Association between Adverse Events and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab: A Multicenter Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Inclusion and Exclusion Criteria
2.3. Treatment Protocol
2.4. Evaluation of the Therapeutic Response
2.5. Assessment of Safety and Liver Function
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Initial and Best Therapeutic Outcomes of Atezo/Beva
3.3. PFS and OS Associated with Atezo/Beva
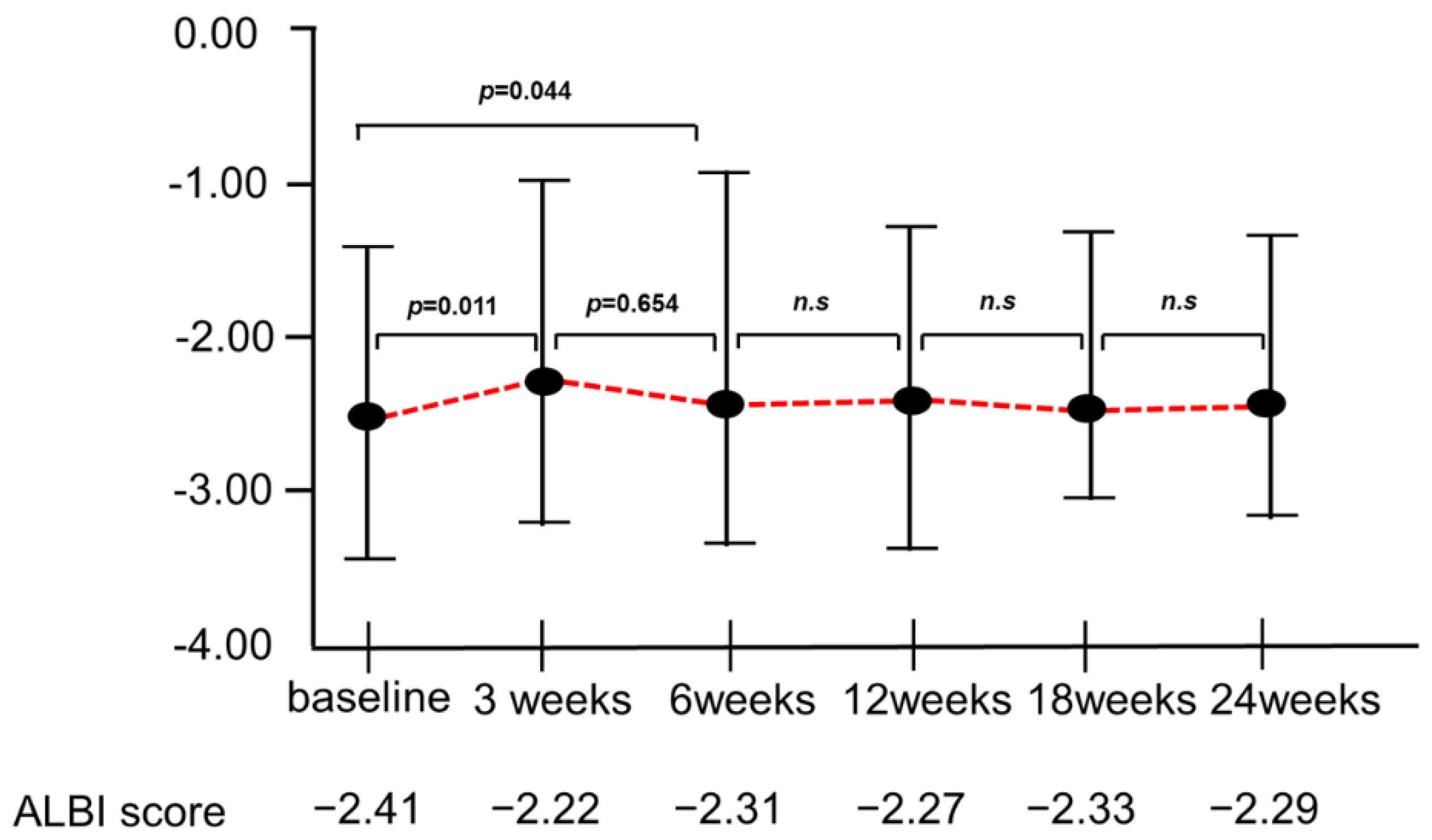
3.4. Changes in the ALBI Score during Atezo/Beva Treatment
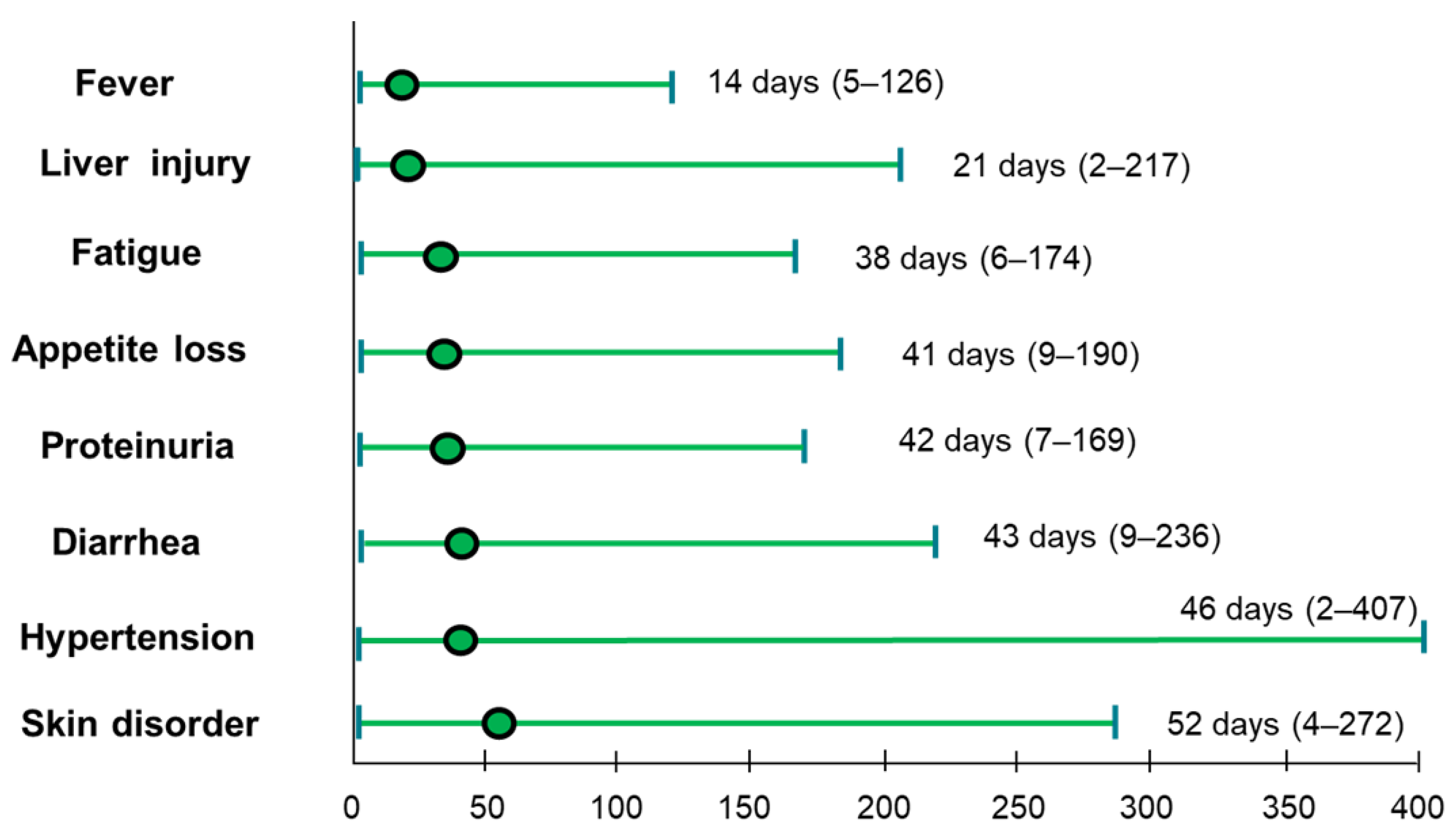
3.5. Adverse Events Profiles and Timing of AEs with Atezo/Beva
3.6. Survival Analysis According to Each AE Profile following Atezo/Beva Treatment
3.7. Survival Analysis According to Early and Late Onset AEs
3.8. Univariate and Multivariate Analyses of Factors Associated with OS
3.9. Changes in ALBI Score in Patients with or without Developed Liver Injury
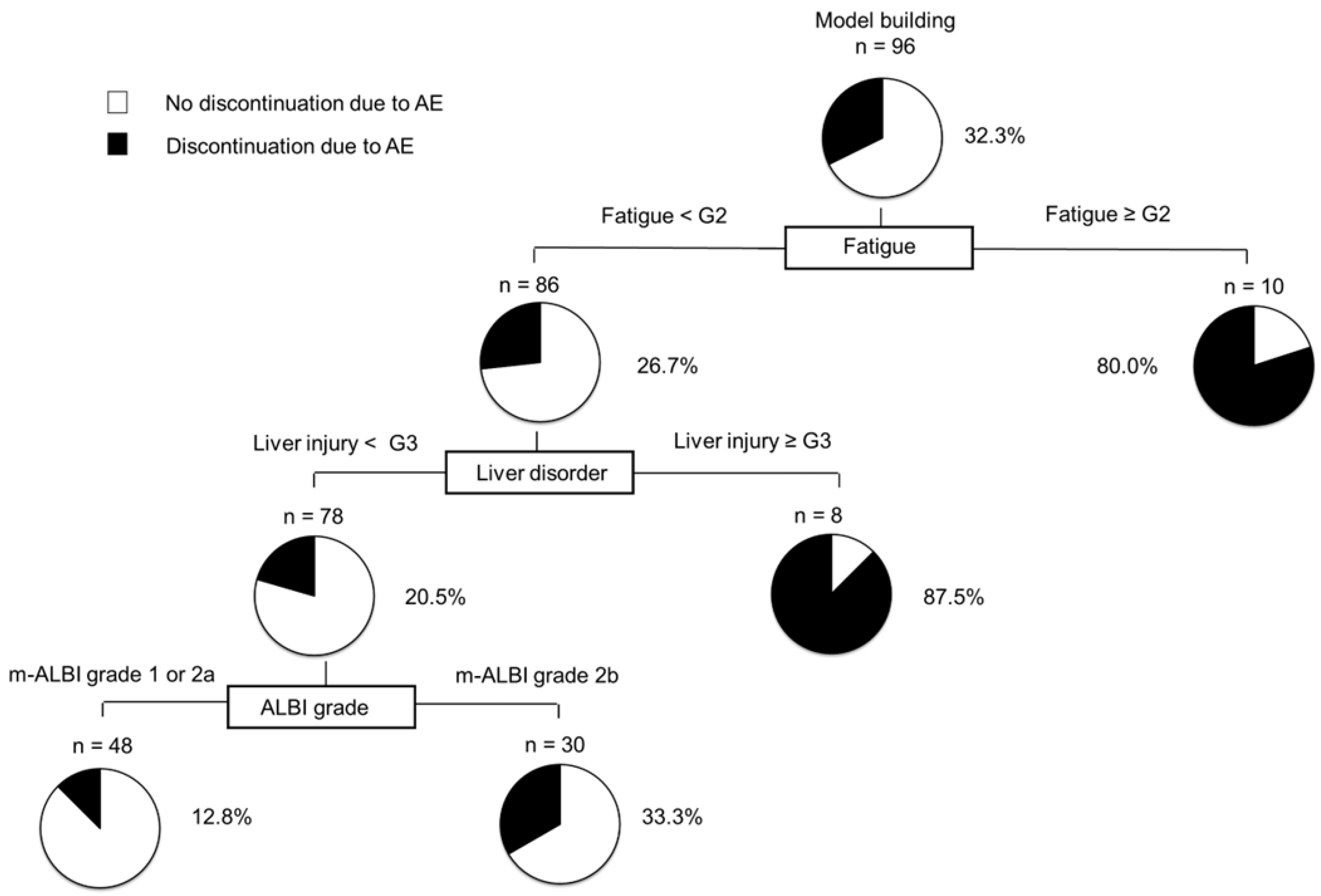
3.10. Decision-Tree Analysis for the Discontinuation of Atezo/Beva Due to AEs
3.11. Logistic Regression Analysis for Discontinuation Due to AEs
3.12. Additional Treatments after the Discontinuation of Atezo/Beva
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Peck-Radosavljevic, M. Systemic treatment of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Management of Hepatocellular Carcinoma in Japan: Current Trends. Liver Cancer 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Chuma, M.; Uojima, H.; Hattori, N.; Arase, Y.; Fukushima, T.; Hirose, S.; Kobayashi, S.; Ueno, M.; Tezuka, S.; Iwasaki, S.; et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: A multicenter analysis. Hepatol. Res. 2022, 52, 269–280. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Early experience of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma BCLC-B stage patients classified as beyond up to seven criteria—Multicenter analysis. Hepatol. Res. 2022, 52, 308–316. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep. 2022, 5, e1464. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Rapposelli, I.G.; Tada, T.; Shimose, S.; Burgio, V.; Kumada, T.; Iwamoto, H.; Hiraoka, A.; Niizeki, T.; Atsukawa, M.; Koga, H.; et al. Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int. 2021, 41, 2997–3008. [Google Scholar] [CrossRef]
- Shimose, S.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; Kuromatsu, R.; et al. Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Cancers 2020, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Michitaka, K.; Toyoda, H.; Tada, T.; Ueki, H.; Kaneto, M.; Aibiki, T.; Okudaira, T.; Kawakami, T.; et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Tanaka, M.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Aino, H.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol. Res. 2019, 49, 919–928. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tokushige, K.; Hyogo, H.; Aikata, H.; Nakajima, T.; Ono, M.; Kawanaka, M.; Sawada, K.; Imajo, K.; Honda, K.; et al. A Data Mining-based Prognostic Algorithm for NAFLD-related Hepatoma Patients: A Nationwide Study by the Japan Study Group of NAFLD. Sci. Rep. 2018, 8, 10434. [Google Scholar] [CrossRef]
- Chan, S.L.; Yip, T.C.; Wong, V.W.; Tse, Y.K.; Yuen, B.W.; Luk, H.W.; Lui, R.N.; Chan, H.L.; Mok, T.S.; Wong, G.L. Pattern and impact of hepatic adverse events encountered during immune checkpoint inhibitors—A territory-wide cohort study. Cancer Med. 2020, 9, 7052–7061. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ito, T.; Hase, T.; Ishigami, M.; Mizuno, K.; Yamamoto, K.; Imai, N.; Ishizu, Y.; Honda, T.; Shibata, H.; et al. Immune-related Liver Injury is a Poor Prognostic Factor in Patients with Nonsmall Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Cancer Investig. 2022, 40, 189–198. [Google Scholar] [CrossRef]
- Yokohama, K.; Asai, A.; Matsui, M.; Okamoto, N.; Yasuoka, H.; Nishikawa, T.; Ohama, H.; Tsuchimoto, Y.; Inoue, Y.; Fukunishi, S.; et al. Liver dysfunction is associated with poor prognosis in patients after immune checkpoint inhibitor therapy. Sci. Rep. 2020, 10, 14470. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [CrossRef] [Green Version]
- Mouri, A.; Kaira, K.; Yamaguchi, O.; Hashimoto, K.; Miura, Y.; Shiono, A.; Shinomiya, S.; Imai, H.; Kobayashi, K.; Kagamu, H. Effect of Systemic Steroid Use for Immune-Related Adverse Events in Patients with Non-Small Cell Lung Cancer Receiving PD-1 Blockade Drugs. J. Clin. Med. 2021, 10, 3744. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.; Lopes, R.G.; Linhares, P.; Costa, A.; Caeiro, C.; Fernandes, A.C.; Tavares, N.; Osório, L.; Vaz, R. Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J. Neurooncol. 2020, 147, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kroetz, D.L. Bevacizumab-induced hypertension: Clinical presentation and molecular understanding. Pharmacol. Ther. 2018, 182, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Briese, W.; Blieninger, J.; Brossart, P.; Bisht, S.; Feldmann, G. Development of Skin Rash Predicts Outcome of Anti-PD-1- and Anti-CTLA4-Based Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer or Squamous Cell Carcinoma of the Head and Neck: A Single-Center Analysis. Oncol. Res. Treat. 2021, 44, 538–546. [Google Scholar] [CrossRef]
- Wu, C.E.; Yang, C.K.; Peng, M.T.; Huang, P.W.; Chang, C.F.; Yeh, K.Y.; Chen, C.B.; Wang, C.L.; Hsu, C.W.; Chen, I.W.; et al. The association between immune-related adverse events and survival outcomes in Asian patients with advanced melanoma receiving anti-PD-1 antibodies. BMC Cancer 2020, 20, 1018. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogasawara, S.; Takahashi, A.; Seko, Y.; Unozawa, H.; Sato, R.; Watanabe, S.; Moriguchi, M.; Morimoto, N.; Tsuchiya, S.; et al. Evolution of Survival Impact of Molecular Target Agents in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2022, 11, 48–60. [Google Scholar] [CrossRef]
- Morimoto, M.; Numata, K.; Kondo, M.; Hidaka, H.; Takada, J.; Shibuya, A.; Kobayashi, S.; Ohkawa, S.; Okuse, C.; Morita, S.; et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol. Res. 2011, 41, 296–302. [Google Scholar] [CrossRef]
- Porta, C.; Levy, A.; Hawkins, R.; Castellano, D.; Bellmunt, J.; Nathan, P.; McDermott, R.; Wagstaff, J.; Donnellan, P.; McCaffrey, J.; et al. Impact of adverse events, treatment modifications, and dose intensity on survival among patients with advanced renal cell carcinoma treated with first-line sunitinib: A medical chart review across ten centers in five European countries. Cancer Med. 2014, 3, 1517–1526. [Google Scholar] [CrossRef]
- Hsu, C.; Rimassa, L.; Sun, H.C.; Vogel, A.; Kaseb, A.O. Immunotherapy in hepatocellular carcinoma: Evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther. Adv. Med. Oncol. 2021, 13, 17588359211031141. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Hatanaka, T.; Tada, T.; Kariyama, K.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; et al. Therapeutic efficacy of lenvatinib as third-line treatment after regorafenib for unresectable hepatocellular carcinoma progression. Hepatol. Res. 2021, 51, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Characteristic | All Patients |
|---|---|
| N | 130 |
| Age (years old) | 72.5 (37–93) |
| Sex (female/male) | 28/102 |
| PS (0/1) | 102/28 |
| Body Mass Index (kg/m2) | 23.1 (15.4–35.2) |
| Etiology (HBV/HCV/Alcohol/NAFLD or NASH | 19/60/30/21 |
| ALBI score (median (range)) | −2.41 (−3.50–−1.55) |
| ALBI grade (1/2a/2b) | 40/45/45 |
| White blood cell (/µL) | 4600 (1900–9800) |
| Neutrophil (%) | 65 (34–86) |
| Lymphocyte (%) | 23.5 (8–53) |
| AST (U/L) | 41 (14–152) |
| ALT (U/L) | 27 (14–179) |
| Tumor diameter (mm) | 33 (10–136) |
| Number of tumors <5/≥5 | 39/91 |
| BCLC stage (B/C) | 69/61 |
| Macrovascular invasion (No/Yes) | 110/20 |
| Extrahepatic spread (No/Yes) | 84/46 |
| AFP (ng/mL) | 39.2 (1.2–284,543) |
| Treatment line (1st/2nd/3rd/4th) | 72/46/8/4 |
| Initial Response | |
|---|---|
| CR | 0 (0.0%) |
| PR | 35 (26.9%) |
| SD | 78 (60.0%) |
| PD | 17 (13.1%) |
| ORR | 35 (26.9%) |
| DCR | 113 (86.9%) |
| Best Response | |
| CR | 0 (0.0%) |
| PR | 42 (32.3%) |
| SD | 52 (40.0%) |
| PD | 17 (13.1%) |
| ORR | 42 (32.3%) |
| DCR | 113 (86.9%) |
| Adverse Event | Any n (%) | Grade 3 ≥ n (%) |
|---|---|---|
| Total adverse events | 126 (96.9%) | 47 (36.1%) |
| Liver injury | 57 (43.8%) | 8 (6.1%) |
| Hypertension | 54 (41.5%) | 9 (6.9%) |
| Proteinuria | 37 (28.5%) | 12 (9.2%) |
| Fatigue | 36 (27.6%) | 1 (0.7%) |
| Skin disorder | 32 (24.6%) | 1 (0.7%) |
| Fever | 30 (23.0%) | 2 (1.5%) |
| Hoarseness | 21 (16%) | 0 (0.0%) |
| Decreased appetite | 20 (15.3%) | 0 (0.0%) |
| Hypothyroidism | 18 (13.8%) | 0 (0.0%) |
| Diarrhea | 17 (13.0%) | 1 (0.7%) |
| Bleeding | 17 (13.0%) | 9 (6.9%) |
| Hypopituitarism | 3 (2.3%) | 3 (2.3%) |
| Heart failure | 3 (2.3%) | 3 (2.3%) |
| Drug-induced pneumonia | 2 (1.5%) | 2 (1.5%) |
| Infusion reaction | 5 (3.8%) | 0 (0.0%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p-Value | Odds Ratio | 95% CI | p-Value | |
| Age, <70 vs. ≥70 | 0.251 | |||
| Sex, male vs. female | 0.246 | |||
| Etiology Viral, vs. non-viral | 0.969 | |||
| m-ALBI grade, 1/2a vs. 2b | 0.048 | 0.4773 | 0.239–0.953 | 0.042 |
| BCLC, B vs. C | 0.834 | |||
| AFP, <200 vs. ≥200 ng/mL | 0.056 | |||
| Liver injury (Presence, vs. Absence) | 0.036 | 2.400 | 1.201–4.798 | 0.016 |
| Hypertension (Presence, vs. Absence) | 0.001 | 0.311 | 0.134–0.720 | 0.006 |
| Skin disorder (Presence, vs. Absence) | 0.047 | 0.371 | 0.157–0.875 | 0.027 |
| Post-progression treatment (Yes, vs. No) | 0.005 | 0.271 | 0.134–0.549 | 0.005 |
| Factors | Unit | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|
| Fatigue grade ≥ 2 | N/A | 12.85 | 2.35–24.12 | <0.001 |
| Liver injury ≥ 3 | N/A | 6.29 | 1.54–19.33 | <0.001 |
| m-ALBI grade 2b | N/A | 3.54 | 1.22–10.27 | 0.017 |
| Subsequent Treatment Rata | 70.8% (68/96) |
|---|---|
| Lenvatinib | 35.2% (24/68) |
| Ramucirumab | 13.2% (9/68) |
| TACE | 13.2% (9/68) |
| HAIC | 8.8% (6/68) |
| Cabozantinib | 7.4% (5/68) |
| Operation | 7.4% (5/68) |
| Sorafenib | 6.0% (4/68) |
| RFA | 2.9% (2/68) |
| Radiation | 1.4% (1/68) |
| others | 4.5% (3/68) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimose, S.; Iwamoto, H.; Tanaka, M.; Niizeki, T.; Kajiwara, M.; Itano, S.; Moriyama, E.; Shirono, T.; Noda, Y.; Kamachi, N.; et al. Association between Adverse Events and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab: A Multicenter Retrospective Study. Cancers 2022, 14, 4284. https://doi.org/10.3390/cancers14174284
Shimose S, Iwamoto H, Tanaka M, Niizeki T, Kajiwara M, Itano S, Moriyama E, Shirono T, Noda Y, Kamachi N, et al. Association between Adverse Events and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab: A Multicenter Retrospective Study. Cancers. 2022; 14(17):4284. https://doi.org/10.3390/cancers14174284
Chicago/Turabian StyleShimose, Shigeo, Hideki Iwamoto, Masatoshi Tanaka, Takashi Niizeki, Masahiko Kajiwara, Satoshi Itano, Etsuko Moriyama, Tomotake Shirono, Yu Noda, Naoki Kamachi, and et al. 2022. "Association between Adverse Events and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab: A Multicenter Retrospective Study" Cancers 14, no. 17: 4284. https://doi.org/10.3390/cancers14174284
APA StyleShimose, S., Iwamoto, H., Tanaka, M., Niizeki, T., Kajiwara, M., Itano, S., Moriyama, E., Shirono, T., Noda, Y., Kamachi, N., Nakano, M., Kuromatsu, R., Koga, H., & Kawaguchi, T. (2022). Association between Adverse Events and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab: A Multicenter Retrospective Study. Cancers, 14(17), 4284. https://doi.org/10.3390/cancers14174284








