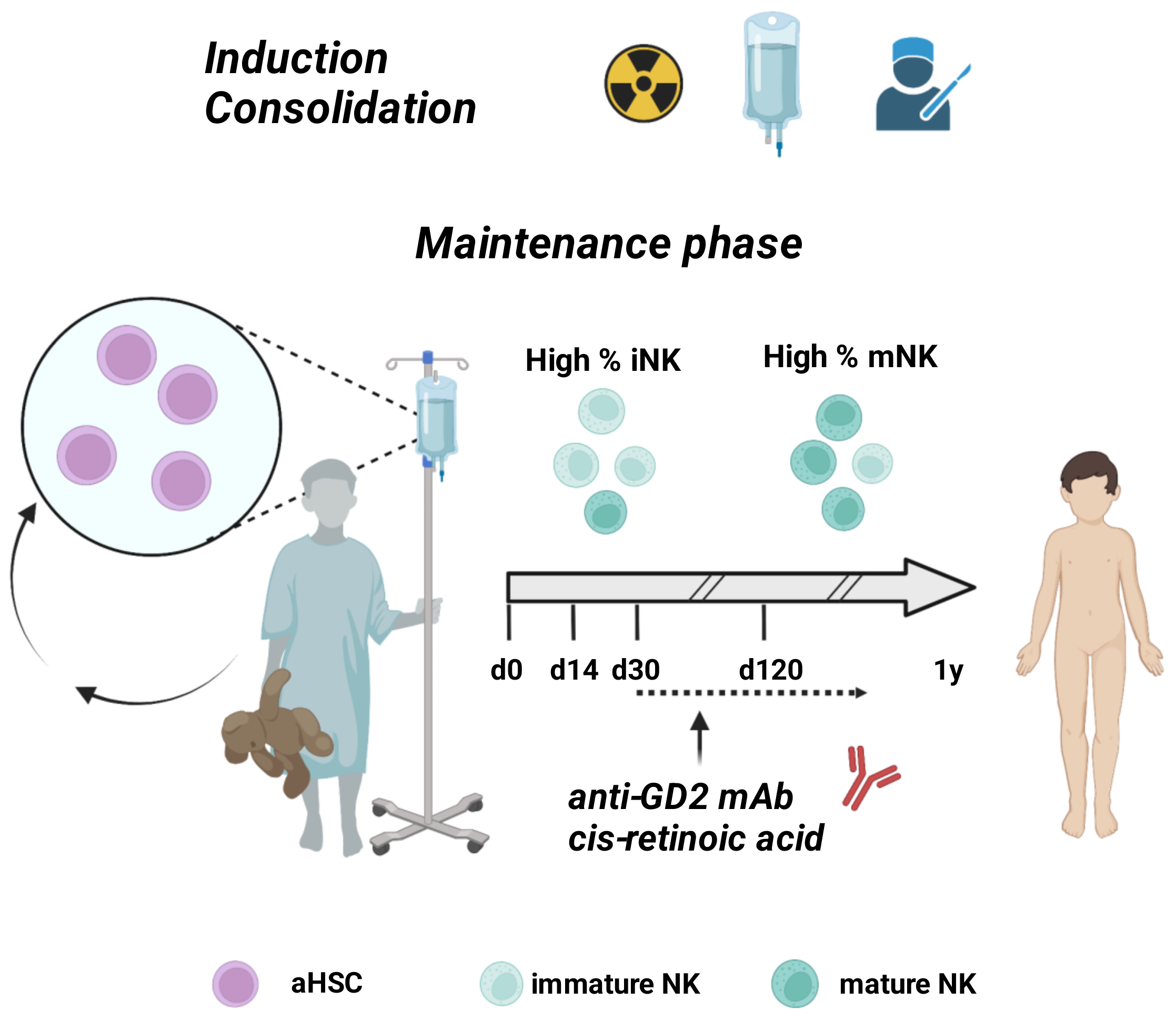

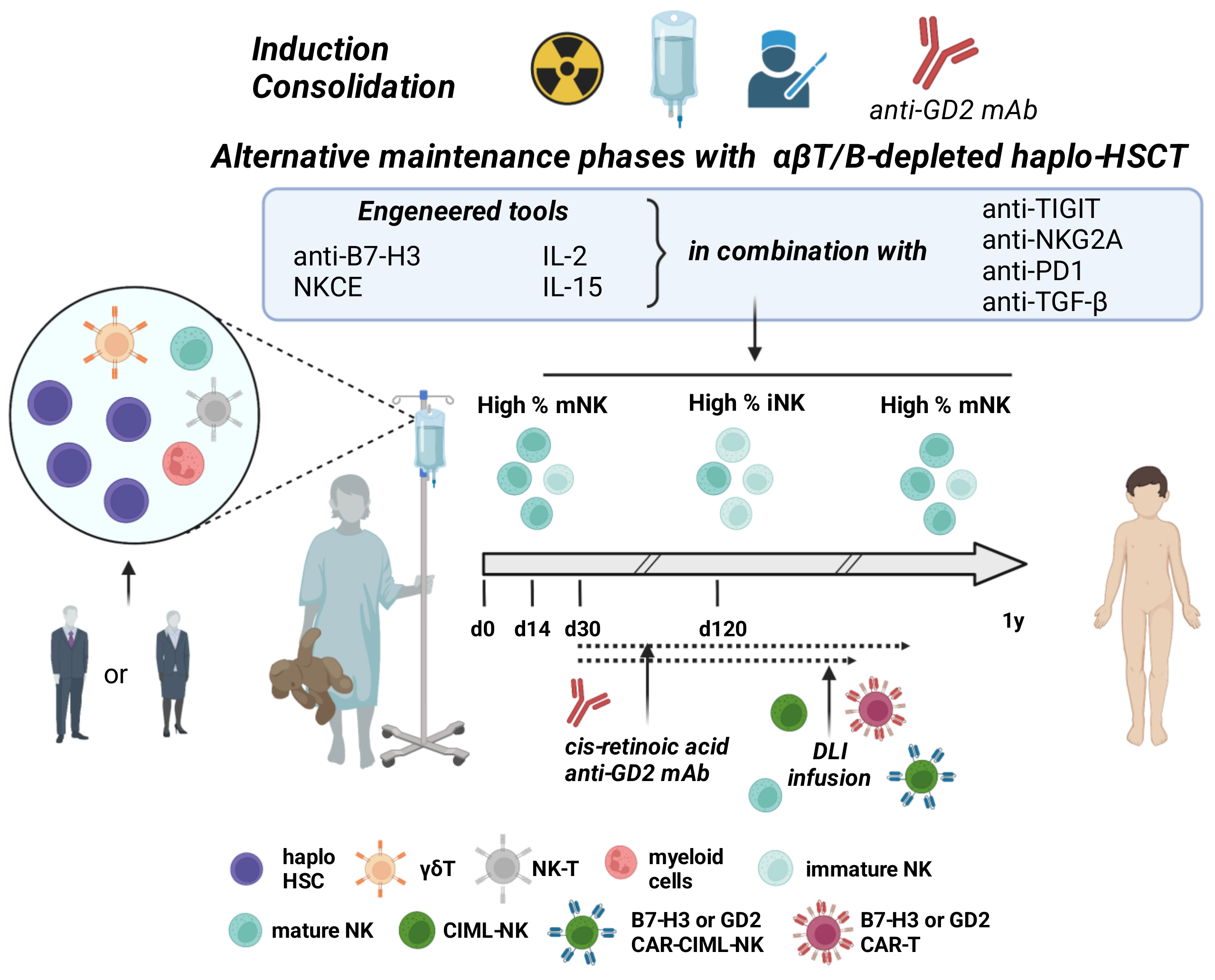
| Setting | NCT Number | Title | Status | Interventions |
|---|---|---|---|---|
| NCT 02573896 | Immunotherapy of Relapsed Refractory Neuroblastoma with Expanded NK Cells | Active, not recruiting | Ex-vivo expanded autologous NK cells + standard dosing of anti-GD2 (ch14.18/CHO) mAb + Lenalidomide | |
| AUTOLOGOUS | NCT04211675 | NK Cells Infusions With Irinotecan, Temozolomide, and Dinutuximab | Not yet recruiting | Ex-vivo expanded autologous NK Cells + Irinotecan, Temozolomide, and Dinutuximab |
| NCT 01875601 | NK White Blood Cells and Interleukin in Children and Young Adults with Advanced Solid Tumors | Completed | Cyclophosphamide lymphodepletion + ex-vivo expanded autologous NK cells + rIL15 | |
| HAPLOIDENTICAL | NCT 00698009 | Haploidentical NK Cells in Patients with Relapsed or Refractory Neuroblastoma | Terminated | HLA- Haploidentical NK cell infusion + Interleukin-2 |
| NCT 03242603 | Immunotherapy of Neuroblastoma Patients Using a Combination of Anti-GD2 and NK Cells | Unknown | Ex-vivo expanded activated HLA- Haploidentical NK cells + anti-GD2 (ch14.18/CHO) mAb | |
| NCT 01156350 | Haplo-identical Hematopoietic Stem Cell Transplantation Following Reduced-Intensity Conditioning in Children with Neuroblastoma | Unknown | HLA- Haploidentical HSCT + CD3/CD19 graft depletion | |
| NCT 00877110 | Anti-GD2 3F8 Antibody and Allogeneic Natural Killer cells for High-Risk Neuroblastoma | Completed | HLA-Haploidentical NK cells + anti-GD2 (3F8) mAb | |
| NCT 02650648 | Humanized Anti-GD2 Antibody Hu3F8 and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | Active, not recruiting | HLA- Haploidentical NK cells + anti-GD2 (humanized 3F8) mAb + rIL-2 | |
| NCT 01857934 | Therapy for Children with Advanced Stage Neuroblastoma | Active, not recruiting | anti-GD2 (hu14.18K322A) mAb + HLA- Haploidentical NK cells + rIL-2 + GM-CSF | |
| NCT 02130869 | A Pilot Study of Immunotherapy Including Haploidentical NK Cell Infusion Following CD133+ Positively-Selected Autologous Hematopoietic Stem Cells in Children with High Risk Solid Tumors or Lymphomas | Completed | CD133pos selected autologous stem cell infusion + anti-GD2 (hu14.18K322A) mAb + rIL-2 + HLA-Haploidentical NK cell + G-CSF + GM-CSF | |
| NCT 00790413 | Haploidentical Stem Cell Transplantation in Neuroblastoma | Active, not recruiting | T-cell depleted HLA-Haploidentical HSCT + DLI (T cells) + Rituximab + mesenchymal stem cells | |
| NCT 02258815 | CH14.18 1021 Antibody and IL2 After Haplo SCT in Children with Relapsed Neuroblastoma | Unknown | HLA-Haploidentical HSCT+ anti-GD2 (CH14.18/CHO) mAb + rIL2 | |
| NCT 01807468 | Haploidentical Stem Cell Transplantation and NK Cell Therapy in Patients with High-risk Solid Tumors | Unknown | HLA-Haploidentical KIR-L mismatch HSCT + ex-vivo expanded donor-derived NK cells + low-doses of rIL-2 | |
| NCT 02100891 | Phase 2 STIR Trial: Haploidentical Transplant and Donor Natural Killer Cells for Solid Tumors | Active, not recruiting | HLA-Haploidentical HSCT + ex-vivo expanded donor-derived NK cells | |
| NCT 01386619 | NK DLI in Patients After Human Leukocyte Antigen (HLA)-Haploidentical Hematopoietic Stem Cell Transplantation (HSCT) | Completed | HLA-Haploidentical HSCT + donor-derived NK cells | |
| NCT 02508038 | Alpha/Beta CD19+ Depleted Haploidentical Transplantation + Zometa for Pediatric Hematologic Malignancies and Solid Tumors | Recruiting | TCR-α/β+ and CD19+ depleted KIR/KIR ligand-mismatched Haploidentical HSCT + zoledronate | |
| NCT 00569283 | Donor Natural Killer Cell Infusion in Preventing Relapse or Graft Failure in Patients Who Have Undergone Donor Bone Marrow Transplant | Completed | HLA-Haploidentical HSCT + donor-derived NK cells | |
| ALLOGENIC | NCT 01576692 | Combination Chemotherapy, Monoclonal Antibody, and Natural Killer Cells in Treating Young Patients with Recurrent or Refractory Neuroblastoma | Completed | anti-GD2 (Hu14.18K322A) mAb + allogeneic NK cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottino, C.; Della Chiesa, M.; Sorrentino, S.; Morini, M.; Vitale, C.; Dondero, A.; Tondo, A.; Conte, M.; Garaventa, A.; Castriconi, R. Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants. Cancers 2022, 14, 4548. https://doi.org/10.3390/cancers14194548
Bottino C, Della Chiesa M, Sorrentino S, Morini M, Vitale C, Dondero A, Tondo A, Conte M, Garaventa A, Castriconi R. Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants. Cancers. 2022; 14(19):4548. https://doi.org/10.3390/cancers14194548
Chicago/Turabian StyleBottino, Cristina, Mariella Della Chiesa, Stefania Sorrentino, Martina Morini, Chiara Vitale, Alessandra Dondero, Annalisa Tondo, Massimo Conte, Alberto Garaventa, and Roberta Castriconi. 2022. "Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants" Cancers 14, no. 19: 4548. https://doi.org/10.3390/cancers14194548
APA StyleBottino, C., Della Chiesa, M., Sorrentino, S., Morini, M., Vitale, C., Dondero, A., Tondo, A., Conte, M., Garaventa, A., & Castriconi, R. (2022). Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants. Cancers, 14(19), 4548. https://doi.org/10.3390/cancers14194548







