Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Literature Search
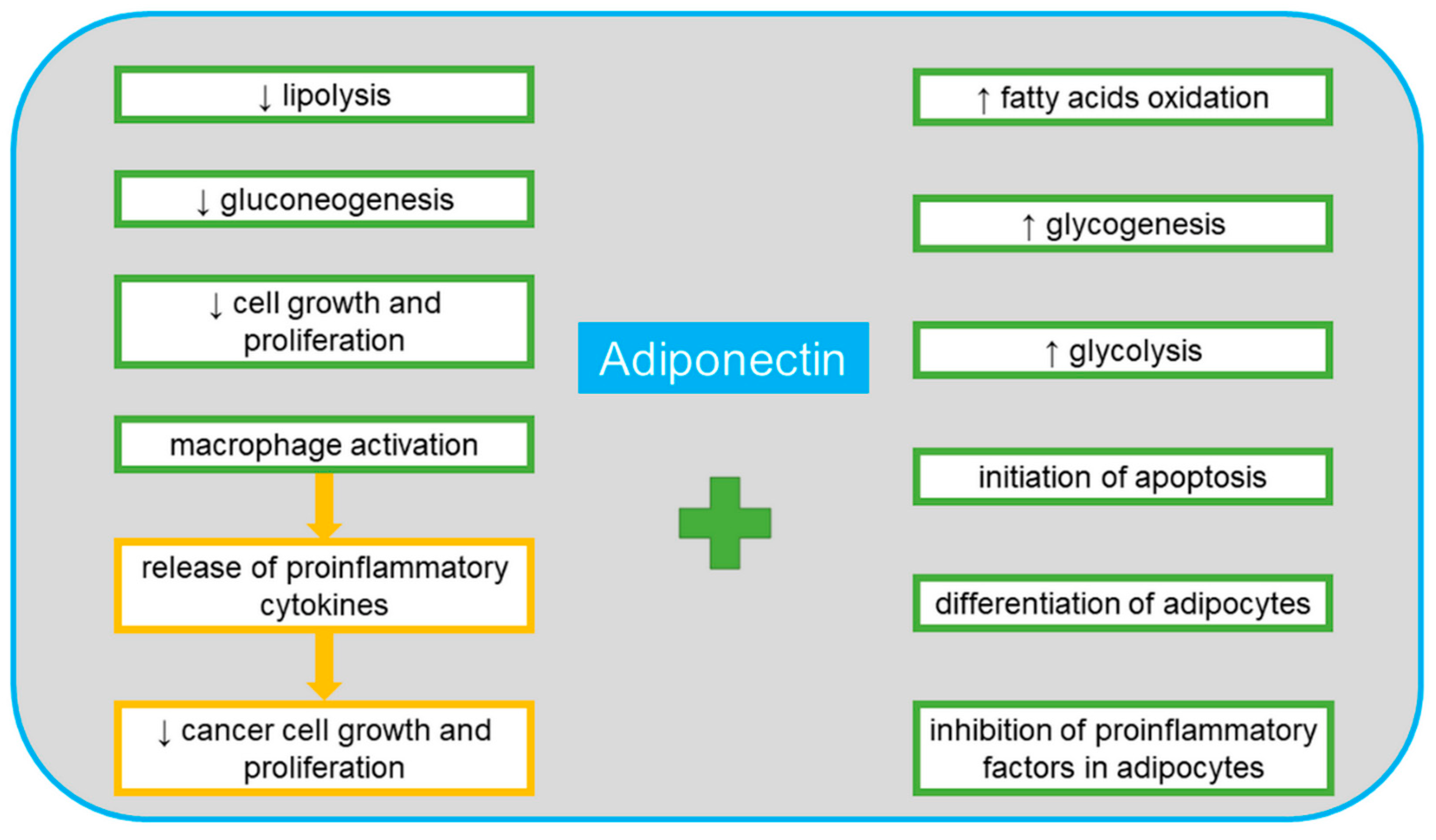
3. Adiponectin
4. Adiponectin in Ovarian Cancer
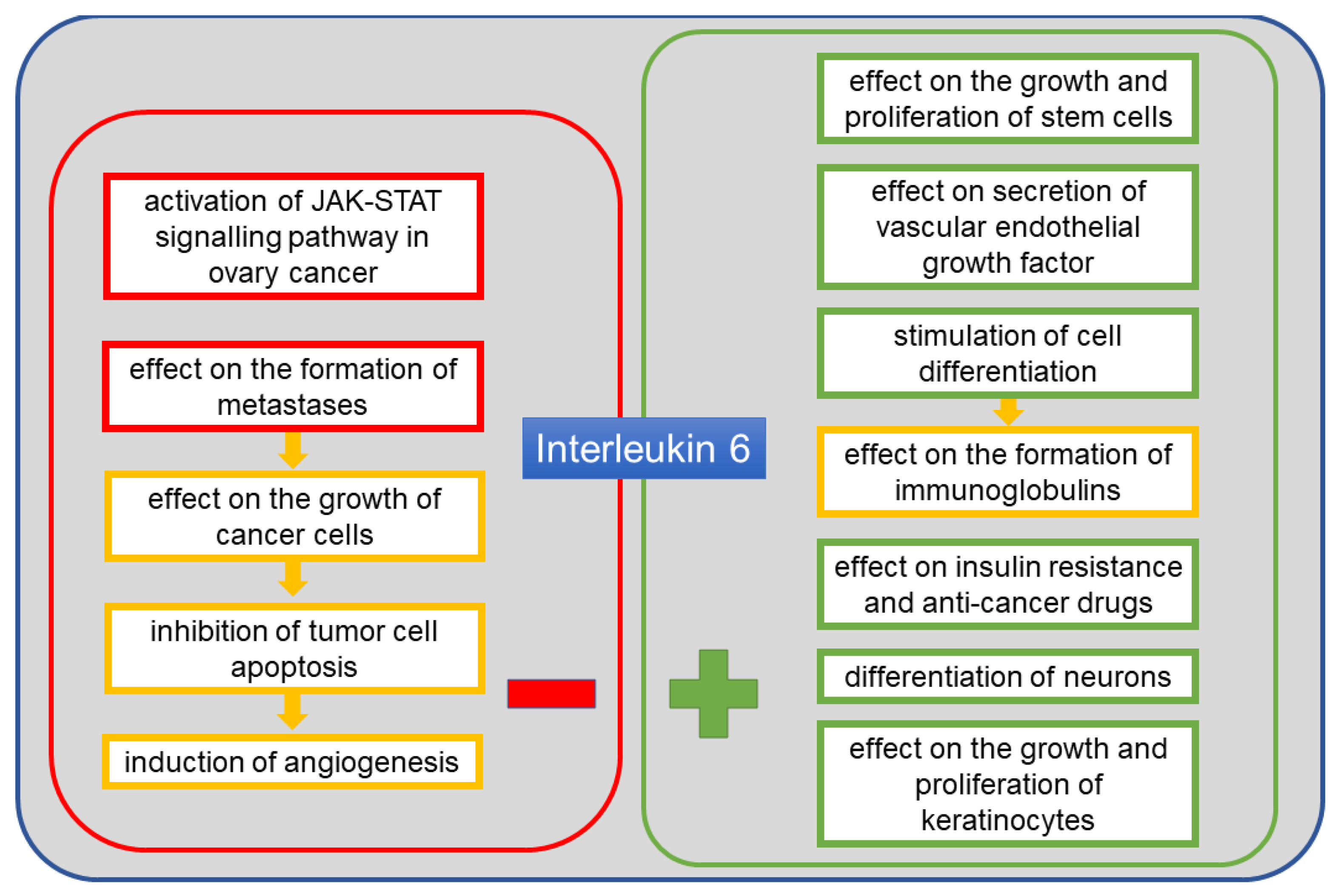
5. Interleukin 6
6. IL-6 in Ovarian Cancer
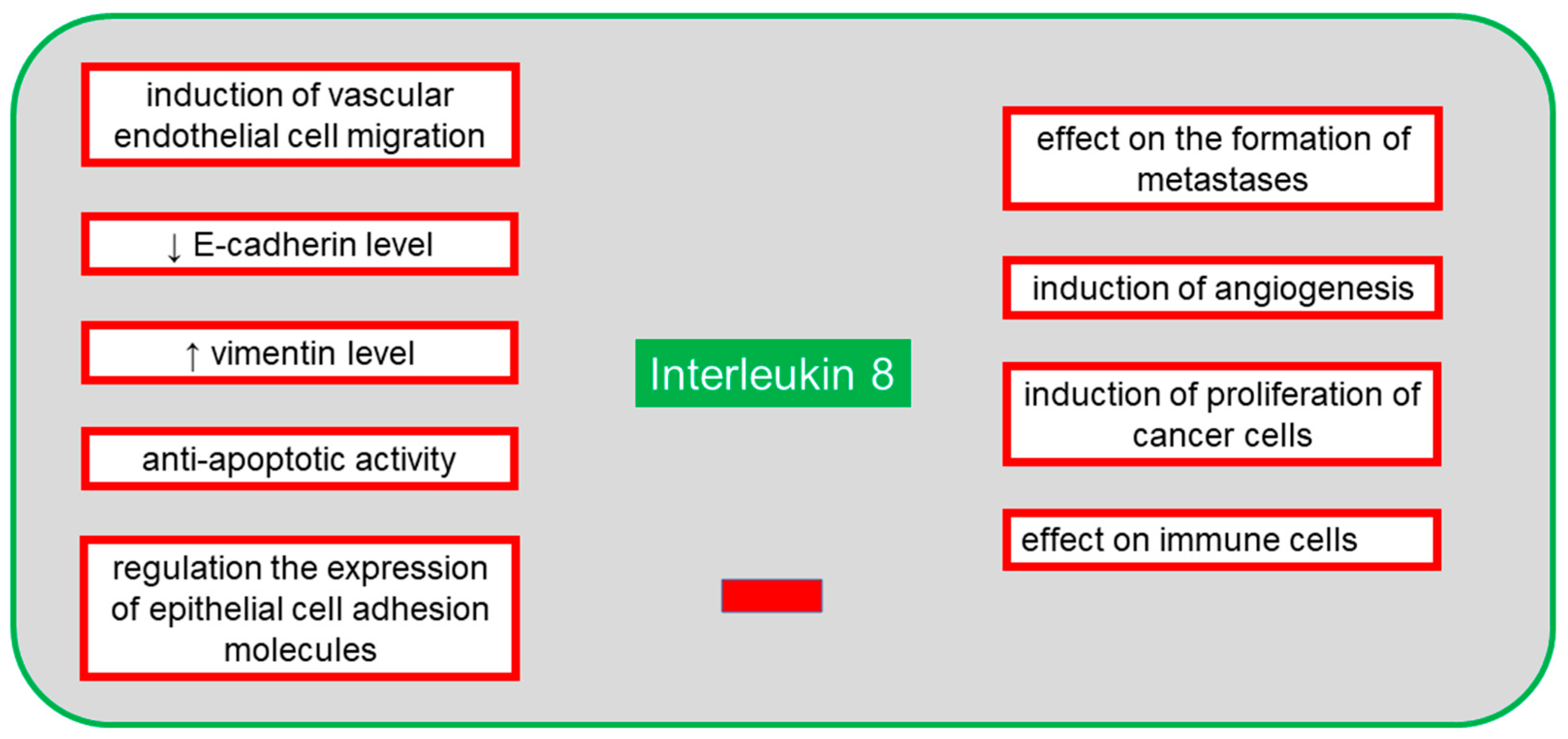
7. Interleukin 8
8. IL-8 in Ovarian Cancer
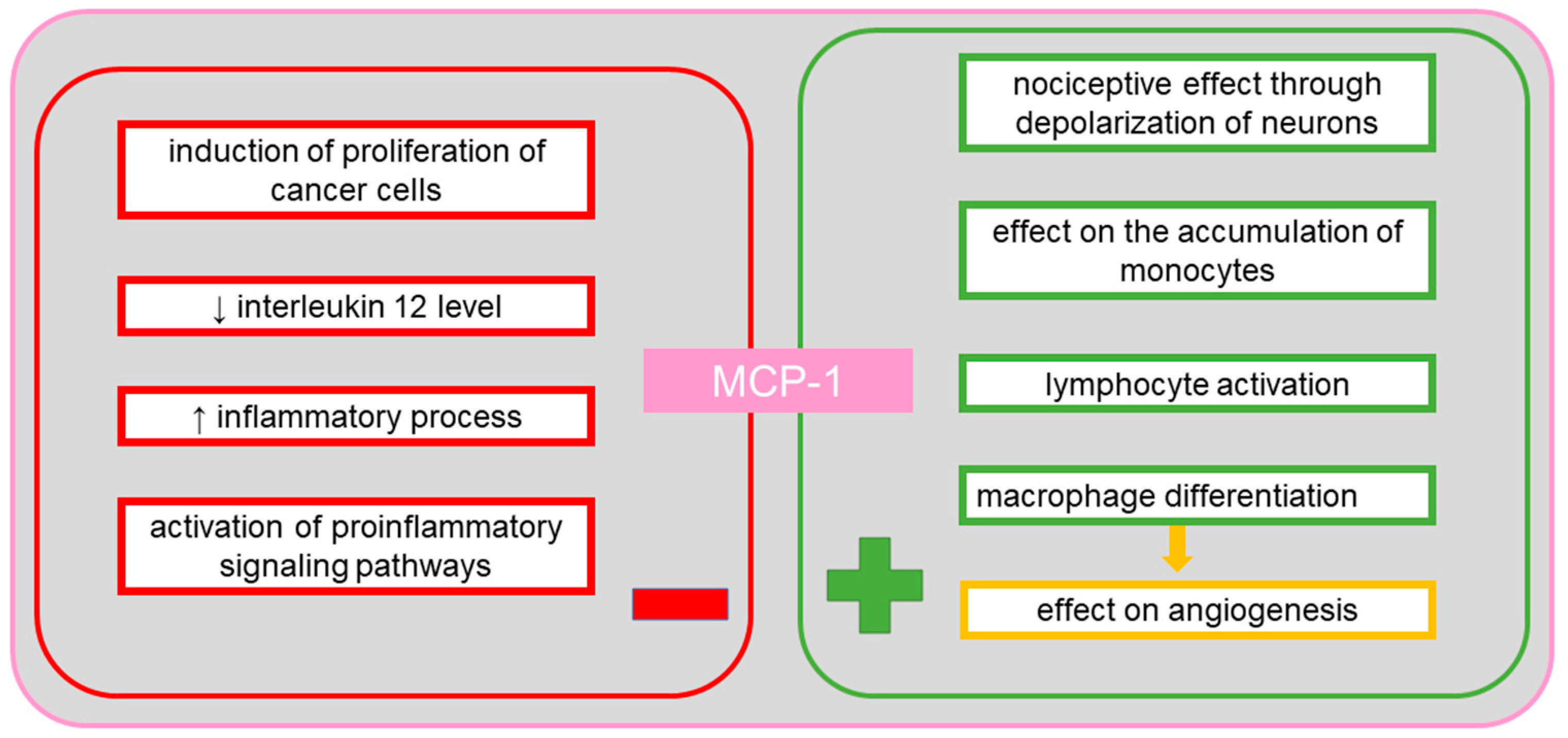
9. Monocyte Chemotactic Protein-1 (MCP-1)
10. MCP-1 in Ovarian Cancer
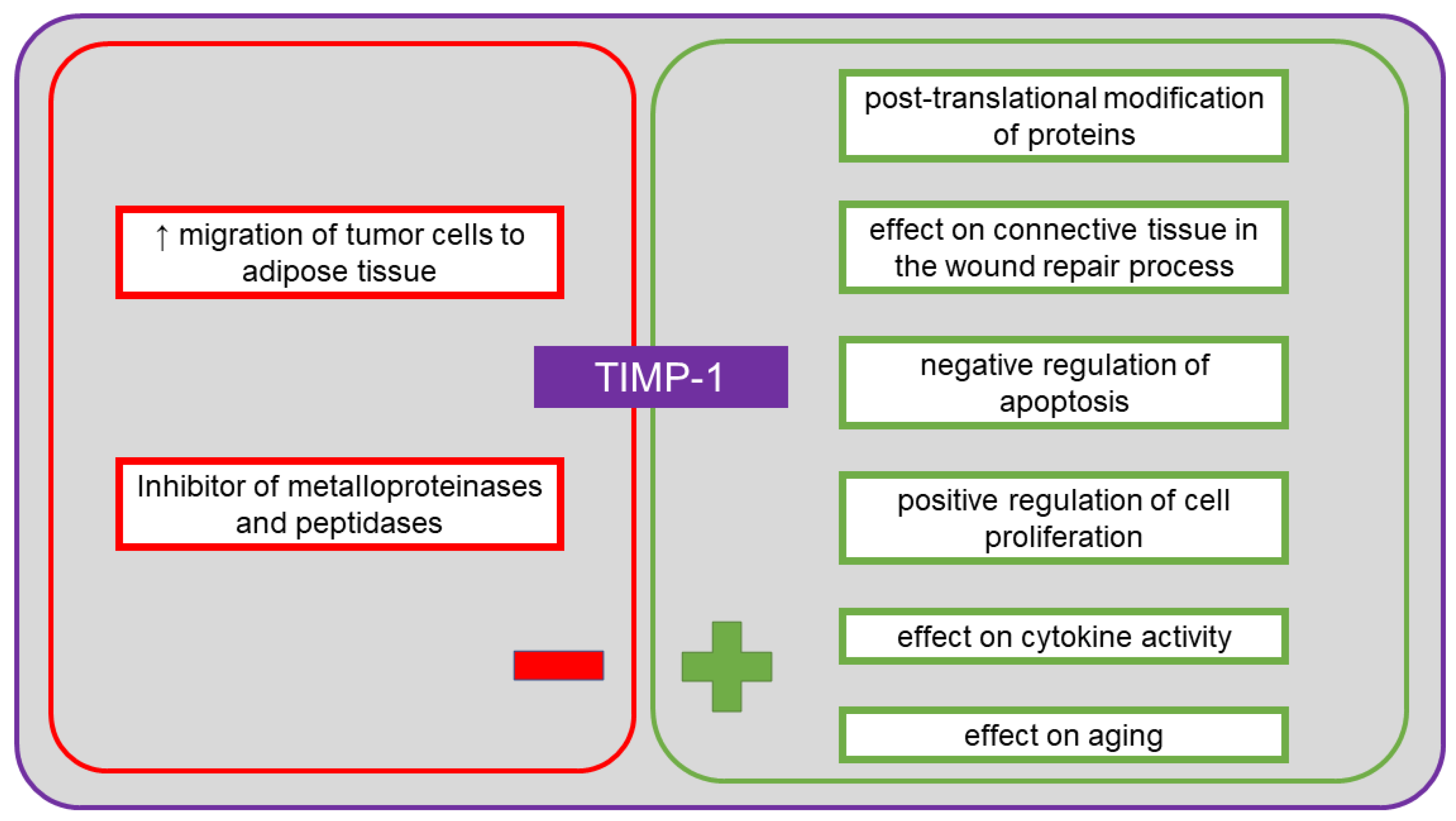
11. Tissue Inhibitor of Metalloproteinase-1 (TIMP-1)
12. TIMP-1 in Ovarian Cancer
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Burden Statistics and Trends Across Europe | European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 13 July 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Vaughan, S.; Road, C.; Ka, L.; Centre, S.; Way, R.; Coukos, G. Rethinking Ovarian Cancer: Recommendations for Improving Outcomes. Nat. Rev. Cancer 2012, 11, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-M.; Kurman, R.J. Ovarian Tumorigenesis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Prat, J. Abridged republication of FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum. Cancer 2015, 121, 3452–3454. [Google Scholar] [CrossRef] [PubMed]
- Thibault, B.; Castells, M.; Delord, J.P.; Couderc, B. Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2014, 33, 17–39. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13. [Google Scholar] [CrossRef]
- Hanash, S.M.; Pitteri, S.J.; Faca, V.M. Mining the plasma proteome for cancer biomarkers. Nature 2008, 452, 571–579. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, F.; Xu, Q.; Han, L.; Xu, J.; Gao, L.; Sun, X.; Li, Y.; Li, Y.; Qian, M.; et al. Revisiting ovarian cancer microenvironment: A friend or a foe? Protein Cell 2018, 9, 674–692. [Google Scholar] [CrossRef]
- Klymenko, Y.; Nephew, K.P. Epigenetic crosstalk between the tumor microenvironment and ovarian cancer cells: A therapeutic road less traveled. Cancers 2018, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Clinical relevance of adipokines. Diabetes Metab. J. 2012, 36, 317–327. [Google Scholar] [CrossRef]
- Terlikowska, K.; Strzyż-Skalij, M.; Kryński, K.; Osmólska, M.; Łada, Z.; Malinowska-Gleń, M.; Kryńska, E.; Terlikowski, R. Ovarian cancer and inflammation. Part 1. Pro-inflammatory cytokines. Prog. Health Sci. 2018, 8, 194–201. [Google Scholar] [CrossRef]
- Wójcik, B.; Górski, J. Brunatna tkanka tłuszczowa u dorosłego człowieka: Występowanie i funkcja (Brown adipose tissue in adult humans: Distribution and function). Endokrynol. Otyłość Zaburzenia Przemiany Mater. 2011, 7, 34–40. [Google Scholar]
- Raucci, R.; Rusolo, F.; Sharma, A.; Colonna, G.; Castello, G.; Costantini, S. Functional and structural features of adipokine family. Cytokine 2013, 61, 1–14. [Google Scholar] [CrossRef]
- El Hadi, H.; di Vincenzo, A.; Vettor, R.; Rossato, M. Food ingredients involved in white-to-brown adipose tissue conversion and in calorie burning. Front. Physiol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cint, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedzka-Rystwej, P.; Trzeciak-Ryczek, A.; Deptuła, W. Tkanka tłuszczowa i jej rola w odporności—Nowe dane. Ann. Allergy Asthma Immunol. 2012, 17, 16–21. [Google Scholar]
- Lehr, S.; Hartwig, S.; Sell, H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin. Appl. 2012, 6, 91–101. [Google Scholar] [CrossRef]
- Blüher, M. Do adipokines link obesity to its related metabolic and cardiovascular diseases? J. Clin. Lipidol. 2010, 5, 95–107. [Google Scholar] [CrossRef]
- Bays, H.E. “Sick Fat”, Metabolic Disease and Atherosclerosis. Am. J. Med. 2009, 122, S26–S37. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Słomian, G.; Nowak, D.; Buczkowska, M.; Głogowska-Gruszka, A.; Słomian, S.P.; Roczniak, W.; Janyga, S.; Nowak, P. The role of adiponectin and leptin in the treatment of ovarian cancer patients. Endokrynol. Pol. 2019. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, H.-J.; Kim, C.Y.; Kim, Y.H.; Ju, W.; Kim, S.C. Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet. Gynecol. Sci. 2016, 59, 279. [Google Scholar] [CrossRef]
- Giuntoli, R.; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-Montes, T.P.; Bristow, R.E.; Oelke, M. Ovarian Cancer-associated Ascites Demonstrates altered immune environment-2009. Anticancer Res. 2009, 29, 2875–2884. [Google Scholar] [PubMed]
- Mikuła-Pietrasik, J.; Uruski, P.; Matuszkiewicz, K.; Szubert, S.; Moszyński, R.; Szpurek, D.; Sajdak, S.; Tykarski, A.; Książek, K. Ovarian cancer-derived ascitic fluids induce a senescence-dependent pro-cancerogenic phenotype in normal peritoneal mesothelial cells. Cell. Oncol. 2016, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, W.; Wang, X. (Wang Xinbo); Wang, X. (Wang Xiaoli); Sun, H. Prognostic value of serum IL-8 and IL-10 in patients with ovarian cancer undergoing chemotherapy. Oncol. Lett. 2019, 17, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Sanguinete, M.M.M.; de Oliveira, P.H.; Martins-Filho, A.; Micheli, D.C.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol. Investig. 2017, 46, 677–688. [Google Scholar] [CrossRef]
- Sadłecki, P.; Walentowicz-Sadłecka, M.; Szymański, W.; Grabiec, M. Comparison of VEGF, IL-8 and β-FGF concentrations in the serum and ascites of patients with ovarian cancer, Porównanie stężeń VEGF, IL-8 oraz β-FGF w surowicy krwi i płynie otrzewnowym u pacjentek leczonych z powodu raka jajnika. Ginekol. Pol. 2011, 82, 498–502. [Google Scholar] [PubMed]
- Rådestad, E.; Klynning, C.; Stikvoort, A.; Mogensen, O.; Nava, S.; Magalhaes, I.; Uhlin, M. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. Oncoimmunology 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Hefler, L.; Tempfer, C.; Heinze, G.; Mayerhofer, K.; Breitenecker, G.; Leodolter, S.; Reinthaller, A.; Kainz, C. Monocyte chemoattractant protein-1 serum levels in ovarian cancer patients. Br. J. Cancer 1999, 81, 855–859. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Proost, P.; Opdenakker, G.; Laureys, G.; Verhasselt, B.; Peperstraete, L.; van de Putte, I.; Saccani, A.; Allavena, P.; et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J. Biol. Chem. 2002, 277, 24584–24593. [Google Scholar] [CrossRef]
- Mahner, S.; Woelber, L.; Eulenburg, C.; Schwarz, J.; Carney, W.; Jaenicke, F.; Milde-Langosch, K.; Mueller, V. TIMP-1 and VEGF-165 serum concentration during first-line therapy of ovarian cancer patients. BMC Cancer 2010, 10, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hao, F.; Wan, W.; Wang, X. Adiponectin exhibits proliferative and anti-apoptotic effects on ovarian cancer cells via PI3K/AKT and Raf/MEK/ERK pathways. Trop. J. Pharm. Res. 2018, 17, 2141–2149. [Google Scholar] [CrossRef]
- Lane, D.; Matte, I.; Rancourt, C.; Piché, A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 2011, 11, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Matte, I.; Garde-Granger, P.; Laplante, C.; Carignan, A.; Rancourt, C.; Piché, A. Inflammation-regulating factors in ascites as predictive biomarkers of drug resistance and progression-free survival in serous epithelial ovarian cancers. BMC Cancer 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chudecka-Głaz, A.M.; Cymbaluk-Płoska, A.A.; Menkiszak, J.L.; Pius-Sadowska, E.; Machaliński, B.B.; Sompolska-Rzechuła, A.; Rzepka-Górska, I.A. Assessment of selected cytokines, proteins, and growth factors in the peritoneal fluid of patients with ovarian cancer and benign gynecological conditions. OncoTargets Ther. 2015, 8, 471–485. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Matte, I.; Lane, D.; Laplante, C.; Rancourt, C.; Piché, A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am. J. Cancer Res. 2012, 2, 566–580. [Google Scholar] [PubMed]
- Cantón-Romero, J.C.; Miranda-Díaz, A.G.; Bañuelos-Ramírez, J.L.; Carrillo-Ibarra, S.; Sifuentes-Franco, S.; Castellanos-González, J.A.; Rodríguez-Carrizalez, A.D. Markers of Oxidative Stress and Inflammation in Ascites and Plasma in Patients with Platinum-Sensitive, Platinum-Resistant, and Platinum-Refractory Epithelial Ovarian Cancer. Oxid. Med. Cell. Longev. 2017, 2017, 2873030. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Madeddu, C.; Massa, D.; Astara, G.; Farci, D.; Melis, G.B.; Mantovani, G. Interleukin-6 and leptin as markers of energy metabolicchanges in advanced ovarian cancer patients. J. Cell. Mol. Med. 2009, 13, 3951–3959. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 13 July 2020).
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Lee, Y.H.; Magkos, F.; Mantzoros, C.S.; Kang, E.S. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism 2011, 60, 1664–1672. [Google Scholar] [CrossRef]
- Kowalska, W.; Waldowska, M.; Bojarska-Junak, A. Zróżnicowanie fenotypowe i funkcjonalne makrofagów w kontekście ich wpływu na odpowiedź przeciwnowotworową. Rev. Res. Cancer 2016, 2, 13–17. [Google Scholar]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef]
- Silveira, L.S.; Antunes, B. de M.M.; Minari, A.L.A.; dos Santos, R.V.T.; Neto, J.C.R.; Lira, F.S. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef]
- Gelsomino, L.; Naimo, G.D.; Catalano, S.; Mauro, L.; Andò, S. The emerging role of adiponectin in female malignancies. Int. J. Mol. Sci. 2019, 20, 2127. [Google Scholar] [CrossRef]
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; deFazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Fasching, P.A.; et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; Dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5.24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kontny, E.; Maśliński, W. Interleukina 6—Znaczenie biologiczne i rola w patogenezie reumatoidalnego zapalenia stawów. Reumatologia 2009, 47, 24–33. [Google Scholar]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Systemic Inflammation in Obese Humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, R.C.; Zhang, X.L.; Niu, X.L.; Qu, Y.; Li, L.Z.; Meng, X.Y. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine 2012, 59, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Katsikogianni, F.; Tsiouda, T.; Sakkas, A.; Katsikogiannis, N.; Zarogoulidis, K. Interleukin-8 and interleukin-17 for cancer. Cancer Invest. 2014, 32, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhao, Z.; Huang, L.; Wang, L.; Miao, Y.; Wu, J. IL-8 promotes cell migration through regulating EMT by activating the Wnt/β-catenin pathway in ovarian cancer. J. Cell. Mol. Med. 2020, 24, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Kapka-Skrzypczak, L.; Popek, S.; Sawicki, K.; Drop, B.; Czajka, M.; Jodłowska-Jędrych, B.; Matysiak-Kucharek, M.; Furman-Toczek, D.; Zagórska-Dziok, M.; Kruszewski, M. IL-6 prevents CXCL8-induced stimulation of EpCAM expression in ovarian cancer cells. Mol. Med. Rep. 2019, 19, 2317–2322. [Google Scholar] [CrossRef]
- Abdollahi, T.; Robertson, N.M.; Abdollahi, A.; Litwack, G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003, 63, 4521–4526. [Google Scholar]
- Farkas, B.; Boldizsar, F.; Bohonyi, N.; Farkas, N.; Marczi, S.; Kovacs, G.L.; Bodis, J.; Koppan, M. Comparative analysis of abdominal fluid cytokine levels in ovarian hyperstimulation syndrome (OHSS). J. Ovarian Res. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Gu, X.; Lu, Y.; Gu, C.; Zheng, Y.; Zhang, Z.; Chen, L.; Yao, Z.; Li, L.Y. Down-modulation of TNFSF15 in ovarian cancer by VEGF and MCP-1 is a pre-requisite for tumor neovascularization. Angiogenesis 2012, 15, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Saccani, A.; Bottazzi, B.; Bernasconi, S.; Allavena, P.; Gaetano, B.; Fei, F.; LaRosa, G.; Scotton, C.; Balkwill, F.; et al. Defective Expression of the Monocyte Chemotactic Protein-1 Receptor CCR2 in Macrophages Associated with Human Ovarian Carcinoma. J. Immunol. 2000, 164, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Gaiotte, L.B.; Cesário, R.C.; Seiva, F.R.F.; de Almeida Chuffa, L.G. The role of Toll-like receptor 4 signaling pathway in ovarian, cervical, and endometrial cancers. Life Sci. 2020, 247, 117435–117444. [Google Scholar] [CrossRef]
- Furukawa, S.; Soeda, S.; Kiko, Y.; Suzuki, O.; Hashimoto, Y.; Watanabe, T.; Nishiyama, H.; Tasaki, K.; Hojo, H.; Abe, M.; et al. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res. 2013, 33, 4785–4790. [Google Scholar]
- Alexander, E.T.; Minton, A.R.; Peters, M.C.; van Ryn, J.; Gilmour, S.K. Thrombin inhibition and cisplatin block tumor progression in ovarian cancer by alleviating the immunosuppressive microenvironment. Oncotarget 2016, 7, 85291–85305. [Google Scholar] [CrossRef] [PubMed]
- Negus, R.P.M.; Stamp, G.W.H.; Relf, M.G.; Burke, F.; Malik, S.T.A.; Bernasconi, S.; Allavena, P.; Sozzani, S.; Mantovani, A.; Balkwill, F.R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J. Clin. Investig. 1995, 95, 2391–2396. [Google Scholar] [CrossRef]
- Furuya, M. Ovarian cancer stroma: Pathophysiology and the roles in cancer development. Cancers 2012, 4, 701–724. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q. Relationship between matrix metalloproteinases and the occurrence and development of ovarian cancer. Braz. J. Med. Biol. Res. 2017, 50, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Escalona, R.M.; Chan, E.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. The many facets of metzincins and their endogenous inhibitors: Perspectives on ovarian cancer progression. Int. J. Mol. Sci. 2018, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Ellerbroek, S.M.; Hudson, L.G.; Stack, M.S. Proteinase requirements of epidermal growth factor–induced ovarian cancer cell invasion. Int. J. Cancer 1998, 78, 331–337. [Google Scholar] [CrossRef]
- Sengupta, S.; Kim, K.S.; Berk, M.P.; Oates, R.; Escobar, P.; Belinson, J.; Li, W.; Lindner, D.J.; Williams, B.; Xu, Y. Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene 2007, 26, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Sonego, M.; Poletto, E.; Pivetta, E.; Nicoloso, M.S.; Pellicani, R.; Vinciguerra, G.L.R.; Citron, F.; Sorio, R.; Mongiat, M.; Baldassarre, G. TIMP-1 Is Overexpressed and Secreted by Platinum Resistant Epithelial Ovarian Cancer Cells. Cells 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Shigemasa, K.; Nagai, N.; Ohama, K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol. 2000, 17, 673–681. [Google Scholar] [CrossRef]
- Huang, L.W.; Garrett, A.P.; Bell, D.A.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C. Differential expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 protein and mRNA in epithelial ovarian tumors. Gynecol. Oncol. 2000, 77, 369–376. [Google Scholar] [CrossRef]
- Kikkawa, F.; Tamakoshi, K.; Nawa, A.; Shibata, K.; Yamagata, S.; Yamagata, T.; Suganuma, N. Positive correlation between inhibitors of matrix metalloproteinase 1 and matrix metalloproteinases in malignant ovarian tumor tissues. Cancer Lett. 1997, 120, 109–115. [Google Scholar] [CrossRef]
- Finkernagel, F.; Reinartz, S.; Schuldner, M.; Malz, A.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Graumann, J.; Pogge von Strandmann, E.; Müller, R. Dual-platform affinity proteomics identifies links between the recurrence of ovarian carcinoma and proteins released into the tumor microenvironment. Theranostics 2019, 9, 6601–6617. [Google Scholar] [CrossRef]
- Živadinović, R.; Petrić, A.; Krtinić, D.; Dinić, S.P.-T.; Živadinović, B. Ascitic Fluid in Ovarian Carcinoma—From Pathophysiology to the Treatment. In Ascites—Physiopathology, Treatment, Complications and Prognosis; IntechOpen: London, UK, 2017; pp. 47–75. [Google Scholar]
- Brun, J.L.; Cortez, A.; Lesieur, B.; Uzan, S.; Rouzier, R.; Daraï, E. Expression of MMP-2,-7,-9, MT1-MMP and TIMP-1 and -2 has no prognostic relevance in patients with advanced epithelial ovarian cancer. Oncol. Rep. 2012, 27, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Journal | No of Cases | Adipokine |
|---|---|---|---|---|
| Słomian et al. [22] | 2019 | Endokrynol. Pol. | 43 | Adiponectin in serum |
| Jin et al. [23] | 2016 | Obstet. Gynecol. Sci. | 52 | Adiponectin in plasma |
| Feng et al. [33] | 2018 | Trop. J. Pharm. Res. | 46 | Adiponectin in serum |
| Adiponectin in ascites | ||||
| Lane et al. [34] | 2011 | BMC Cancer | 39 | IL-6 in ascites |
| Lane at al. [35] | 2015 | BMC Cancer | 53 | IL-6 in ascites |
| Chudecka-Głaz et al. [36] | 2015 | Onco. Targets. Ther. | 36 | IL-6 in ascites |
| Kampan et al. [37] | 2020 | Sci. Rep. | 18 | IL-6 in ascites |
| 51 | IL-6 in serum | |||
| Matte et al. [38] | 2012 | Am. J. Cancer Res. | 38 | IL-6 in ascites |
| Canton-Romero et al. [39] | 2017 | Oxid. Med. Cell. Longev. | 21 | IL-6 in ascites |
| Maccio et al. [40] | 2009 | J. Cell. Mol. Med. | 104 | IL-6 in plasma |
| Giuntoli et al. [24] | 2009 | Anticancer. Res. | 37 | IL-6 in ascites |
| 33 | IL-6 in serum | |||
| Mikuła-Pietrasik et al. [25] | 2016 | Cell. Oncol. | 16 | IL-8 in ascites |
| 8 | MCP-1 in ascites | |||
| Zhang et al. [26] | 2019 | Oncol. Lett. | 92 | IL-8 in serum |
| Sanguinete et al. [27] | 2017 | Immunol. Invest. | 26 | IL-8 in serum |
| Sadłecki et al. [28] | 2011 | Ginekol. Pol. | 21 | IL-8 in ascites |
| 21 | IL-8 in serum | |||
| 8 | MCP-1 in ascites | |||
| Radestad et al. [29] | 2019 | Oncoimmunology | 35 | IL-8 in ascites |
| 35 | IL-8 in serum | |||
| Hefler et al. [30] | 1999 | Br. J. Cancer | 86 | MCP-1 in serum |
| Schutyser et al. [31] | 2002 | J. Biol. Chem. | 12 | MCP-1 in ascites |
| Mahner et al. [32] | 2010 | BMC Cancer | 37 | TIMP-1 in serum |
| Disease | Concentration [ng/mL] | No. of Cases | Ref. |
|---|---|---|---|
| Ovarian cancer prior to chemotherapy | 8830.00 ± 3190.00 | 43 | [22] |
| Ovarian cancer after chemotherapy | 10,370.00 ± 4180.00 | ||
| Ovarian cancer | 8250.00 ± 970.00 | 52 | [23] |
| Control group—no ovary conditions | 11,440.00 ± 1130.00 | 18 | |
| FIGO I-IV ovarian cancer | range (~3900.00 ± 400.00 to ~4800.00 ± 700.00) | 46 | [33] |
| Control group—no ovary conditions | ~3400.00 ± 300.00 | 17 |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| Ovarian cancer | 6419.00 ± 1409.00 | 39 | [34] |
| FIGO III/IV ovarian cancer | 1820.00 (range 279.00–4327.00) | 53 | [35] |
| Control group—benign gynecological conditions | 15.00 (range 6.00–65.00) | 10 | |
| Malignant ovarian cancer | 379.00 (range 9.70–528.10) | 36 | [36] |
| Control group—benign gynecological conditions | 137.80 (range 0.08–528.10) | 38 | |
| Advanced ovarian cancer | 18,050.00 (range 5162.00–122,883.00) | 18 | [37] |
| FIGO III/IV ovarian cancer | 3089.00 (range 0.00–31,142.00) | 26 | [38] |
| FIGO I/II ovarian cancer | 455.00 (range 0.00–29,669.00) | 12 | |
| Platinum-refractory FIGO III–IV ovarian cancer | 1382.30 ± 257.60 | 7 | [39] |
| Platinum-resistant FIGO III–IV ovarian cancer | 969.60 ± 76.30 | 4 | |
| Platinum-sensitive FIGO I–IV ovarian cancer | 1582.00 ± 346.10 | 10 | |
| FIGO III/IV ovarian cancer | 14,845.28 ± 4658.05 | 37 | [24] |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| Advanced ovarian cancer | 28.30 | 33 | [37] |
| Ovarian cancer with accompanying ascites | 53.00 (range 11.20–216.00) | 18 | |
| Control group—benign gynecological conditions | 7.40 | 12 | |
| Control group—no ovary conditions | 1.20 | 21 | |
| FIGO III/IV ovarian cancer | 29.97 ± 5.14 | 33 | [24] |
| Platinum-refractory FIGO III–IV ovarian cancer | 396.60 ± 105.00 | 7 | [39] |
| Platinum-resistant FIGO III–IV ovarian cancer | 834.20 ± 31.00 | 4 | |
| Platinum-sensitive FIGO I–IV ovarian cancer | 936.40 ± 284.60 | 10 | |
| Control group—no ovary conditions | 448.34 ± 28.00 | 6 | |
| FIGO III/IV ovarian cancer | 18.60 ± 18.70 | 75 | [40] |
| FIGO I/II ovarian cancer | 8.10 ± 10.40 | 29 | |
| Control group—no ovary conditions | 3.00 ± 3.20 | 95 |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| Ovarian cancer | 396.60 * | 21 | [28] |
| FIGO III and IV ovarian cancer | 231.30 (74.70–6669.30) * | 35 | [29] |
| FIGO IIIC and IV ovarian cancer | 536.00 ± 82.00 # | 8 | [25] |
| Benign gynecological conditions | 450.00 ± 72.00 # | 8 |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| FIGO IIIC ovarian cancer | 233.35 (38.60–1222.50) * | 5 | [27] |
| FIGO I–IIIB ovarian cancer | 95.20 (12.40–1222.50) * | 21 | |
| FIGO III–IV ovarian cancer | 228.41 ± 6.79 | 72 | [26] |
| FIGO I–II ovarian cancer | 181.70 ± 13.54 # | 20 | |
| Benign gynecological conditions | 79.68 ± 9.53 # | 64 | |
| Control group—no ovary conditions | 54.31 ± 10.26 # | 58 | |
| Ovarian cancer | 22.70 * | 21 | [28] |
| FIGO III–IV ovarian cancer | 101.70 (10.70–614.20) * | 35 | [29] |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| FIGO IIIC and IV ovarian cancer | 330.00 ± 25.00 * | 8 | [25] |
| Benign gynecological conditions | 325.00 ± 5.00 * | 8 | |
| FIGO IC and IV ovarian cancer | 3200.00 ± 400.00 $ | 12 | [31] |
| Non-gynecological cancers and benign ovary conditions | 3300.00 ± 1000.00 $ | 12 |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| FIGO I–III ovarian cancer | 535.60 (129.60–1200.00) * | 48 | [30] |
| Relapsing ovarian cancer | 427.30 (193.40–1101.00) * | 38 | |
| Benign gynecological conditions | 371.20 (222.00–986.80) * | 67 | |
| Control group of healthy women | 318.70 (241.30–681.40) | 45 |
| Disease | Concentration [pg/mL] | No. of Cases | Ref. |
|---|---|---|---|
| FIGO I–IV ovarian cancer before cytoreductive procedure | 403.00 (273.00–887.00) * | 37 | [32] |
| FIGO I–IV ovarian cancer after cytoreductive procedure | 529.00 (323.00–1000.00) * | ||
| FIGO I–IV ovarian cancer during chemotherapy | 351.00 (204.00–616.00) * | ||
| FIGO I–IV ovarian cancer after chemotherapy | 333.00 (209.00–990.00) * |
| Adipokine | Ovarian Cancer | |
|---|---|---|
| Blood | Ascites | |
| Adiponectin | ↓ or ↑ | N/A |
| Interleukin 6 (IL-6) | ↑ | ↑↑ |
| Interleukin 8 (IL-8) | ↑ | ↑ |
| Monocyte chemotactic protein-1 (MCP-1) | ↑ | ↑ # |
| Tissue inhibitor of metalloproteinase-1 (TIMP-1) | ↑ * | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewski, M.; Szewczyk-Golec, K.; Hołyńska-Iwan, I.; Wróblewska, J.; Woźniak, A. Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients. Cancers 2021, 13, 4702. https://doi.org/10.3390/cancers13184702
Wróblewski M, Szewczyk-Golec K, Hołyńska-Iwan I, Wróblewska J, Woźniak A. Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients. Cancers. 2021; 13(18):4702. https://doi.org/10.3390/cancers13184702
Chicago/Turabian StyleWróblewski, Marcin, Karolina Szewczyk-Golec, Iga Hołyńska-Iwan, Joanna Wróblewska, and Alina Woźniak. 2021. "Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients" Cancers 13, no. 18: 4702. https://doi.org/10.3390/cancers13184702
APA StyleWróblewski, M., Szewczyk-Golec, K., Hołyńska-Iwan, I., Wróblewska, J., & Woźniak, A. (2021). Characteristics of Selected Adipokines in Ascites and Blood of Ovarian Cancer Patients. Cancers, 13(18), 4702. https://doi.org/10.3390/cancers13184702







