LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethics Approval Statement
2.2. Study Population and Design
2.3. Liver Maximum Capacity (LiMAx®) Measurement
2.4. Radioembolization
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
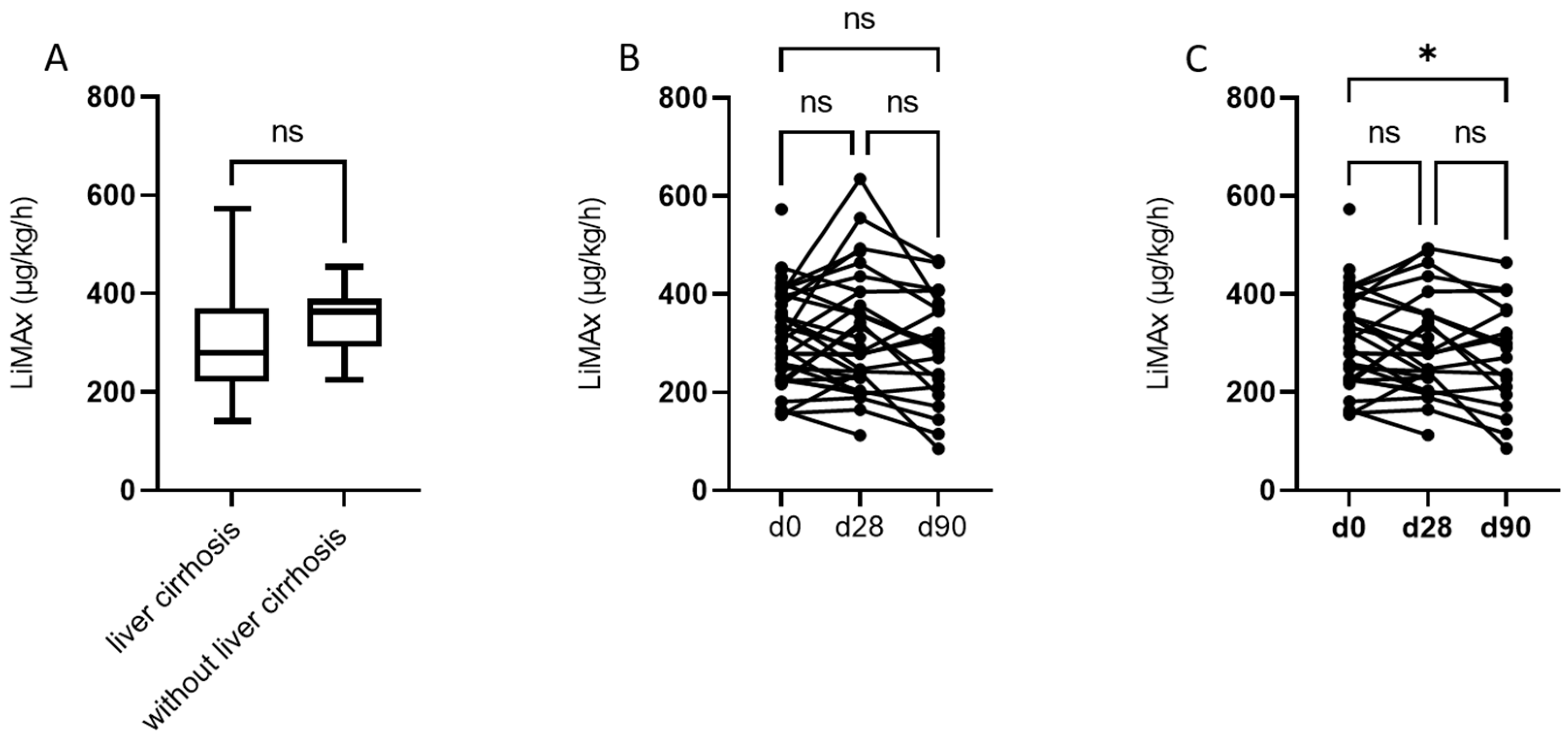
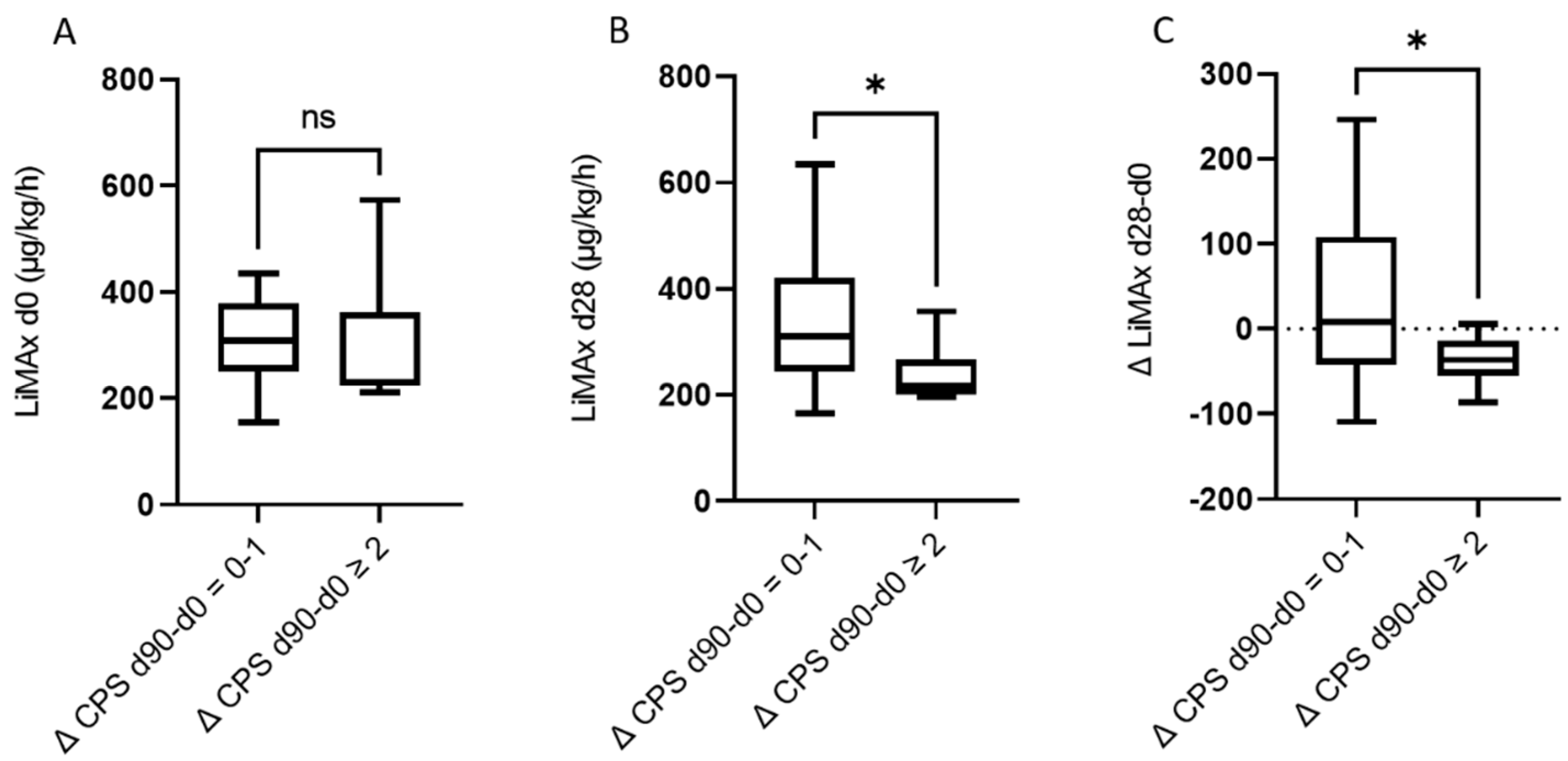
3.2. Assessment of LiMAx® and Liver Function at Baseline and 28 and 90 Days after RE
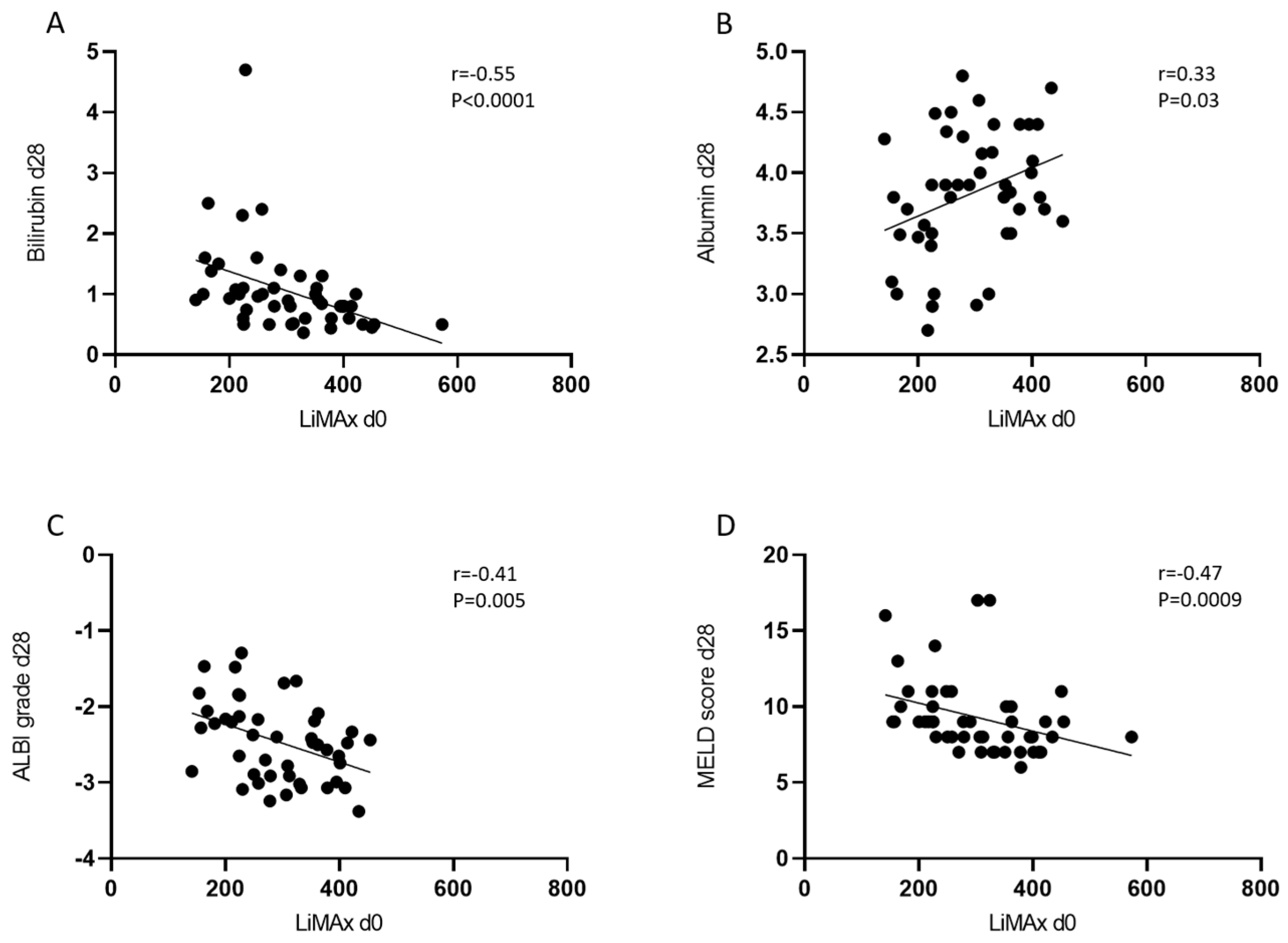
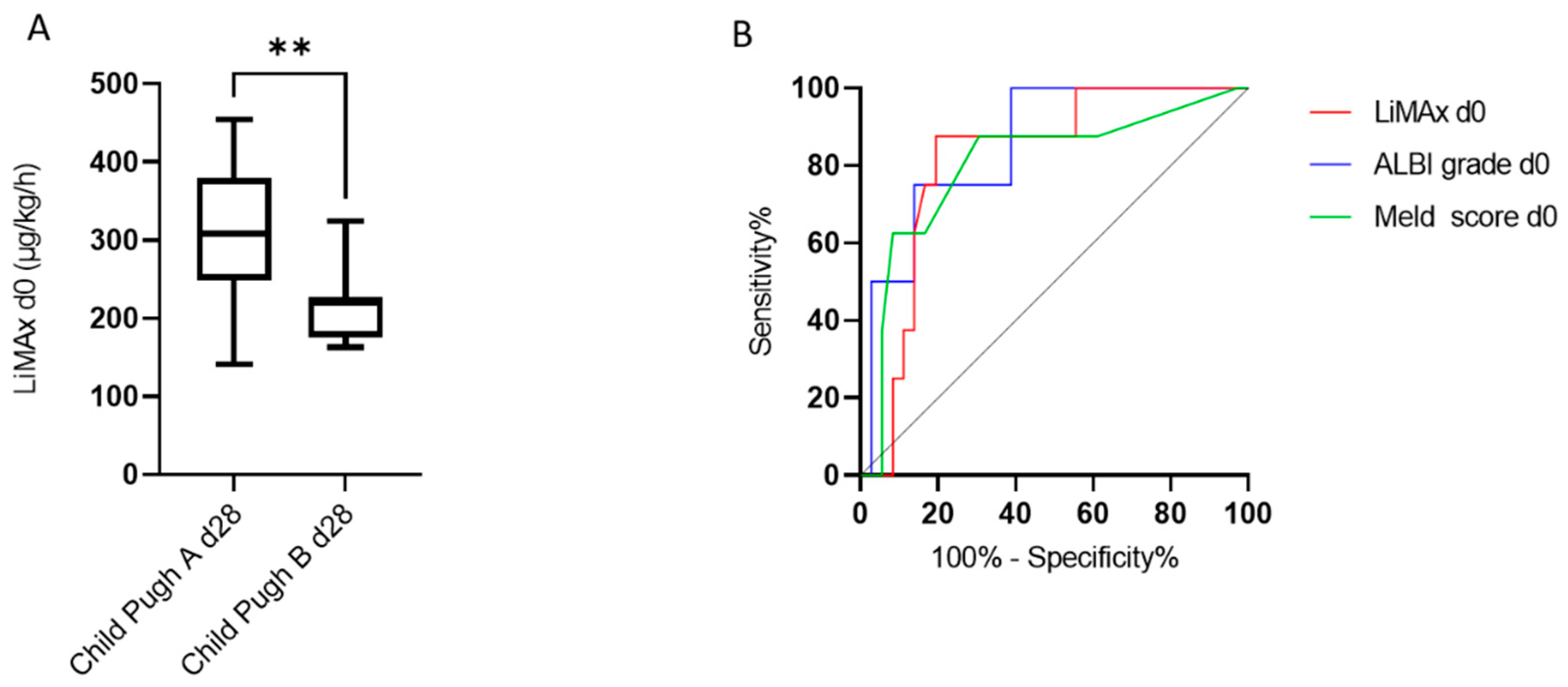
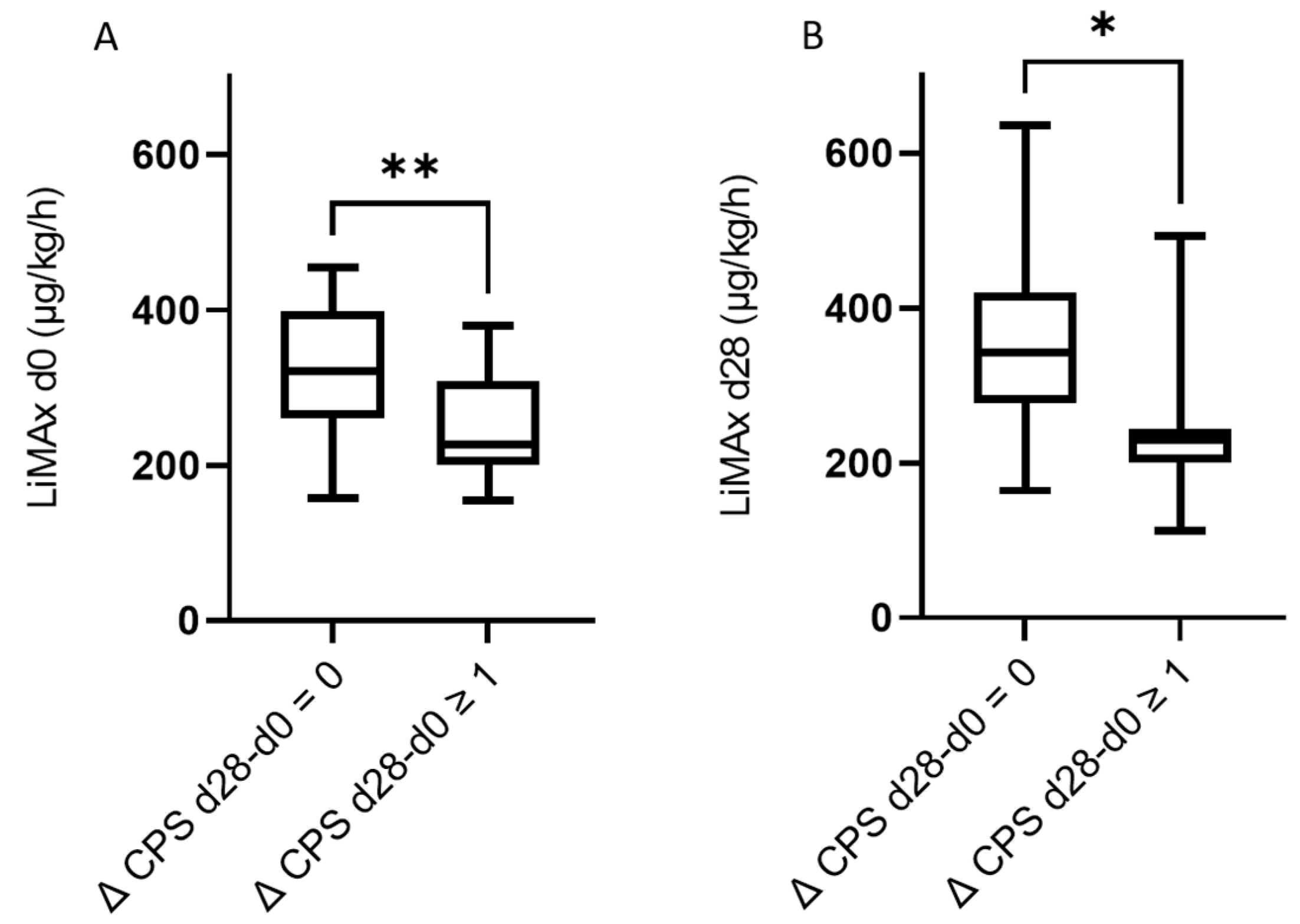
3.3. The Predictive Value of LiMAx for Hepatic Decompensation 28 and 90 Days after RE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E.; Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; et al. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Kulik, L.; Wang, E.; Riaz, A.; Ryu, R.K.; Sato, K.T.; Gupta, R.; Nikolaidis, P.; Miller, F.H.; et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2011, 140, 497–507.e2. [Google Scholar] [CrossRef]
- Salem, R.; Gilbertsen, M.; Butt, Z.; Memon, K.; Vouche, M.; Hickey, R.; Baker, T.; Abecassis, M.M.; Atassi, R.; Riaz, A.; et al. Increased Quality of Life Among Hepatocellular Carcinoma Patients Treated With Radioembolization, Compared With Chemoembolization. Clin. Gastroenterol. Hepatol. 2013, 11, 1358–1365.e1. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Gadaleta-Caldarola, G.; Brandi, G. First-Line Immune Checkpoint Inhibitor-Based Combinations in Unresectable Hepatocellular Carcinoma: Current Management and Future Challenges. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1245–1251. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Riecke, B.; Heyne, K.; Martus, P.; Fricke, M.; Lehmann, S.; Niehues, S.M.; Schwabe, M.; Lemke, A.-J.; et al. Prediction of Postoperative Outcome After Hepatectomy With a New Bedside Test for Maximal Liver Function Capacity. Ann. Surg. 2009, 250, 119–125. [Google Scholar] [CrossRef]
- Stockmann, M.; Lock, J.F.; Malinowski, M.; Niehues, S.M.; Seehofer, D.; Neuhaus, P. The LiMAx Test: A New Liver Function Test for Predicting Postoperative Outcome in Liver Surgery. HPB 2010, 12, 139–146. [Google Scholar] [CrossRef]
- Anger, F.; Klein, I.; Löb, S.; Wiegering, A.; Singh, G.; Sperl, D.; Götze, O.; Geier, A.; Lock, J.F. Preoperative Liver Function Guiding HCC Resection in Normal and Cirrhotic Liver. Visc. Med. 2021, 37, 94–101. [Google Scholar] [CrossRef]
- Kreimeyer, H.; Buechter, M.; Best, J.; Gieseler, R.K.; Katsounas, A.; Sowa, J.-P.; Gerken, G.; Canbay, A.; Manka, P.; Bechmann, L.P. Performance of the LiMAx Test, Fibrinogen, and Transient Elastography in Patients with Acute Liver Injury. Dig. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.C.; Massmann, A.; Schulz, A.; Buecker, A.; Glanemann, M.; Lammert, F.; Malinowski, M. Volume–Function Analysis (LiMAx Test) in Patients with HCC and Cirrhosis Undergoing TACE—A Feasibility Study. Dig. Dis. Sci. 2021, 66, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Barzakova, E.S.; Schulze-Hagen, M.; Zimmermann, M.; Lurje, G.; Bednarsch, J.; Pedersoli, F.; Isfort, P.; Kuhl, C.; Bruners, P. Monitoring Liver Function of Patients Undergoing Transarterial Chemoembolization (TACE) by a 13C Breath Test (LiMAx). Cardiovasc. Interv. Radiol. 2019, 42, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Rizzo, A.; Cusmai, A.; Gadaleta-Caldarola, G.; Palmiotti, G. Which Role for Predictors of Response to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma? Expert Rev. Gastroenterol. Hepatol. 2022, 16, 333–339. [Google Scholar] [CrossRef]
- Vilgrain, V.; Abdel-Rehim, M.; Sibert, A.; Ronot, M.; Lebtahi, R.; Castéra, L.; Chatellier, G. Radioembolisation with Yttrium—90 Microspheres versus Sorafenib for Treatment of Advanced Hepatocellular Carcinoma (SARAH): Study Protocol for a Randomised Controlled Trial. Trials 2014, 15, 474. [Google Scholar] [CrossRef][Green Version]
- Chow, P.K.H.; Gandhi, M.; Tan, S.-B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of Combined Selective Internal Radiation Therapy and Sorafenib on Survival in Advanced Hepatocellular Carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef]
- Venerito, M.; Pech, M.; Canbay, A.; Donghia, R.; Guerra, V.; Chatellier, G.; Pereira, H.; Gandhi, M.; Malfertheiner, P.; Chow, P.K.H.; et al. NEMESIS: Noninferiority, Individual-Patient Metaanalysis of Selective Internal Radiation Therapy with 90Y Resin Microspheres Versus Sorafenib in Advanced Hepatocellular Carcinoma. J. Nucl. Med. 2020, 61, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Schotten, C.; Bechmann, L.P.; Manka, P.; Theysohn, J.; Dechêne, A.; El Fouly, A.; Barbato, F.; Neumann, U.; Radünz, S.; Sydor, S.; et al. NAFLD-Associated Comorbidities in Advanced Stage HCC Do Not Alter the Safety and Efficacy of Yttrium-90 Radioembolization. Liver Cancer 2019, 8, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Martínez-Urbistondo, D.; Bester, L.; Bilbao, J.I.; Coldwell, D.M.; Flamen, P.; Kennedy, A.; Ricke, J.; Sharma, R.A. Prevention and Treatment of Complications of Selective Internal Radiation Therapy: Expert Guidance and Systematic Review: Sangro et Al. Hepatology 2017, 66, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Büchter, M.; Manka, P.; Thimm, J.; Best, J.; Gerken, G.; Baba, H.; Kahraman, A. Enzymatic liver function measured by LiMAx correlates well with histology in patients with chronic liver disease. Z. Gastroenterol. 2018, 56, E2–E89. [Google Scholar] [CrossRef]
- Blüthner, E.; Bednarsch, J.; Malinowski, M.; Binder, P.; Pratschke, J.; Stockmann, M.; Kaffarnik, M. Dynamic Liver Function Is an Independent Predictor of Recurrence-Free Survival after Curative Liver Resection for HCC—A Retrospective Cohort Study. Int. J. Surg. 2019, 71, 56–65. [Google Scholar] [CrossRef]
- Lock, J.F.; Schwabauer, E.; Martus, P.; Videv, N.; Pratschke, J.; Malinowski, M.; Neuhaus, P.; Stockmann, M. Early Diagnosis of Primary Nonfunction and Indication for Reoperation after Liver Transplantation. Liver Transpl. 2010, 16, 172–180. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, J.; Kahraman, A.; Gerken, G.; Theysohn, J.M.; Willuweit, K.; Hoyer, D.P.; Lange, C.M.; Buechter, M. Enzymatic Liver Function Measured by LiMAx Is Superior to Current Standard Methods in Predicting Transplant-Free Survival after TIPS Implantation. Sci. Rep. 2021, 11, 13834. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Lescure, C.; Estrade, F.; Pedrono, M.; Campillo-Gimenez, B.; Le Sourd, S.; Pracht, M.; Palard, X.; Bourien, H.; Muzellec, L.; Uguen, T.; et al. ALBI Score Is a Strong Predictor of Toxicity Following SIRT for Hepatocellular Carcinoma. Cancers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Van Doorn, D.J.; Hendriks, P.; Burgmans, M.C.; Rietbergen, D.D.D.; Coenraad, M.J.; van Delden, O.M.; Bennink, R.J.; Labeur, T.A.; Klümpen, H.-J.; Eskens, F.A.L.M.; et al. Liver Decompensation as Late Complication in HCC Patients with Long-Term Response Following Selective Internal Radiation Therapy. Cancers 2021, 13, 5427. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ros, N.; Silva, N.; Bilbao, J.I.; Iñarrairaegui, M.; Benito, A.; D’Avola, D.; Rodriguez, M.; Rotellar, F.; Pardo, F.; Sangro, B. Partial Liver Volume Radioembolization Induces Hypertrophy in the Spared Hemiliver and No Major Signs of Portal Hypertension. HPB 2014, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Goh, B.K.P.; Cheah, F.K.; Allen, J.C.; Lo, R.H.G.; Ng, D.C.E.; Goh, A.S.W.; Khor, A.Y.K.; Sim, H.S.; Ng, J.J.; et al. Underlying Liver Disease Influences Volumetric Changes in the Spared Hemiliver after Selective Internal Radiation Therapy with 90Y in Patients with Hepatocellular Carcinoma: Greater Hypertrophy in Hepatitis B Patients. J. Dig. Dis. 2014, 15, 444–450. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, S.; Tovoli, F.; Barbera, M.A.; Garuti, F.; Palloni, A.; Frega, G.; Garajovà, I.; Rizzo, A.; Trevisani, F.; Brandi, G. Metronomic Capecitabine vs. Best Supportive Care in Child-Pugh B Hepatocellular Carcinoma: A Proof of Concept. Sci. Rep. 2018, 8, 9997. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Durvalumab plus Tremelimumab in Unresectable Hepatocellular Carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 592–596. [Google Scholar] [CrossRef]
| Parameter/Grade/Stage | Whole Cohort (N = 50) | Prospective Cohort (N = 37) | |
|---|---|---|---|
| Age at first SIRT | years | 70 (64–75) | 68 (64–73) |
| Sex | Male/female | 44 (88)/6 (12) | 33 (89)/4 (11) |
| ECOG PS a | 0 | 30 (61) | 26 (70) |
| I | 17 (35) | 10 (27) | |
| II | 2 (4) | 1 (3) | |
| Cirrhosis | 41 (82) | 32 (87) | |
| Etiology | ASH | 18 (36) | 14 (38) |
| NASH | 13 (26) | 7 (19) | |
| Hepatitis B | 6 (12) | 5 (14) | |
| Hepatitis C | 3 (6) | 3 (8) | |
| Hemochromatosis | 2 (4) | 2 (5) | |
| Other | 8 (16) | 6 (16) | |
| Child–Pugh score | A | 47 (94) | 35 (95) |
| B | 3 (6) | 2 (5) | |
| ALBI grade | 1 | 30 (60) | 22 (60) |
| 2 | 19 (38) | 15 (40) | |
| 3 | 1 (2) | 0 | |
| Ascites | Absent Grade I-II | 47 (94) 3 (6) | 34 (92) 3 (8) |
| Encephalopathy | history | 0 | 0 |
| BCLC | A | 2 (4) | 0 |
| B | 35 (70) | 26 (70) | |
| C | 13 (26) | 11 (30) | |
| Macrovascular invasion | 13 (26) | 7 (19) | |
| Portal vein thrombosis | Main Branch | 3 (6) 2 (4) | 3 (8) 1 (3) |
| Tumor manifestations | Unilobar | 17 (34) | 10 (27) |
| Bilobar | 33 (66) | 27 (73) | |
| Number of lesions a | 1 | 9 (18) | 7 (19) |
| 1–3 | 4 (8) | 2 (5) | |
| >3 | 36 (73) | 28 (76) | |
| Tumor volume, mL | 205.1 (142.9–586.5) | 174.5 (127.4–339.0) | |
| Number of RE sessions | 1 2 | 33 (66) 17 (34) | 24 (65) 13 (35) |
| LiMAx®, µg/kg/h | d0 d28 d90 | 305 (224–378.3) | 309 (226.5–398) 286 (222.5–405) 290 (203–375.5) |
| Parameter | Whole Cohort | Prospective Cohort | |||||
|---|---|---|---|---|---|---|---|
| Baseline N = 50 | day28 N = 44 | day 90 N = 43 | Baseline N = 37 | day 28 N = 33 | day 90 N = 31 | ||
| Child–Pugh score | A | 47 (94) | 36 (82) | 32 (74) | 35 (95) | 27(82) | 23(74) |
| B | 3 (6) | 8 (18) | 5 (12) | 2 (5) | 6 (18) | 3 (10) | |
| C | 0 | 0 | 1 (2) | 0 | 0 | 1 (3) | |
| deceased | n.a. | 0 | 5 (12) | n.a. | 0 | 4 (13) | |
| ALBI grade | 1 | 30 (60) | 18 (40) a | 17 (40) | 22 (60) | 13 (38) b | 12 (39) |
| 2 | 19 (38) | 26 (58) a | 20 (47) | 15 (40) | 20 (59) b | 14 (45) | |
| 3 | 1 (2) | 1 (2) a | 1 (2) | 0 | 1 (3) b | 1 (3) | |
| deceased | n.a. | n.a. | 5 (12) | n.a. | 0 | 4 (13) | |
| Meld score | 8 (7–10) | 9 (8–10) | 10 (8–12) | 8 (7–9) | 9 (8–10) | 10 (8–12) | |
| Bilirubin | mg/dL | 0.7 (0.5–1.1) | 0.9 (0.6–1.1) | 1.0 (0.6–1.4) | 0.8 (0.5–1.2) | 0.95 (0.6–1.3) | 1.2 (0.7–1.6) |
| Albumin | g/dL | 4.1 (3.7–4.4) | 3.8 (3.5–4.3) | 4.0 (3.6–4.3) | 4.1 (3.8–4.4) | 3.9 (3.5–4.3) | 4.0 (3.5–4.3) |
| Parameter | Youden Index | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|
| LiMAx® day 0 | 229 | 87.50 | 80.56 | 0.818 |
| ALBI grade day 0 | −2.430 | 75.00 | 86.11 | 0.854 |
| MELD score day 0 | 8.5 | 87.50 | 69.44 | 0.804 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyh, C.; Heucke, N.; Schotten, C.; Büchter, M.; Bechmann, L.P.; Wichert, M.; Dechêne, A.; Herrmann, K.; Heider, D.; Sydor, S.; et al. LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis. Cancers 2022, 14, 4584. https://doi.org/10.3390/cancers14194584
Leyh C, Heucke N, Schotten C, Büchter M, Bechmann LP, Wichert M, Dechêne A, Herrmann K, Heider D, Sydor S, et al. LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis. Cancers. 2022; 14(19):4584. https://doi.org/10.3390/cancers14194584
Chicago/Turabian StyleLeyh, Catherine, Niklas Heucke, Clemens Schotten, Matthias Büchter, Lars P. Bechmann, Marc Wichert, Alexander Dechêne, Ken Herrmann, Dominik Heider, Svenja Sydor, and et al. 2022. "LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis" Cancers 14, no. 19: 4584. https://doi.org/10.3390/cancers14194584
APA StyleLeyh, C., Heucke, N., Schotten, C., Büchter, M., Bechmann, L. P., Wichert, M., Dechêne, A., Herrmann, K., Heider, D., Sydor, S., Lemmer, P., Ludwig, J. M., Pospiech, J., Theysohn, J., Damm, R., March, C., Powerski, M., Pech, M., Özcürümez, M., ... Manka, P. P. (2022). LiMAx Prior to Radioembolization for Hepatocellular Carcinoma as an Additional Tool for Patient Selection in Patients with Liver Cirrhosis. Cancers, 14(19), 4584. https://doi.org/10.3390/cancers14194584







