Simple Summary
Increased options for cancer treatment have made the prediction of prognosis an important factor in therapeutic decision making. The aim of this study was to assess the clinical significance of prognosis–scoring systems and to identify predictors for 6–month mortality after palliative surgery. The median actual survival period was longer than the predicted life expectancy based on the revised Tokuhashi score and new Katagiri score. However, 21.3% of patients died of cancers within 6 months after palliative surgery. A statistical analysis showed that a higher CRP/albumin ratio (odds ratio: 0.39; cut–off 0.409) and absence of postoperative adjuvant therapy (odds ratio: 7.15) were independent risk factors for poor survival. Our findings suggest the need for careful consideration to determine if palliative surgery is the best option for a patient with these negative prognostic factors, regardless of life expectancy predicted based on a prognosis score.
Abstract
Prediction of prognosis is a key factor in therapeutic decision making due to recent the development of therapeutic options for spinal metastases. The aim of the study was to examine predictive scoring systems and identify prognostic factors for 6–month mortality after palliative surgery. The participants were 75 patients with spinal metastases who underwent palliative surgery and had a minimum follow–up period of 1 year. Associations of actual survival with categories based on the revised Tokuhashi score and new Katagiri score were evaluated. Univariate and multivariate analyses were performed to identify prognostic factors for 6–month mortality after palliative surgery. The median actual survival period was longer than those predicted using the scoring systems. However, 21.3% of patients died of cancers within 6 months after surgery. A higher CRP/albumin ratio (odds ratio: 0.39; cut–off 0.409) and absence of postoperative adjuvant therapy (odds ratio: 7.15) were independent risk factors for 6–month mortality. There was no association of mortality with primary site, severity of sarcopenia, or other biomarkers. These results suggest that careful consideration is needed to determine whether palliative surgery is the best option for patients with a high preoperative CRP/albumin ratio and/or absence of postoperative adjuvant therapy, regardless of predictions made from scoring systems.
1. Introduction
The number of patients with cancer and deaths due to cancer have increased worldwide. Statistics from the International Agency for Research on Cancer, an external research organization of the World Health Organization, indicated 19.3 million new cases of cancer and estimated 10.0 million cancer deaths worldwide in 2020, a significant increase from the 2002 estimates of 10.9 million new cases and 6.7 million cancer deaths, respectively [1,2]. A previous study suggested that approximately 30% of patients with advanced cancer develop spinal metastasis [3]. In a cadaver study, spinal metastases were found in 36% of 832 deceased patients with a terminal diagnosis of malignant neoplasm [4]. Furthermore, 5–20% of these patients suffered from metastatic spinal cord compression (MSCC) [5,6]. Spinal metastasis can cause a number of sequelae, including pain, instability, and neurological deficit. In addition, patients with MSCC may have progression of myelopathy that results in the loss of motor, sensory, and autonomic functions.
Many reports have suggested the superiority of palliative surgery over nonsurgical treatment for the improvement in performance status (PS), activities of daily living (ADL), and neurological status [7,8]. The cost–utility of surgical treatment for spinal metastasis is also considered to be acceptable, especially for patients who are nonambulatory due to acute neurologic compromise [9,10]. With recent developments in therapeutic options for spinal metastases, including surgery, radiotherapy, chemotherapy, and targeted therapy, spine surgeons and oncologists need to consider the best option for remission of symptoms based on the prediction of life expectancy and the benefits of treatment.
At our hospital, patients with progressive neurological deficits or intractable pain were selected as candidates for palliative surgery if the life expectancy was predicted to be over approximately 6 months by using prognosis–scoring systems, opinions from oncologists, and the neurologic, oncologic, mechanical, and systemic (NMOS) framework [11]. However, contrary to our preoperative predictions, some patients survived for ≤6 months after palliative surgery. Thus, the aim of the study was to evaluate whether there is a discrepancy between prognosis prediction scores and actual prognosis, and to identify predictors of poor survival of ≤6 months after surgery.
2. Materials and Methods
2.1. Study Design
Between 2005 and 2021, 83 patients with spinal metastases underwent palliative surgery at our hospital. The study cohort included 75 patients who had a minimum follow–up period of 1 year, including those who died within 1 year after surgery (follow–up rate: 90.4%). The surgical indication was progressive neurological deficits due to MSCC and/or intractable pain associated with spinal metastases. Patients in poor general condition for general anesthesia and those with short life expectancy (<3–6 months) assessed by using prognosis–scoring systems and the opinions of oncologists were excluded from surgery. All patients underwent one–stage posterior decompression with or without fusion with reference to the spinal instability neoplastic score (SINS). To maintain case homogeneity, patients who received total en bloc spondylectomy as curative treatment were excluded. The study protocol was approved by the Human Ethics Review Committee of Fukui University Medical Faculty and strictly followed the Clinical Research Guidelines of the Ministry of Health, Labor, and Welfare of the Japanese Government.
2.2. Clinical Assessments
Data were collected on patient background (age, sex, and body mass index (BMI)), primary site, preoperative Frankel grade [12], Eastern Cooperative Oncology Group (ECOG) PS [13] at 3 months after surgery assessed by oncologists, affected level, indication for surgery (neurological deficit, intractable pain), surgical procedure (decompression and fusion, decompression without fusion), surgery–related factors (operation time, blood loss, emergency operation, perioperative complications of Clavien–Dindo classification grade 2 or higher [14]), and pre– and postoperative adjuvant therapy (radiation, chemotherapy, hormonal therapy, and molecular targeted drugs). Both the revised Tokuhashi score [15] and new Katagiri score [16] were used for the prediction of prognosis and assessment of the severity of spinal metastases. SINS was used to assess the degree of spinal instability and to guide the need for fusion surgery. Sarcopenia was evaluated using the sum of the left and right psoas area on an axial image in the mid–L3 vertebral body divided by the L3 vertebral body area, calculated using a picture archiving and communication system [17]. Radiological assessments were performed by two observers. The preoperative inflammatory response and nutritional status were evaluated using the serum C–reactive protein (CRP)/albumin ratio (CAR), neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) [18]. The postoperative survival period was defined as the time from the date of surgery to the final follow–up or death, whichever was earlier. Information on death was obtained from the patient’s family or from a transfer hospital.
2.3. Statistical Analysis
Data were presented as a median [interquartile range]. Categorical variables were compared by Mann–Whitney U–test or chi–square test, with p < 0.05 considered to be significant. Significant factors in univariate analysis and those reported in the literature were included in a multivariate regression model. The estimated odds ratio and 95% confidence interval (CI) were calculated to identify independent predictors of survival >6 months. For measurement of radiological parameters, inter– and intraobserver reliabilities were assessed by calculating intraclass correlation coefficients (ICCs), with ICC (1,3) and ICC (2,3) > 0.75 considered to represent good to excellent reliability. The median survival period based on the predictive scoring systems were calculated using Kaplan–Meier survivorship analysis. The cut–off was defined as the point nearest to the upper–left corner of a receiver operating characteristic (ROC) curve, and the area under the curve (AUC) was used to assess the accuracy of the parameter as a predictor. EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [19], a graphical user interface for R (The R foundation for Statistical Computing, Vienna, Austria), was used for all analyses.
3. Results
3.1. Comparison of Preoperative Prediction and Actual Median Survival Time after Palliative Surgery
A total of 75 patients (median age: 67.0 years; male 53, female 22) were enrolled in the study. The primary site and median survival period are shown in Table 1. There was no significant difference in survival based on the primary site. The overall Kaplan–Meier median survival for all patients was 20 months (12–36) (Figure 1A). Kaplan–Meier survival curves based on the revised Tokuhashi score (good, ≥12; intermediate, 9–11; poor, 0–8) and new Katagiri score (good, 0–3; intermediate, 4–6; poor, ≥7) for the prognosis are shown in Figure 1B,C, respectively. The median survival periods could be clustered using these scoring systems: 36.0, 19.0, and 10.5 months using the revised Tokuhashi score (p = 0.13 by log rank test) and 35.0, 18.0, and 10.0 months using the new Katagiri score (p = 0.055 by log rank test). The 1–year survival rates in the poor and intermediate groups were 77.8% and 38.1% using the revised Tokuhashi score and 30.8% and 65.0% using the new Katagiri score, respectively. Of the 75 patients, 16 (21.3%) died of various cancers within 6 months of surgery, contrary to our preoperative predictions. Table 2 shows the relationship between the revised Tokuhashi score and the new Katagiri score with 6–month mortality. In some cases, a discrepancy between these predictive scoring systems and the actual survival was observed; even patients clustered based on intermediate and good groups died (19.4%/22.5% and 5.9%/9.1% of patients, respectively) within 6 months of surgery. There was no correlation between the median survival period and SINS: 14, 35 and 18 months in stable (0–6 points), potentially unstable (7–12 points), and unstable (≥13 points) cases, respectively (p = 0.38 by log rank test) were observed.

Table 1.
Primary site and median survival times in patients with spinal metastasis.
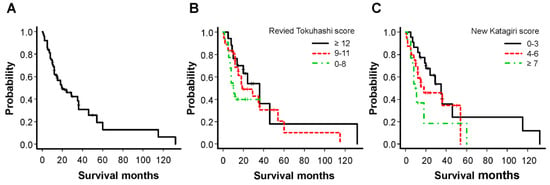
Figure 1.
Kaplan−Meier analyses of overall survival after palliative surgery in all patients (A) and categories based on the revised Tokuhashi score (B) and new Katagiri score (C).

Table 2.
Relationship between the predictive prognostic scores with 6–month mortality.
3.2. Prognostic Factors for Poor Survival of Less than 6 Months after Palliative Surgery
A comparison of patients with and without survival >6 months is shown in Table 3. In univariate analysis, preoperative Frankel grade, CAR, and postoperative adjuvant therapy differed significantly between these groups. However, in terms of preoperative Frankel grade, there was no significant difference in the survival period between nonambulatory (Frankel A–C) and ambulatory (Frankel D and E) cases (p = 0.56). The median survival of 6 patients with a survival period of ≤6 months without any postoperative adjuvant therapy was 1.75 [1.5–2.0] months, which was a very poor survival rate. In three of these six patients, the primary site was first detected owing to spinal metastasis, and no pathological diagnosis was performed preoperatively (lung cancer without molecularly targeted drugs (n = 2), hormone–independent prostate cancer (n = 1)). The remaining three cases showed rapidly progressing cancer (colorectal cancer, gastric cancer, and neck cancer); unfortunately, they could not achieve the expected physical performance postoperatively. There was no difference in the prognostic scores (revised Tokuhashi score, new Katagiri score) between the two groups, or in patient background, primary site, postoperative ECOG PS, affected level, severity of sarcopenia, NLR, PLR, indication for surgery, surgical procedures, operation factors, and preoperative adjuvant therapy. Of the 75 patients, 11 (14.7%) experienced perioperative complications within 14 days of surgery (surgical site infection (n = 3), motor weakness (n = 3), pneumonia (n = 2), severe anemia (n = 2), and deep vein thrombosis (n = 1)), which were not responsible for their mortality. Multivariate logistic regression analysis including significant variables from univariate analysis and prognostic scores was used to identify independent prognostic factors in patients with the survival of >6 months. In this analysis, CAR (odds ratio 0.39) and postoperative adjuvant therapy (odds ratio 7.15) were identified as independent prognostic factors (Table 4). In addition, prediction of the preoperative CAR for 6–month mortality was calculated using an ROC curve. The AUC for preoperative CAR was 0.832, and the cut–off value for 6–month mortality after palliative surgery derived from the ROC curve was 0.409 (sensitivity, 89.7%; specificity, 82.4%) (Figure 2).

Table 3.
Comparison of patients with and without survival >6 months after palliative surgery.

Table 4.
Multivariate analysis of predictors of survival >6 months after palliative surgery.
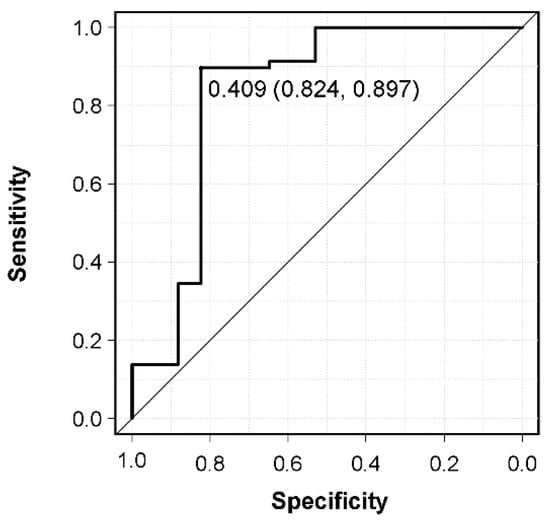
Figure 2.
Receiver operating characteristic curve of preoperative CRP/albumin ratio (CAR) for 6–month mortality after palliative surgery and determination of the CAR cut–off value.
3.3. Association between Prognostic Scoring Systems and CRP/Albumin Ratio (CAR)
In the multivariate analysis, a higher CRP/albumin ratio (cut–off: 0.409) was found to be an independent risk factor for 6–month mortality. Next, the relationships between prognostic–scoring systems and preoperative CAR were assessed (Table 5). For the revised Tokuhashi score, the preoperative CAR in poor survival cases (score 0–8) was significantly higher than in those with predicted intermediate (score 9–11) and good (score ≥ 12) survival (p = 0.024). However, the median preoperative CAR (0.37) was below the cut–off value (0.409). There was no association between the new Katagiri score for prognosis and the preoperative CAR (p = 0.22).

Table 5.
Relationships of predictive prognostic scores with the preoperative CRP/albumin ratio.
4. Discussion
Palliative surgery for patients with spinal metastases is likely to increase owing to advances in cancer treatment that have increased the life expectancy for primary tumors, including molecular targeted drugs for lung and renal cancers and hormonal therapy for breast and prostate cancers. Predicting the prognosis for patients with spinal metastases is an important factor in therapeutic decision making. Candidates for palliative surgery are identified using prognostic scores and opinions of oncologists; however, 21.3% of the patients in the current study had survival of ≤6 months after palliative surgery. Thus, we retrospectively examined the revised Tokuhashi and new Katagiri scores and identified the prognostic factors for poor survival in patients with the survival of ≤6 months after palliative surgery, contrary to preoperative assessments.
Prognostic scores are used to categorize patients with spinal metastases based on the survival time and are useful guides in therapeutic decision making [20,21,22]. In our patients, the revised Tokuhashi and new Katagiri scores were effective for categorizing their prognosis into poor, intermediate, and good survival, although without statistical significance. The original article on the revised Tokuhashi score found life expectancies of patients in the poor (score 0–8) and intermediate (score 9–11) groups of <6 and >6 months, respectively [15]. However, the respective median survival periods in the current study were 10.5 and 18 months for these groups, which differed from the original study. In contrast, 19.4% and 5.9% of patients categorized in the intermediate and good survival groups, respectively, based on the revised Tokuhashi score died within 6 months of surgery. Previous studies have suggested that the agreement of the actual survival with that predicted using the revised Tokuhashi score is approximately 60%, and that the predictive accuracy decreases for patients with a life expectancy shorter than 1 year [23,24,25]. The new Katagiri score has advantages of including classification of the growth rate of the primary cancer and effects of molecular targeted drugs and sensitivity to hormonal therapy. The original study on the new Katagiri score reported 1–year survival rates of 6% in the poor group (score ≥ 7) and 49% in the intermediate group (score 4–6) [16]. However, the 1–year survival rates in the current study were 30.8% and 65.0% in these respective groups, showing a similar discrepancy from the original study to that for the revised Tokuhashi score. Another study on the survival rate after palliative surgery using the new Katagiri score found a rate of 22% in the poor group [26]. The Tokuhashi score was published in 2005 and the Katagiri score was applied to patients between 2005 and 2008; this is one of the shortcomings of using fixed algorithmic scoring systems. Our results suggest that recent developments in cancer treatment have prolonged the life expectancy. On the other hand, 22.5% and 9.1% of patients categorized in the intermediate and good survival groups based on the new Katagiri score died within 6 months of surgery. Regarding SINS, the association with the prognosis for survival is controversial [27,28]. The current study did not show any association of SINS or surgical procedures with the survival period. SINS is an important scoring system to assess spinal instability and determine the need for fusion surgery [29]; however, it may not predict the survival after palliative surgery based on the results of the current study.
Preoperative ambulatory status has been identified as a significant prognostic factor in patients with spinal metastases in a meta–analysis, with the suggestion that this status should be considered when choosing the treatment modality [30]. In the current study, the preoperative Frankel grade differed significantly between patients with and without survival for >6 months in univariate analysis; however, no significant difference was observed in the survival period between nonambulatory (Frankel A–C) and ambulatory (Frankel D and E) cases. Frailty and/or elderly age may also increase the risk of mortality, postoperative complications and chemotherapy intolerance [31,32]. A cohort study suggested that the psoas size, as a surrogate for frailty/sarcopenia, predicts the overall mortality more strongly than the Tokuhashi score [33]. However, in the current study, the survival period was not correlated with age, BMI, or psoas size at the L3 vertebra body. A modified frailty index used in this study may not capture the systemic condition and functional status of the patients. A previous article suggested that a frailty measure specific to spinal metastatic disease should be developed for highlighting systemic variables and patient factors that affect clinical outcomes [17].
Inflammatory mediators and cytokines produced by inflammatory cells in the tumor microenvironment are increasingly recognized as important contributors to cancer progression through their effects on the promotion of proliferation, angiogenesis, and metastasis, reduction in responses to hormones and chemotherapeutic agents, and subversion of adaptive immunity [34]. The association between inflammatory response and tumor progression also suggests the possible prognostic significance of preoperative inflammatory biomarkers, including CAR, NLR, and PLR. Several cancer studies have suggested the accuracy of CAR. One of the studies even reported CAR to be the most sensitive prognostic factor for esophageal squamous cell carcinoma [35]. In the current study, CAR was an independent prognostic factor for the survival period of over 6 months, and the cut–off for CAR was 0.409. In another study on patients with spinal metastasis from clear cell renal cell carcinoma, NLR and PLR were found to be significantly correlated with the overall survival [18]. Further studies are needed to identify the optimal biomarker for each primary site; however, preoperative CAR may be a reliable inflammatory biomarker to predict life expectancy. Importantly, the findings of the current study also indicated that CAR was not clearly associated with the revised Tokuhashi and new Katagiri scores. Thus, careful consideration should be given to whether palliative surgery is the best option for a patient with a high CAR, especially in a case with relatively good prognosis scores.
The prolonged prognosis of cancer patients is largely due to recent developments in chemotherapy, hormonal therapy and molecular targeted therapy; however, not all patients are eligible for these therapies. In the original study of the new Katagiri score, previous chemotherapy was a negative prognostic factor for survival rate (odds ratio: 1.39) [16]. In the current study, a history of preoperative adjuvant therapy was not associated with a poor survival period; however, the absence of a postoperative adjuvant therapy was identified as an independent negative factor for 6–month mortality after palliative surgery. Importantly, the hazard ratio differed significantly between treatment with molecular targeted drugs or hormonal therapy and between sensitive and resistant lesions, as well as sensitivity to chemotherapy and radiotherapy, even for the same primary cancer [26]. Thus, preoperative information from oncologists on the indication for postoperative adjuvant therapy is particularly important when determining the prognosis for a primary cancer, especially for considering the indication for palliative surgery. In cases of spinal metastases with motor paralysis and/or intractable pain, preoperative PS does not necessarily reflect the general condition. Therefore, oncologists and spine surgeons should use decision making frameworks such as NOMS in their decision making [11]. Nevertheless, in cases of spinal metastases that require urgent palliative surgery without any preoperative detailed pathological diagnosis because of spinal metastasis, the informed consent about the prognosis should be obtained more carefully.
This study has certain limitations, including its retrospective and single-center design, small number of patients, and inclusion of cases receiving surgical treatment only. The number of cases for each primary site might be too small for identification of prognostic factors and treatment strategies for each site. Thus, larger prospective and multicenter clinical studies are needed to provide further evidence in support of our findings. Despite these limitations, the results of the study provide important insights and guidance on therapeutic decision making for patients with spinal metastases.
5. Conclusions
Recent developments in cancer treatment and the variety of treatment options have made the prediction of prognosis an important factor in therapeutic decision making. The revised Tokuhashi score and the new Katagiri score are useful for predicting the prognosis of patients with spinal metastasis; however, the median life expectancy in the current study was longer than the survival period predicted using these scoring systems. Contrary to the preoperative predictions, 21.3% of patients had a survival period of <6 months after palliative surgery. A higher CRP/albumin ratio (CAR) and absence of postoperative adjuvant therapy were independent risk factors for 6–month mortality, regardless of preoperative life expectancy based on scoring systems and/or the opinions of oncologists. Our findings suggest that patients with more favorable prognostic scores, but with high CAR (cut–off value = 0.409) and/or lack of eligibility for postoperative adjuvant therapy, may have shorter survival than expected and that careful consideration should be given to determine whether palliative surgery is the best option for such patients.
Author Contributions
Conceptualization, H.N.; methodology, H.N., S.W., K.H., Y.I. and T.T.; formal analysis, H.N., S.W., K.H. and Y.I.; investigation, H.N., S.W., K.H., Y.I. and Y.W.; data curation, H.N., S.W., K.H., Y.I. and Y.W.; writing—original draft preparation, H.N.; writing—review and editing, T.T. and A.M.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the Human Ethics Review Committee of our University Medical Faculty (Approval Number 2014046), and it strictly followed the Clinical Research Guidelines of the Ministry of Health, Labor, and Welfare of the Japanese Government.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author and subject to the ethical approvals in place and materials transfer agreements.
Acknowledgments
We are grateful to all patients for the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wibmer, C.; Leithner, A.; Hofmann, G.; Clar, H.; Kapitan, M.; Berghold, A.; Windhager, R. Survival Analysis of 254 Patients after Manifestation of Spinal Metastases: Evaluation of Seven Preoperative Scoring Systems. Spine 2011, 36, 1977–1986. [Google Scholar] [CrossRef]
- Wong, D.A.; Fornasier, V.L.; MacNab, I. Spinal Metastases: The Obvious, the Occult, and the Impostors. Spine 1990, 15, 1–4. [Google Scholar] [CrossRef]
- Byrne, T.N. Spinal Cord Compression from Epidural Metastases. N. Engl. J. Med. 1992, 327, 614–619. [Google Scholar]
- Jacobs, W.B.; Perrin, R.G. Evaluation and Treatment of Spinal Metastases: An Overview. Neurosurg. Focus 2001, 11, e10. [Google Scholar] [CrossRef] [Green Version]
- Kakutani, K.; Sakai, Y.; Maeno, K.; Takada, T.; Yurube, T.; Kurakawa, T.; Miyazaki, S.; Terashima, Y.; Ito, M.; Hara, H.; et al. Prospective Cohort Study of Performance Status and Activities of Daily Living After Surgery for Spinal Metastasis. Clin. Spine Surg. 2017, 30, E1026–E1032. [Google Scholar] [CrossRef]
- Kanda, Y.; Kakutani, K.; Sakai, Y.; Yurube, T.; Miyazaki, S.; Takada, T.; Hoshino, Y.; Kuroda, R. Prospective Cohort Study of Surgical Outcome for Spinal Metastases in Patients Aged 70 Years or Older. Bone Jt. J. 2020, 102-B, 1709–1716. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kakutani, K.; Sakai, Y.; Ejima, Y.; Maeno, K.; Takada, T.; Yurube, T.; Terashima, Y.; Ito, M.; Kakiuchi, Y.; et al. Quality of Life and Cost-Utility of Surgical Treatment for Patients with Spinal Metastases: Prospective Cohort Study. Int. Orthop. 2017, 41, 1265–1271. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bensen, G.P.; Blucher, J.A.; Ferrone, M.L.; Balboni, T.A.; Schwab, J.H.; Harris, M.B.; Katz, J.N.; Losina, E. The Cost-Effectiveness of Surgical Intervention for Spinal Metastases: A Model-Based Evaluation. J. Bone Jt. Surg. Am. 2021, 103, 2221–2228. [Google Scholar] [CrossRef]
- MacLean, M.A.; Touchette, C.J.; Georgiopoulos, M.; Brunette-Clément, T.; Abduljabbar, F.H.; Ames, C.P.; Bettegowda, C.; Charest-Morin, R.; Dea, N.; Fehlings, M.G.; et al. AO Spine Knowledge Forum Tumor. Systemic considerations for the surgical treatment of spinal metastatic disease: A scoping literature review. Lancet Oncol. 2022, 23, e321–e333. [Google Scholar] [CrossRef]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.; Walsh, J.J. The Value of Postural Reduction in the Initial Management of Closed Injuries of the Spine with Paraplegia and Tetraplegia. I. Paraplegia 1969, 7, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Ajiro, Y.; Umezawa, N. Outcome of Treatment for Spinal Metastases Using Scoring System for Preoperative Evaluation of Prognosis. Spine 2009, 34, 69–73. [Google Scholar] [CrossRef]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New Prognostic Factors and Scoring System for Patients with Skeletal Metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef]
- Bourassa-Moreau, É.; Versteeg, A.; Moskven, E.; Charest-Morin, R.; Flexman, A.; Ailon, T.; Dalkilic, T.; Fisher, C.; Dea, N.; Boyd, M.; et al. Sarcopenia, but Not Frailty, Predicts Early Mortality and Adverse Events after Emergent Surgery for Metastatic Disease of the Spine. Spine J. 2020, 20, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Li, J.; Hu, M.; Zhang, H.; Yang, J.; Gong, H.; Li, B.; Wan, W.; Xiao, J. Prognostic Significance of Preoperative Inflammatory Biomarkers and Traditional Clinical Parameters in Patients with Spinal Metastasis from Clear Cell Renal Cell Carcinoma: A Retrospective Study of 95 Patients in a Single Center. Cancer Manag. Res. 2020, 12, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Bollen, L.; Wibmer, C.; Van der Linden, Y.M.; Pondaag, W.; Fiocco, M.; Peul, W.C.; Marijnen, C.A.M.; Nelissen, R.G.H.H.; Leithner, A.; Dijkstra, S.P.D. Predictive Value of Six Prognostic Scoring Systems for Spinal Bone Metastases: An Analysis Based on 1379 Patients. Spine 2016, 41, E155–E162. [Google Scholar] [CrossRef]
- Cassidy, J.T.; Baker, J.F.; Lenehan, B. The Role of Prognostic Scoring Systems in Assessing Surgical Candidacy for Patients with Vertebral Metastasis: A Narrative Review. Global Spine J. 2018, 8, 638–651. [Google Scholar] [CrossRef]
- Massaad, E.; Hadzipasic, M.; Alvarez-Breckenridge, C.; Kiapour, A.; Fatima, N.; Schwab, J.H.; Saylor, P.; Oh, K.; Schoenfeld, A.J.; Shankar, G.M.; et al. Predicting Tumor-Specific Survival in Patients with Spinal Metastatic Renal Cell Carcinoma: Which Scoring System Is Most Accurate? J. Neurosurg. Spine 2020, 33, 529–539. [Google Scholar] [CrossRef]
- Mezei, T.; Horváth, A.; Pollner, P.; Czigléczki, G.; Banczerowski, P. Research on the Predicting Power of the Revised Tokuhashi System: How Much Time Can Surgery Give to Patients with Short Life Expectancy? Int. J. Clin. Oncol. 2020, 25, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Quraishi, N.A.; Manoharan, S.R.; Arealis, G.; Khurana, A.; Elsayed, S.; Edwards, K.L.; Boszczyk, B.M. Accuracy of the Revised Tokuhashi Score in Predicting Survival in Patients with Metastatic Spinal Cord Compression (MSCC). Eur. Spine J. 2013, 22, S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Skoch, J.; Walter, C.M.; Torabi, M.; Borgstrom, M.; Baaj, A.A. The Tokuhashi Score: Effectiveness and Pitfalls. Eur. Spine J. 2016, 25, 673–678. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ando, K.; Nakashima, H.; Sato, K.; Kanemura, T.; Yoshihara, H.; Hirasawa, A.; Kato, F.; Ishiguro, N.; Imagama, S. Prognostic Factors in the New Katagiri Scoring System After Palliative Surgery for Spinal Metastasis. Spine 2020, 45, E813–E819. [Google Scholar] [CrossRef]
- Zadnik, P.L.; Goodwin, C.R.; Karami, K.J.; Mehta, A.I.; Amin, A.G.; Groves, M.L.; Wolinsky, J.-P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L.; et al. Outcomes Following Surgical Intervention for Impending and Gross Instability Caused by Multiple Myeloma in the Spinal Column. J. Neurosurg. Spine 2015, 22, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Masuda, K.; Ebata, K.; Yasuhara, Y.; Enomoto, A.; Saito, T. Outcomes and Prognosis of Neurological Decompression and Stabilization for Spinal Metastasis: Is Assessment with the Spinal Instability Neoplastic Score Useful for Predicting Surgical Results? Asian Spine J. 2018, 12, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Lenschow, M.; Lenz, M.; von Spreckelsen, N.; Ossmann, J.; Meyer, J.; Keßling, J.; Nadjiri, L.; Telentschak, S.; Zarghooni, K.; Knöll, P.; et al. Impact of Spinal Instrumentation on Neurological Outcome in Patients with Intermediate Spinal Instability Neoplastic Score (SINS). Cancers 2022, 14, 2193. [Google Scholar] [CrossRef]
- Yang, X.-G.; Han, Y.; Wang, F.; Liu, Y.-H.; Hu, Y.-C.; Lun, D.-X.; Feng, J.-T.; Hua, K.-C.; Yang, L.; Zhang, H.; et al. Is Ambulatory Status a Prognostic Factor of Survival in Patients with Spinal Metastases? An Exploratory Meta-Analysis. Orthop. Surg. 2018, 10, 173–180. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The Prevalence and Outcomes of Frailty in Older Cancer Patients: A Systematic Review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Rades, D.; Huttenlocher, S.; Evers, J.N.; Bajrovic, A.; Karstens, J.H.; Rudat, V.; Schild, S.E. Do Elderly Patients Benefit from Surgery in Addition to Radiotherapy for Treatment of Metastatic Spinal Cord Compression? Strahlenther. Onkol. 2012, 188, 424–430. [Google Scholar] [CrossRef]
- Zakaria, H.M.; Wilkinson, B.M.; Pennington, Z.; Saadeh, Y.S.; Lau, D.; Chandra, A.; Ahmed, A.K.; Macki, M.; Anand, S.K.; Abouelleil, M.A.; et al. Sarcopenia as a Prognostic Factor for 90-Day and Overall Mortality in Patients Undergoing Spine Surgery for Metastatic Tumors: A Multicenter Retrospective Cohort Study. Neurosurgery 2020, 87, 1025–1036. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Xu, X.L.; Yu, H.Q.; Hu, W. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS ONE 2015, 10, e0138657. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).