Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Treatment Protocol
2.3. Evaluation of Liver Functional Reserve during Treatment
2.4. Statistical Analysis
3. Results
3.1. Overview of Patient Characteristics According to Eligibility Criteria of IMbrave150
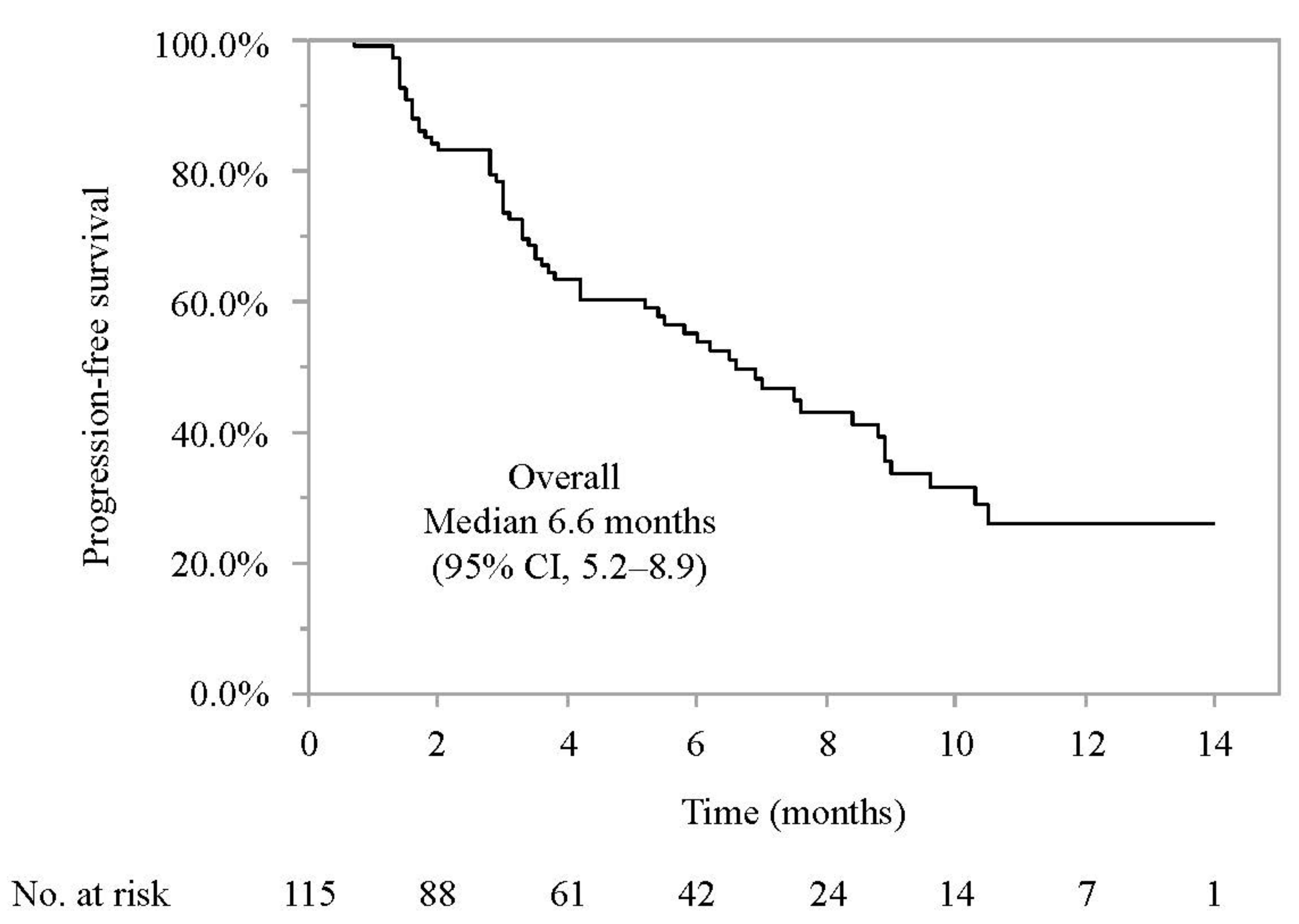
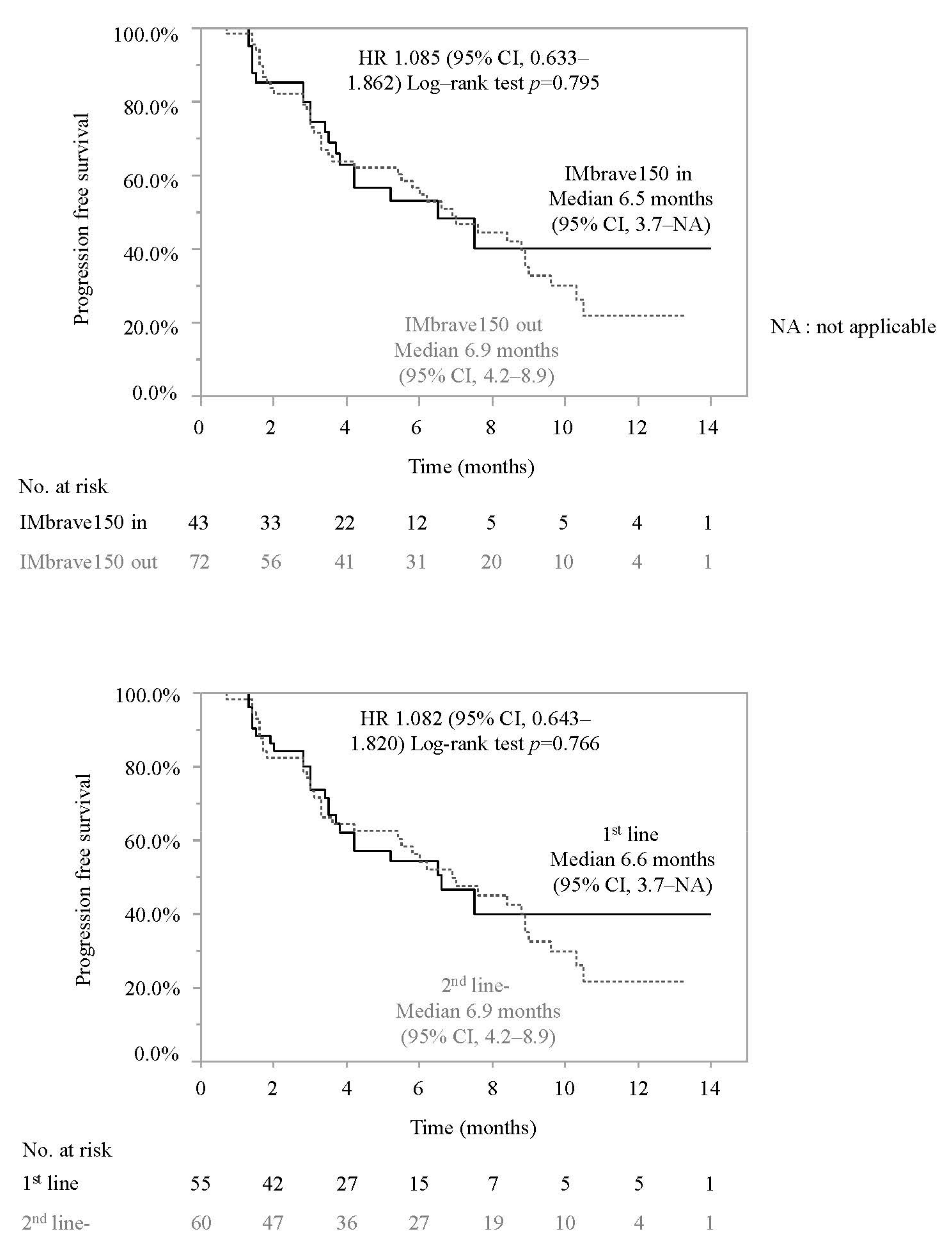
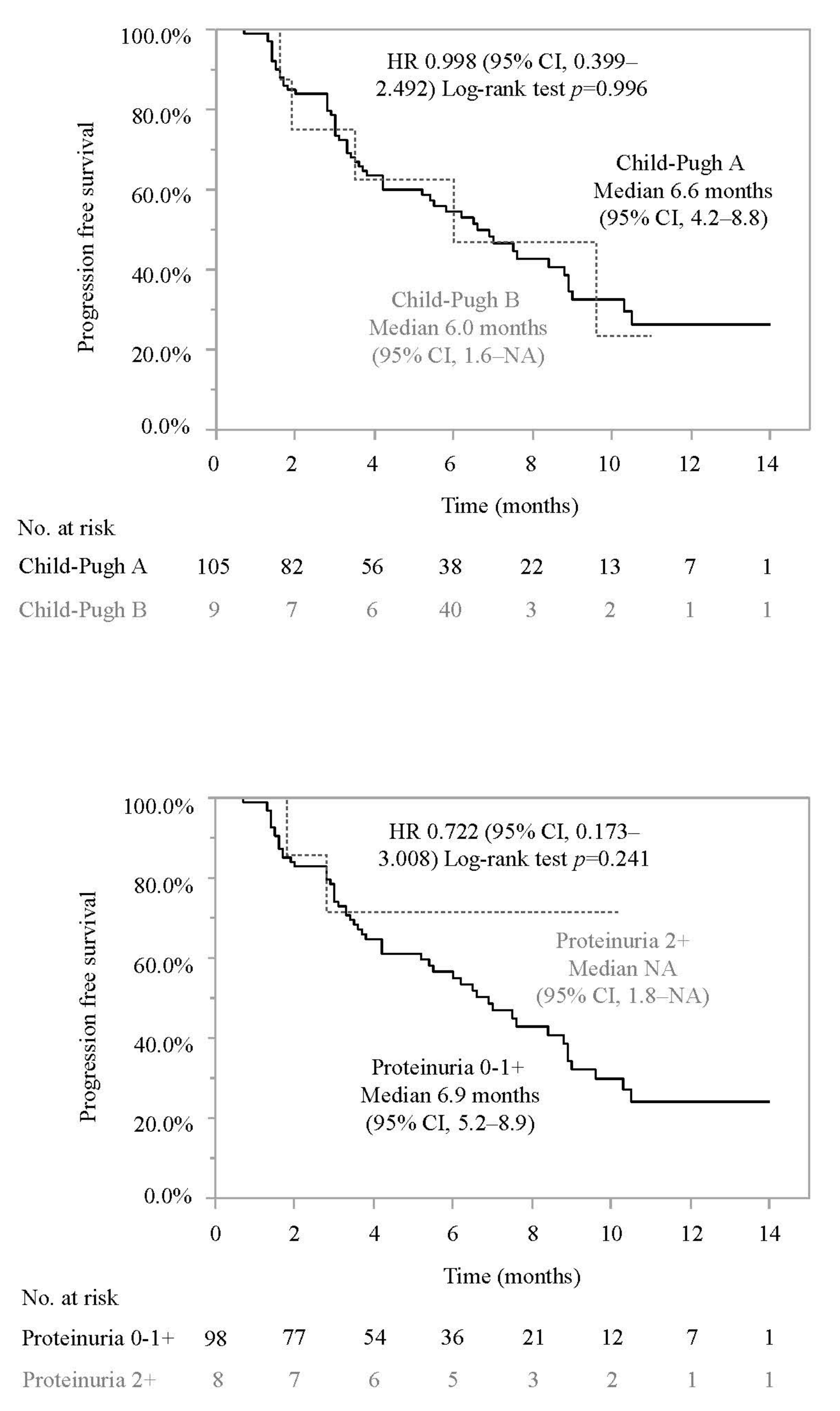
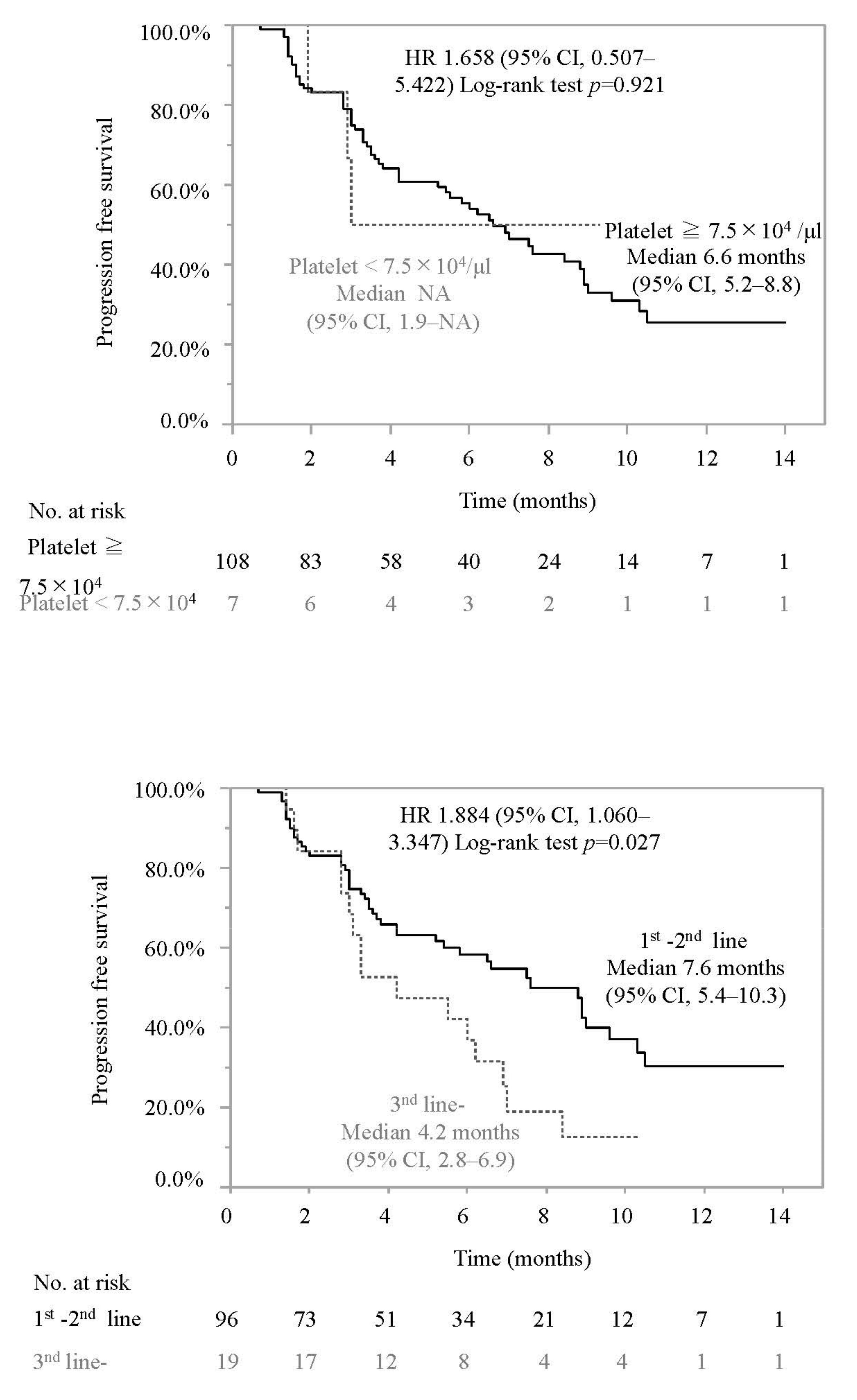
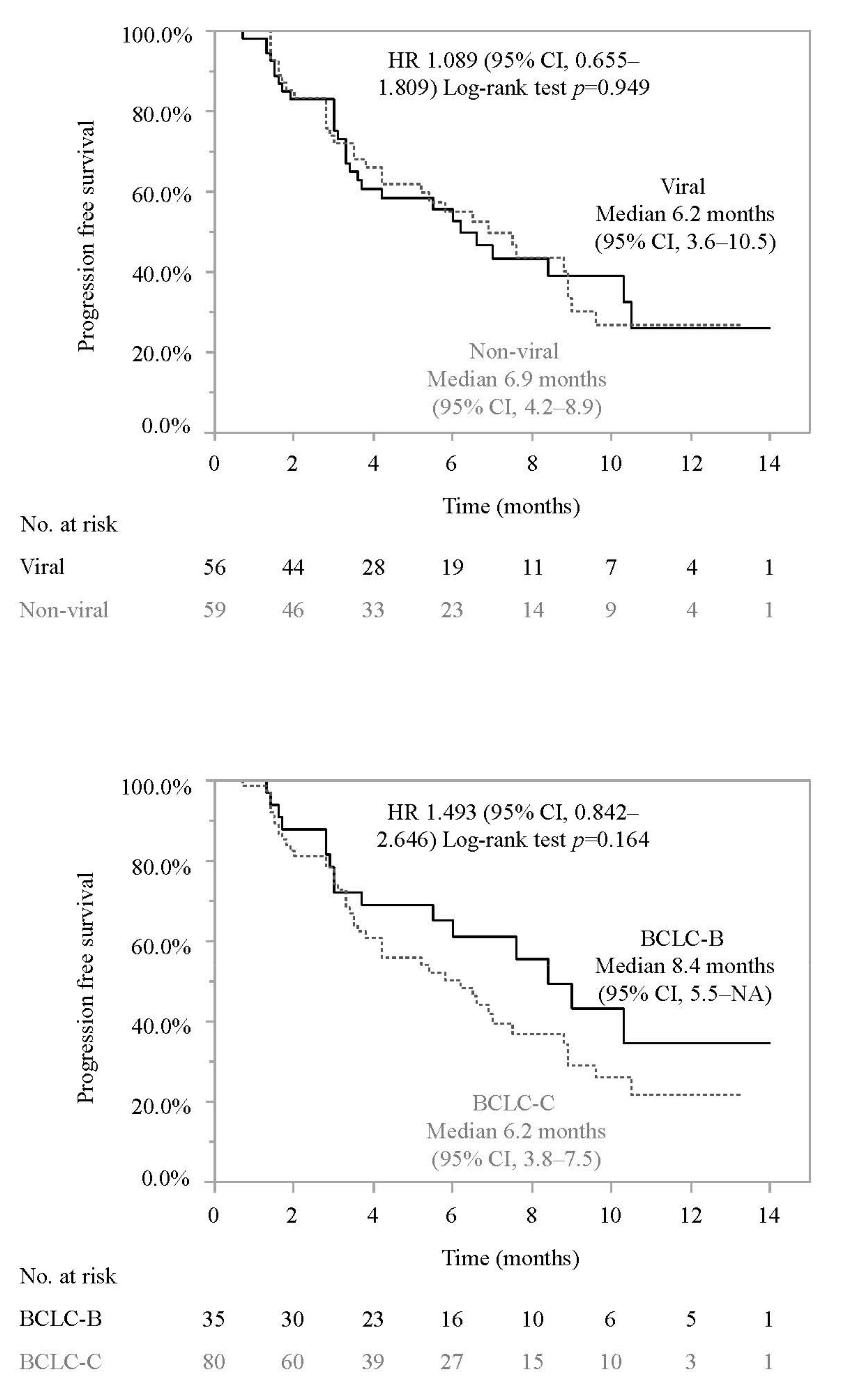
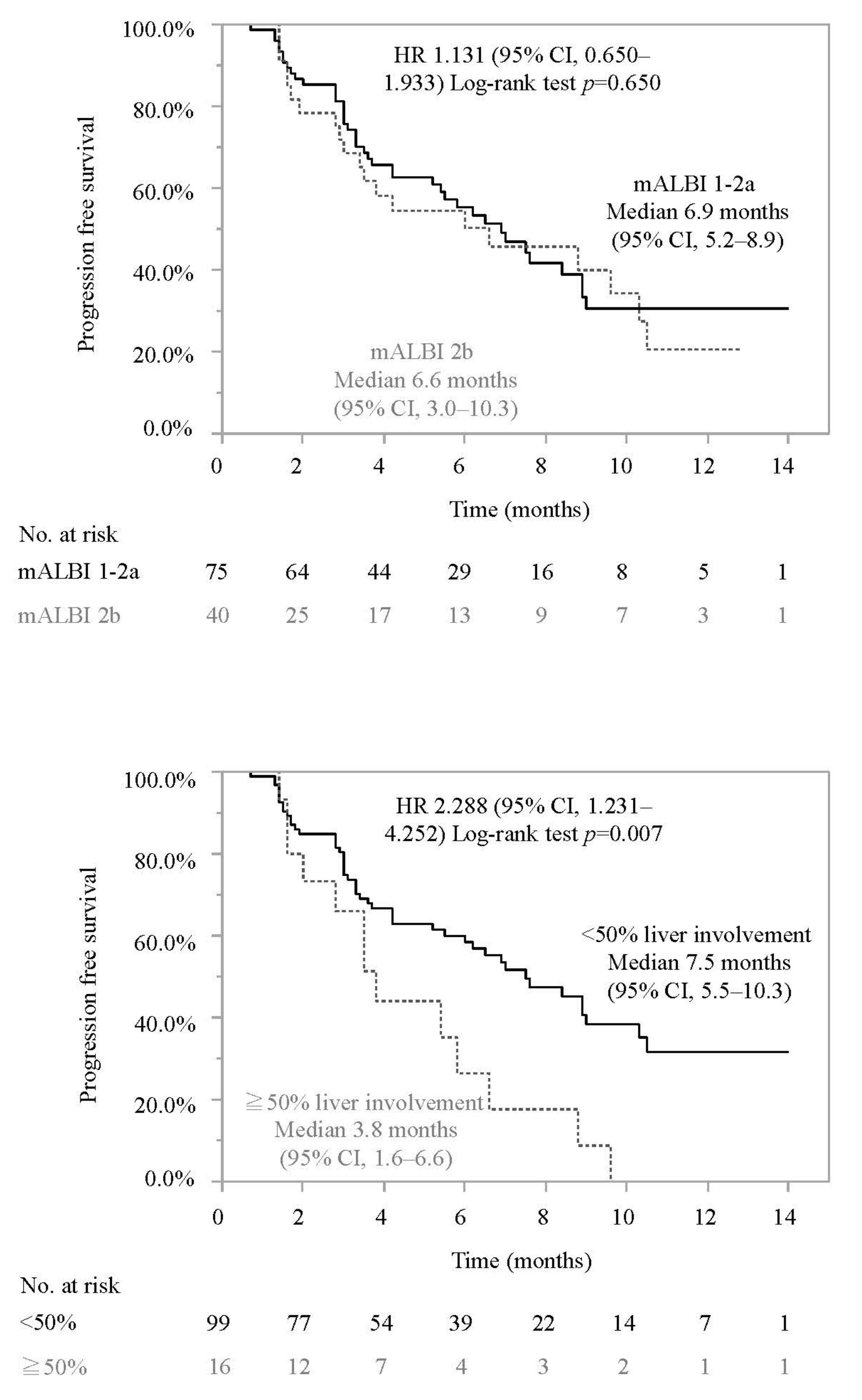
3.2. Progression-Free Survival and Associated Factors in Patients Who Did or Did Not Meet the IMbrave150 Eligibility Criteria
Treatment Response
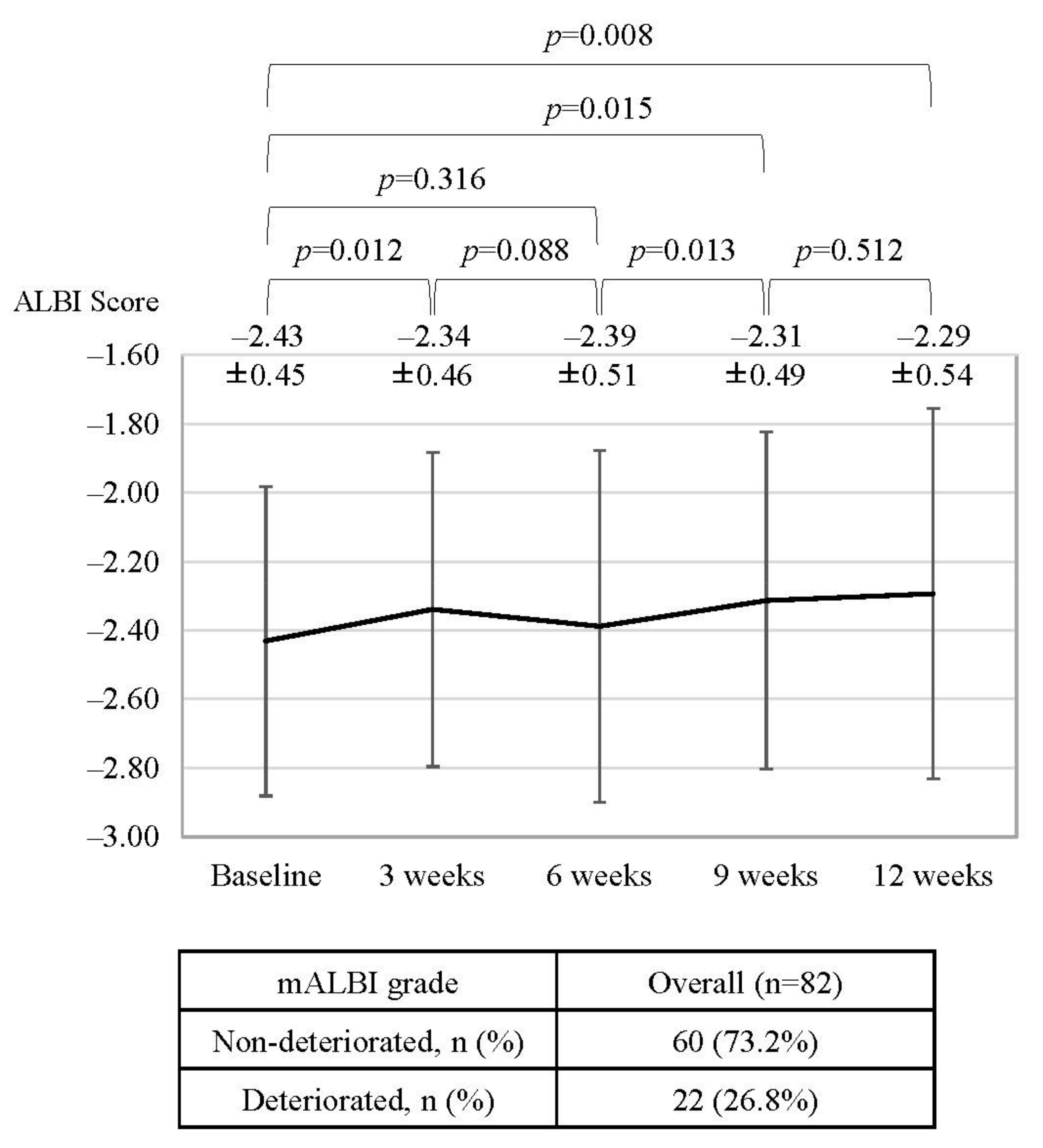
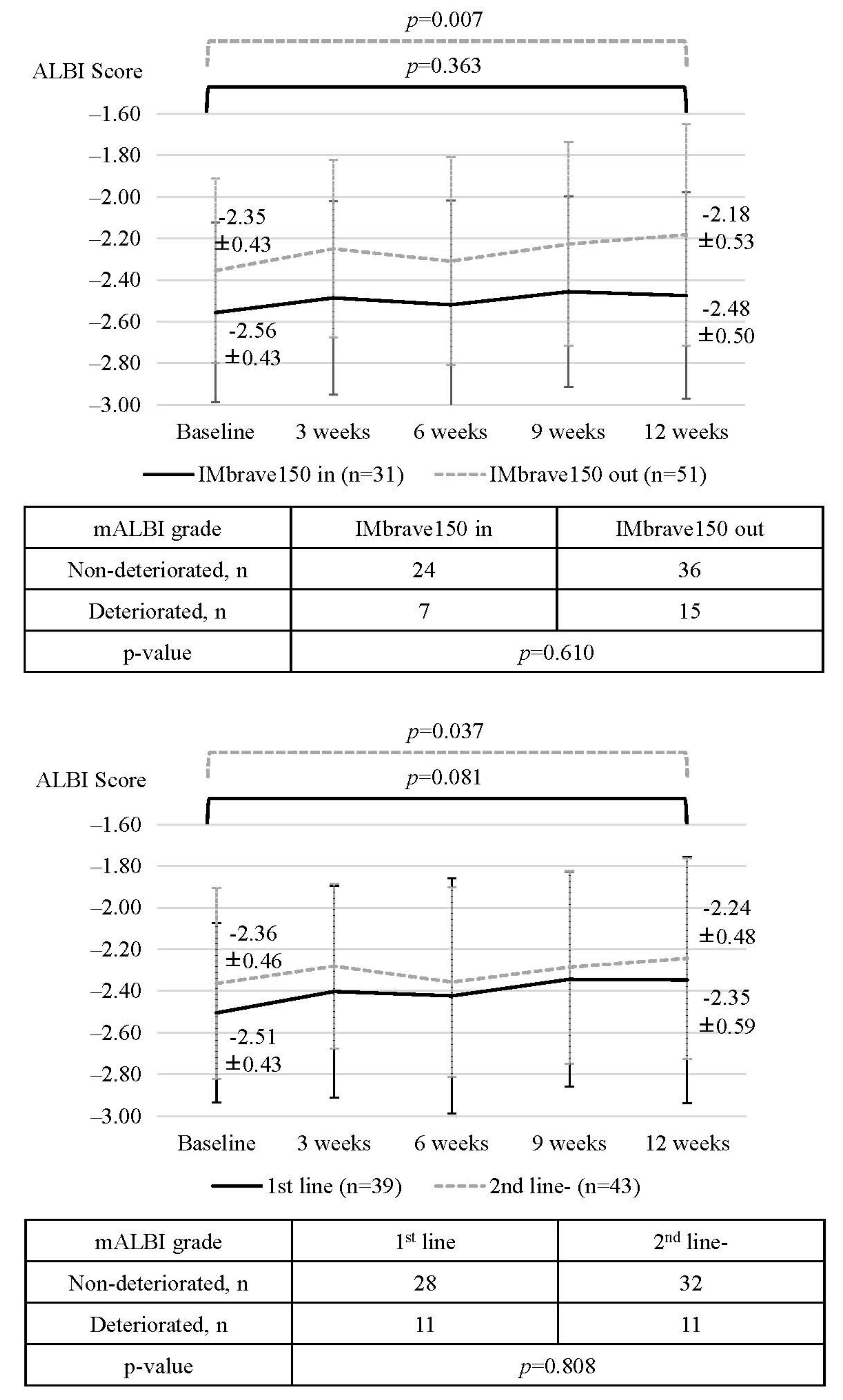
3.3. Changes in ALBI Score, mALBI Grade, and the Rate of Treatment Interruption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Yang, Z.; Suda, G.; Maehara, O.; Ohara, M.; Yoshida, S.; Hosoda, S.; Kimura, M.; Kubo, A.; Tokuchi, Y.; Fu, Q.; et al. Changes in Serum Growth Factors during Lenvatinib Predict the Post Progressive Survival in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 232. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Kudo, M.; Tsuchiya, K.; Kato, N.; Hagihara, A.; Numata, K.; Aikata, H.; Inaba, Y.; Kondo, S.; Motomura, K.; Furuse, J.; et al. Cabozantinib in Japanese patients with advanced hepatocellular carcinoma: A phase 2 multicenter study. J. Gastroenterol. 2021, 56, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Ogawa, K.; Kimura, M.; Kubo, A.; Tokuchi, Y.; Kitagataya, T.; Maehara, O.; Ohnishi, S.; Shigesawa, T.; et al. Early response and safety of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet IMbrave150 eligibility criteria. Hepatol. Res. 2021, 51, 979–989. [Google Scholar] [CrossRef] [PubMed]
- de Castro, T.; Jochheim, L.S.; Bathon, M.; Welland, S.; Scheiner, B.; Shmanko, K.; Roessler, D.; Khaled, N.B.; Jeschke, M.; Ludwig, J.M.; et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: A real-world experience. Ther. Adv. Med. Oncol. 2022, 14, 17588359221080298. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Takata, N.; Nakagawa, H.; Toyama, T.; Arai, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol. Res. 2016, 46, 650–656. [Google Scholar] [CrossRef]
- Suzuki, K.; Suda, G.; Yamamoto, Y.; Furuya, K.; Baba, M.; Nakamura, A.; Miyoshi, H.; Kimura, M.; Maehara, O.; Yamada, R.; et al. Tenofovir-disoproxil-fumarate modulates lipid metabolism via hepatic CD36/PPAR-alpha activation in hepatitis B virus infection. J. Gastroenterol. 2021, 56, 168–180. [Google Scholar] [CrossRef]
- Ohara, M.; Suda, G.; Kimura, M.; Maehara, O.; Shimazaki, T.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Kawagishi, N.; Nakai, M.; et al. Analysis of the optimal psoas muscle mass index cut-off values, as measured by computed tomography, for the diagnosis of loss of skeletal muscle mass in Japanese people. Hepatol. Res. 2020, 50, 715–725. [Google Scholar] [CrossRef]
- Ogawa, K.; Kobayashi, T.; Furukawa, J.I.; Hanamatsu, H.; Nakamura, A.; Suzuki, K.; Kawagishi, N.; Ohara, M.; Umemura, M.; Nakai, M.; et al. Tri-antennary tri-sialylated mono-fucosylated glycan of alpha-1 antitrypsin as a non-invasive biomarker for non-alcoholic steatohepatitis: A novel glycobiomarker for non-alcoholic steatohepatitis. Sci. Rep. 2020, 10, 321. [Google Scholar] [CrossRef]
- Suzuki, K.; Suda, G.; Yamamoto, Y.; Furuya, K.; Baba, M.; Kimura, M.; Maehara, O.; Shimazaki, T.; Yamamoto, K.; Shigesawa, T.; et al. Entecavir treatment of hepatitis B virus-infected patients with severe renal impairment and those on hemodialysis. Hepatol. Res. 2019, 49, 1294–1304. [Google Scholar] [CrossRef]
- Suda, G.; Furusyo, N.; Toyoda, H.; Kawakami, Y.; Ikeda, H.; Suzuki, M.; Arataki, K.; Mori, N.; Tsuji, K.; Katamura, Y.; et al. Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: A nationwide retrospective study in Japan. J. Gastroenterol. 2018, 53, 119–128. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep. 2022, 5, e1464. [Google Scholar] [CrossRef]
- D’Alessio, A.; Fulgenzi, C.A.M.; Nishida, N.; Schonlein, M.; von Felden, J.; Schulze, K.; Wege, H.; Gaillard, V.E.; Saeed, A.; Wietharn, B.; et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology 2022, in press. [Google Scholar] [CrossRef]
- Tanaka, T.; Hiraoka, A.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol. Res. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, N.; Sugimoto, M.; Yorozu, K.; Kurasawa, M.; Kondoh, O. AntiVEGF antibody triggers the effect of antiPDL1 antibody in PDL1(low) and immune desertlike mouse tumors. Oncol. Rep. 2022, 47, 1–10. [Google Scholar] [CrossRef]
- Iwai, T.; Sugimoto, M.; Patil, N.S.; Bower, D.; Suzuki, M.; Kato, C.; Yorozu, K.; Kurasawa, M.; Shames, D.S.; Kondoh, O. Both T cell priming in lymph node and CXCR3-dependent migration are the key events for predicting the response of atezolizumab. Sci. Rep. 2021, 11, 13912. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Combination Immunotherapy with Anti-PD-1/PD-L1 Antibody plus Anti-VEGF Antibody May Promote Cytotoxic T Lymphocyte Infiltration in Hepatocellular Carcinoma, Including in the Noninflamed Subclass. Liver Cancer 2022, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Overall Cohort (n = 115) | Met the IMbrave150 Criteria (n = 43) | Did Not Meet the IMbrave150 Criteria (n = 72) | p-Value |
|---|---|---|---|---|
| Age, years (range) | 72 (31–89) | 73 (31–84) | 72 (37–89) | 0.744 |
| Sex | ||||
| Male/Female | 95 (82.6%)/20 (17.4%) | 38 (88.4%)/5 (11.6%) | 57 (79.2%)/15 (20.8%) | 0.309 |
| Etiology | ||||
| HBV | 35 (30.4%) | 11 (25.6%) | 24 (33.3%) | 0.411 |
| HCV | 21 (18.3%) | 10 (23.3%) | 11 (15.3%) | 0.324 |
| Non-viral | 59 (51.3%) | 22 (51.2%) | 37 (51.4%) | 1.000 |
| ECOG PS | ||||
| 0/1–2 | 92 (80.0%)/23 (20.0%) | 39 (90.7%)/4 (9.3%) | 53 (73.6%)/19 (26.4%) | 0.031 |
| BMI, kg/m2 | 23.6 (15.9–37.7) | 24.0 (18.7–37.7) | 23.3 (15.9–33.0) | 0.152 |
| Proteinuria | ||||
| 0–1+/2+ | 98 (85.2%)/8 (7.0%) | 41 (89.1%)/0 (0.0%) | 57 (79.2%)/8 (11.1%) | 0.022 |
| White blood cell, mm3 | 4920 (1970–12780) | 5300 (2950–12,780) | 4900 (1970–11,800) | 0.043 |
| Neutrophil count, mm3 | 3203 (1185–9971) | 3380 (1503–9204) | 2978 (1185–9971) | 0.098 |
| Lymphocyte count, mm3 | 1168 (140–2881) | 1207 (559–2657) | 1100 (140–2881) | 0.056 |
| Neutrophil/Lymphocyte ratio | 2.70 (0.83–18.68) | 3.04 (0.83–7.92) | 2.57 (0.98–18.68) | 0.967 |
| Platelet, ×109/L | 162 (36–586) | 154 (77–558) | 167 (36–586) | 0.773 |
| Prothrombin time, % | 92.9 (35.3–150.0) | 90.0 (42.6–116.9) | 94.6 (35.3–150.0) | 0.317 |
| NH3, µg/dL | 42 (8–136) | 42 (17–116) | 42 (8–136) | 0.538 |
| Albumin, g/dL | 3.7 (2.6–4.8) | 3.8 (2.9–4.8) | 3.7 (2.6–4.8) | 0.030 |
| Total bilirubin, mg/dL | 0.8 (0.2–3.8) | 0.8 (0.2–2.9) | 0.8 (0.3–3.8) | 0.979 |
| mALBI grade | ||||
| ½ | 38 (33.0%)/77 (67.0%) | 17 (39.5%)/26 (60.5%) | 21 (29.2%)/51 (70.8%) | 0.307 |
| 1 | 38 (33.0%) | 17 (39.5%) | 21 (29.2%) | 0.307 |
| 2a | 37 (32.2%) | 15 (34.8%) | 22 (30.6%) | 0.683 |
| 2b | 40 (34.8%) | 11 (25.6%) | 29 (40.3%) | 0.156 |
| AST, IU/L | 42 (14–672) | 35 (16–128) | 47 (14–672) | 0.164 |
| ALT, IU/L | 28 (7–278) | 25 (8–122) | 33 (7–289) | 0.256 |
| Child-Pugh Grade | ||||
| A/B | 106 (92.2%)/9 (7.8%) | 43 (100.0%)/0 (0.0%) | 63 (87.5%)/9 (12.5%) | 0.025 |
| Child-Pugh Score | ||||
| 5 | 62 (53.9%) | 24 (55.8%) | 38 (52.8%) | 0.847 |
| 6 | 44 (38.3%) | 19 (44.9%) | 25 (34.7%) | 0.329 |
| 7 | 6 (5.2%) | 0 (0.0%) | 6 (8.3%) | 0.082 |
| 8 | 3 (2.6%) | 0 (0.0%) | 3 (4.2%) | 0.292 |
| AFP, ng/mL * | 74.2 (0.8–1,450,000.0) | 51.6 (0.8–591,315.4) | 77.2 (1.1–14,500,000.0) | 0.817 |
| AFP > 400 | 40 (34.8%) | 15 (34.9%) | 25 (34.7%) | 1.000 |
| DCP, mAU/mL * | 924 (11–245,000) | 509 (21–213,066) | 1787 (11–245,000) | 0.035 |
| Maximum intrahepatic tumor size, mm | 36 (0–220) | 30 (0–167) | 38 (0–220) | 0.307 |
| More than 50% liver involvement | 16 (17.7%) | 4 (9.3%) | 12 (16.7%) | 0.405 |
| Diffuse type | 15 (15.6%) | 5 (11.6%) | 10 (13.9%) | 0.784 |
| Number of intrahepatic tumors | ||||
| None | 14 (12.2%) | 5 (11.6%) | 9 (12.5%) | 1.000 |
| 1 | 11 (9.6%) | 5 (11.6%) | 6 (8.3%) | 0.745 |
| Multiple | 90 (78.3%) | 33 (76.7%) | 57 (79.2%) | 0.817 |
| BCLC stage | ||||
| B/C | 35 (30.4%)/80 (69.6%) | 13 (30.2%)/30 (69.8%) | 22 (30.6%)/50 (69.4%) | 1.000 |
| Up-to-7 in/out | 30 (26.1%)/85 (73.9%) | 10 (23.3%)/33 (76.7%) | 20 (27.8%)/52 (72.2%) | 0.665 |
| Positive for Vp | 23 (20.0%) | 11 (25.6%) | 12 (16.7%) | 0.335 |
| Vp4 | 4 (3.5%) | 2 (4.7%) | 2 (2.8%) | 0.629 |
| Positive for Vv | 5 (4.3%) | 1 (2.3%) | 4 (5.6%) | 0.649 |
| Positive for bile duct invasion | 4 (3.5%) | 1 (2.3%) | 3 (4.2%) | 1.000 |
| Positive for LN metastasis | 20 (17.4%) | 7 (16.8%) | 13 (18.1%) | 1.000 |
| Positive for EHM | 46 (40.0%) | 17 (39.5%) | 29 (40.3%) | 1.000 |
| History of varices treatment | 8 (7.0%) | 1 (2.3%) | 7 (9.7%) | 0.255 |
| History of hypertension | 69 (60.0%) | 27 (62.8%) | 42 (58.3%) | 0.697 |
| Naïve/recurrence | 14 (20.9%)/91 (79.1%) | 15 (34.9%)/28 (65.1%) | 9 (12.5%)/63 (87.5%) | 0.008 |
| History of operation | 59 (51.3%) | 21 (48.8%) | 38 (52.8%) | 0.707 |
| History of RFA | 41 (35.7%) | 14 (32.6%) | 25 (34.7%) | 0.842 |
| History of TACE | 58 (50.4%) | 14 (32.6%) | 44 (61.1%) | 0.004 |
| 1st line systemic chemotherapy | 55 (47.8%) | 43 (100.0%) | 12 (16.7%) | <0.001 |
| 2nd line | 41 (35.7%) | 0 (0.0%) | 41 (56.9%) | |
| 3rd line | 19 (16.5%) | 0 (0.0%) | 19 (26.4%) | |
| History of TKI | 60 (52.2%) | 0 (0.0%) | 60 (83.3%) | |
| Sorafenib | 19 (16.5%) | 0 (0.0%) | 19 (27.5%) | |
| Regorafenib | 8 (7.0%) | 0 (0.0%) | 8 (11.1%) | |
| Lenvatinib | 59 (51.3%) | 0 (0.0%) | 59 (81.9%) | |
| Observation period, months * | 6.8 (0.1–15.4) | 5.6 (0.1–14.9) | 7.2 (0.3–15.4) | 0.140 |
| RECIST v1.1 | mRECIST | |||
|---|---|---|---|---|
| 6 weeks | Best response | 6 weeks | Best response | |
| CR, n (%) | 0 (0.0) | 3 (2.9) | 2 (1.9) | 9 (8.7) |
| PR, n (%) | 9 (8.7) | 17 (16.3) | 18 (17.3) | 20 (19.2) |
| SD, n (%) | 74 (71.2) | 63 (60.6) | 61 (58.7) | 51 (49.0) |
| PD, n (%) | 18 (17.3) | 21 (20.2) | 12 (12.5) | 16 (15.4) |
| NE, n (%) | 3 (2.9) | 0 (0.0) | 10 (9.6) | 8 (7.7) |
| ORR, n (%) | 9 (8.7) | 20 (19.2) | 20 (19.2) | 29 (27.9) |
| DCR, n (%) | 83 (79.8) | 83 (79.8) | 81 (77.9) | 80 (76.9) |
| RECIST v1.1 | mRECIST | |||||
|---|---|---|---|---|---|---|
| IMbrave150 in (n = 38) | IMbrave150 out (n = 66) | p-Value | IMbrave150 in (n = 38) | IMbrave150 out (n = 66) | p-Value | |
| ORR (%) | 18.4% | 19.7% | 1.000 | 29.0% | 27.3% | 1.000 |
| DCR (%) | 81.6% | 78.8% | 0.804 | 73.7% | 78.8% | 0.631 |
| 1st line (n = 48) | 2nd line- (n = 56) | p-value | 1st line (n = 48) | 2nd line- (n = 56) | p-value | |
| ORR (%) | 18.8% | 19.6% | 1.000 | 31.3% | 25.0% | 0.517 |
| DCR (%) | 81.3% | 78.6% | 0.809 | 75.0% | 78.6% | 0.816 |
| Child-Pugh A (n = 96) | Child-Pugh B (n = 8) | p-value | Child-Pugh A (n = 96) | Child-Pugh B (n = 8) | p-value | |
| ORR (%) | 20.8% | 0.0% | 0.349 | 28.1% | 25.0% | 1.000 |
| DCR (%) | 79.2% | 87.5% | 1.000 | 77.1% | 75.0% | 1.000 |
| Proteinuria 0-1+ (n = 91) | Proteinuria 2+ (n = 7) | p-value | Proteinuria 0-1+ (n = 91) | Proteinuria 2+ (n = 7) | p-value | |
| ORR (%) | 18.7% | 28.6% | 0.618 | 27.5% | 28.6% | 1.000 |
| DCR (%) | 82.4% | 85.7% | 1.000 | 79.1% | 85.7% | 1.000 |
| Platelet ≧ 7.5 × 104/μL (n = 98) | Platelet < 7.5 × 104/μL (n = 6) | p-value | Platelet ≧ 7.5 × 104/μL (n = 98) | Platelet < 7.5 × 104/μL (n = 6) | p-value | |
| ORR (%) | 18.4% | 33.3% | 0.325 | 26.5% | 50.0% | 0.345 |
| DCR (%) | 79.6% | 83.3% | 1.000 | 77.6% | 66.7% | 0.620 |
| 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | |
| ORR (%) | 21.2% | 10.5% | 0.355 | 31.8% | 10.5% | 0.089 |
| DCR (%) | 78.8% | 84.2% | 0.758 | 74.1% | 89.5% | 0.230 |
| Viral (n = 51) | Non-viral (n = 53) | p-value | Viral (n = 51) | Non-viral (n = 53) | p-value | |
| ORR (%) | 17.7% | 20.8% | 0.805 | 23.5% | 32.1% | 0.386 |
| DCR (%) | 80.4% | 79.3% | 1.000 | 72.6% | 81.1% | 0.356 |
| BCLC-B (n = 41) | BCLC-C (n = 63) | p-value | BCLC-B (n = 41) | BCLC-C (n = 63) | p-value | |
| ORR (%) | 19.5% | 19.0% | 1.000 | 34.2% | 23.8% | 0.271 |
| DCR (%) | 87.5% | 76.4% | 0.290 | 87.5% | 72.2% | 0.130 |
| mALBI 1-2a (n = 73) | mALBI 2b (n = 31) | p-value | mALBI 1-2a (n = 73) | mALBI 2b (n = 31) | p-value | |
| ORR (%) | 21.9% | 12.9% | 0.416 | 31.5% | 19.4% | 0.240 |
| DCR (%) | 78.1% | 83.9% | 0.600 | 76.7% | 77.4% | 1.000 |
| <50% liver involvement (n = 90) | ≧50% liver involvement (n = 14) | p-value | <50% liver involvement (n = 90) | ≧50% liver involvement (n = 14) | p-value | |
| ORR (%) | 20.0% | 14.3% | 1.000 | 26.0% | 14.3% | 0.340 |
| DCR (%) | 80.0% | 78.6% | 1.000 | 76.7% | 78.6% | 1.000 |
| Up-to-7 in (n = 27) | Up-to-7 out (n = 77) | p-value | Up-to-7 in (n = 27) | Up-to-7 out (n = 77) | p-value | |
| ORR (%) | 40.7% | 11.7% | 0.003 | 48.2% | 20.8% | 0.002 |
| DCR (%) | 88.9% | 76.6% | 0.265 | 85.2% | 74.0% | 0.296 |
| Vp − (n = 88) | Vp + (n = 16) | p-value | Vp − (n = 88) | Vp + (n = 16) | p-value | |
| ORR (%) | 21.6% | 6.3% | 0.298 | 31.8% | 6.3% | 0.037 |
| DCR (%) | 83.0% | 62.5% | 0.087 | 79.6% | 62.5% | 0.194 |
| Overall | |||
|---|---|---|---|
| Discontinuation due to AE n, (%) | 7 (6.1) | ||
| Interruption of Atezo n, (%) | 17 (14.8) | ||
| Interruption of Bev n, (%) | 30 (26.1) | ||
| IMbrave150 in (n = 43) | IMbrave150 out (n = 72) | p-value | |
| Discontinuation due to AE n, (%) | 1 (2.3) | 6 (8.3) | 0.254 |
| Interruption of Atezo n, (%) | 1 (2.3) | 16 (22.2) | 0.003 |
| Interruption of Bev n, (%) | 8 (18.6) | 22 (30.6) | 0.191 |
| 1st line (n = 55) | 2nd line (n = 60) | p-value | |
| Discontinuation due to AE n, (%) | 4 (7.3) | 3 (5.0) | 0.710 |
| Interruption of Atezo n, (%) | 5 (9.1) | 12 (20.0) | 0.120 |
| Interruption of Bev n, (%) | 13 (23.6) | 17 (28.3) | 0.672 |
| Child-Pugh A (n = 106) | Child-Pugh B (n = 9) | p-value | |
| Discontinuation due to AE n, (%) | 6 (5.7) | 1 (11.1) | 0.444 |
| Interruption of Atezo n, (%) | 13 (12.3) | 4 (44.4) | 0.026 |
| Interruption of Bev n, (%) | 26 (24.5) | 4 (44.4) | 0.237 |
| Proteinuria 0-1+ (n = 98) | Proteinuria 2+ (n = 8) | p-value | |
| Discontinuation due to AE n, (%) | 6 (6.1) | 1 (12.5) | 0.423 |
| Interruption of Atezo n, (%) | 14 (14.3) | 2 (25.0) | 0.347 |
| Interruption of Bev n, (%) | 26 (26.5) | 3 (37.5) | 0.681 |
| Platelet ≧ 7.5 × 104/μL (n =108) | Platelet < 7.5 × 104/μL (n = 7) | p-value | |
| Discontinuation due to AE n, (%) | 7 (6.5) | 0 (0.0) | 1.000 |
| Interruption of Atezo n, (%) | 14 (13.0) | 3 (42.9) | 0.065 |
| Interruption of Bev n, (%) | 26 (24.1) | 4 (57.1) | 0.075 |
| 1st–2nd line (n = 85) | 3rd line (n = 19) | p-value | |
| Discontinuation due to AE n, (%) | 7 (7.3) | 0 (0.0) | 0.598 |
| Interruption of Atezo n, (%) | 13 (13.5) | 4 (21.1) | 0.478 |
| Interruption of Bev n, (%) | 22 (22.9) | 8 (42.1) | 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sho, T.; Suda, G.; Yamamoto, Y.; Furuya, K.; Baba, M.; Ogawa, K.; Kubo, A.; Tokuchi, Y.; Fu, Q.; Yang, Z.; et al. Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150. Cancers 2022, 14, 3938. https://doi.org/10.3390/cancers14163938
Sho T, Suda G, Yamamoto Y, Furuya K, Baba M, Ogawa K, Kubo A, Tokuchi Y, Fu Q, Yang Z, et al. Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150. Cancers. 2022; 14(16):3938. https://doi.org/10.3390/cancers14163938
Chicago/Turabian StyleSho, Takuya, Goki Suda, Yoshiya Yamamoto, Ken Furuya, Masaru Baba, Koji Ogawa, Akinori Kubo, Yoshimasa Tokuchi, Qingjie Fu, Zijian Yang, and et al. 2022. "Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150" Cancers 14, no. 16: 3938. https://doi.org/10.3390/cancers14163938
APA StyleSho, T., Suda, G., Yamamoto, Y., Furuya, K., Baba, M., Ogawa, K., Kubo, A., Tokuchi, Y., Fu, Q., Yang, Z., Kimura, M., Kitagataya, T., Maehara, O., Ohnishi, S., Nakamura, A., Yamada, R., Ohara, M., Kawagishi, N., Natsuizaka, M., ... Sakamoto, N. (2022). Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150. Cancers, 14(16), 3938. https://doi.org/10.3390/cancers14163938






