Clinical Utility and Limitation of Diagnostic Ability for Different Degrees of Dysplasia of Intraductal Papillary Mucinous Neoplasms of the Pancreas Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
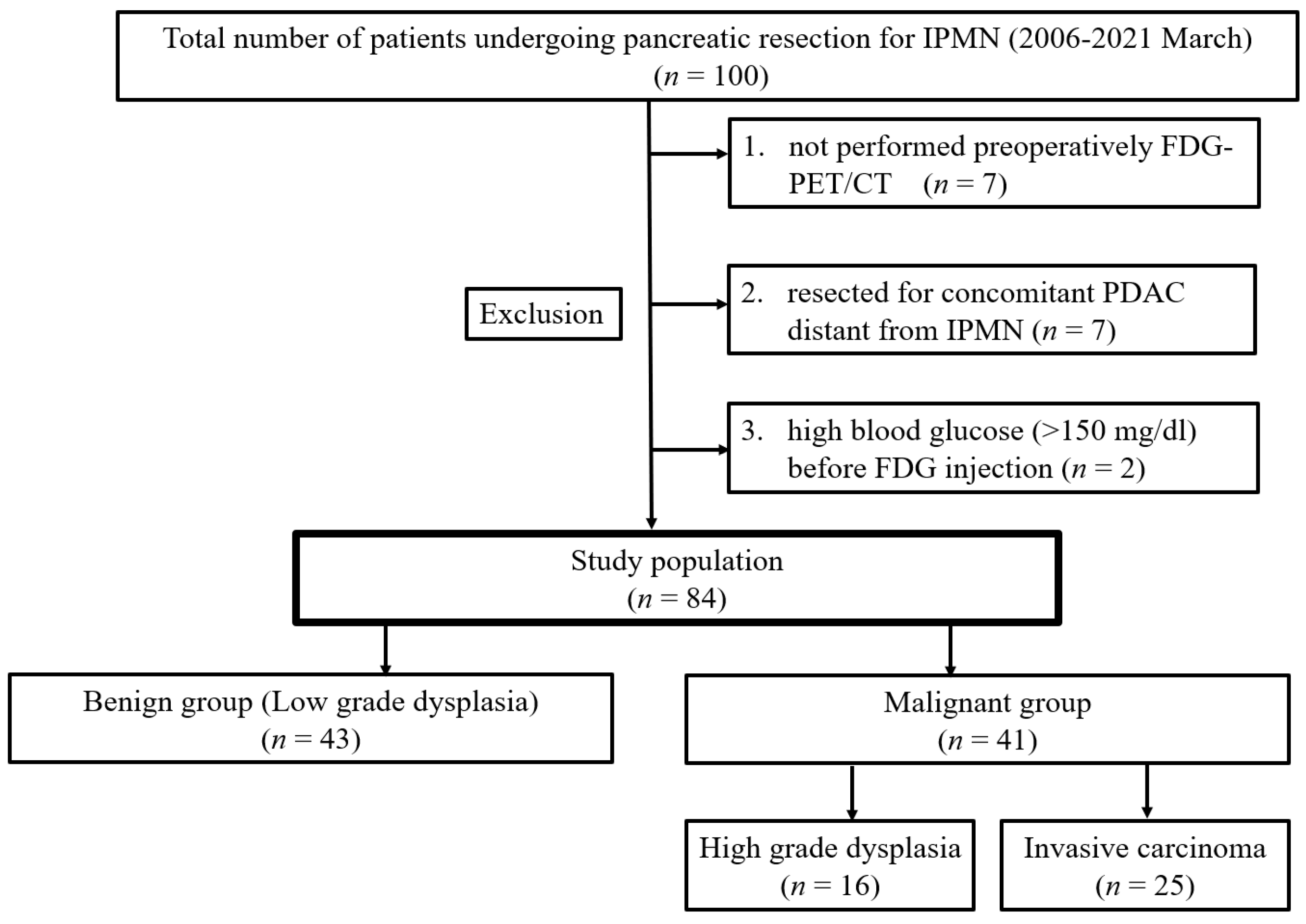
2.1. Study Design and Patient Population
2.2. Surgical Indications
2.3. Imaging Modalities Protocol
2.4. Evaluation of Mural Nodule Height
2.5. Histopathological Diagnosis
2.6. Immunohistochemistry (IHC) and Evaluation of IHC
2.7. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of the Patients with IPMN
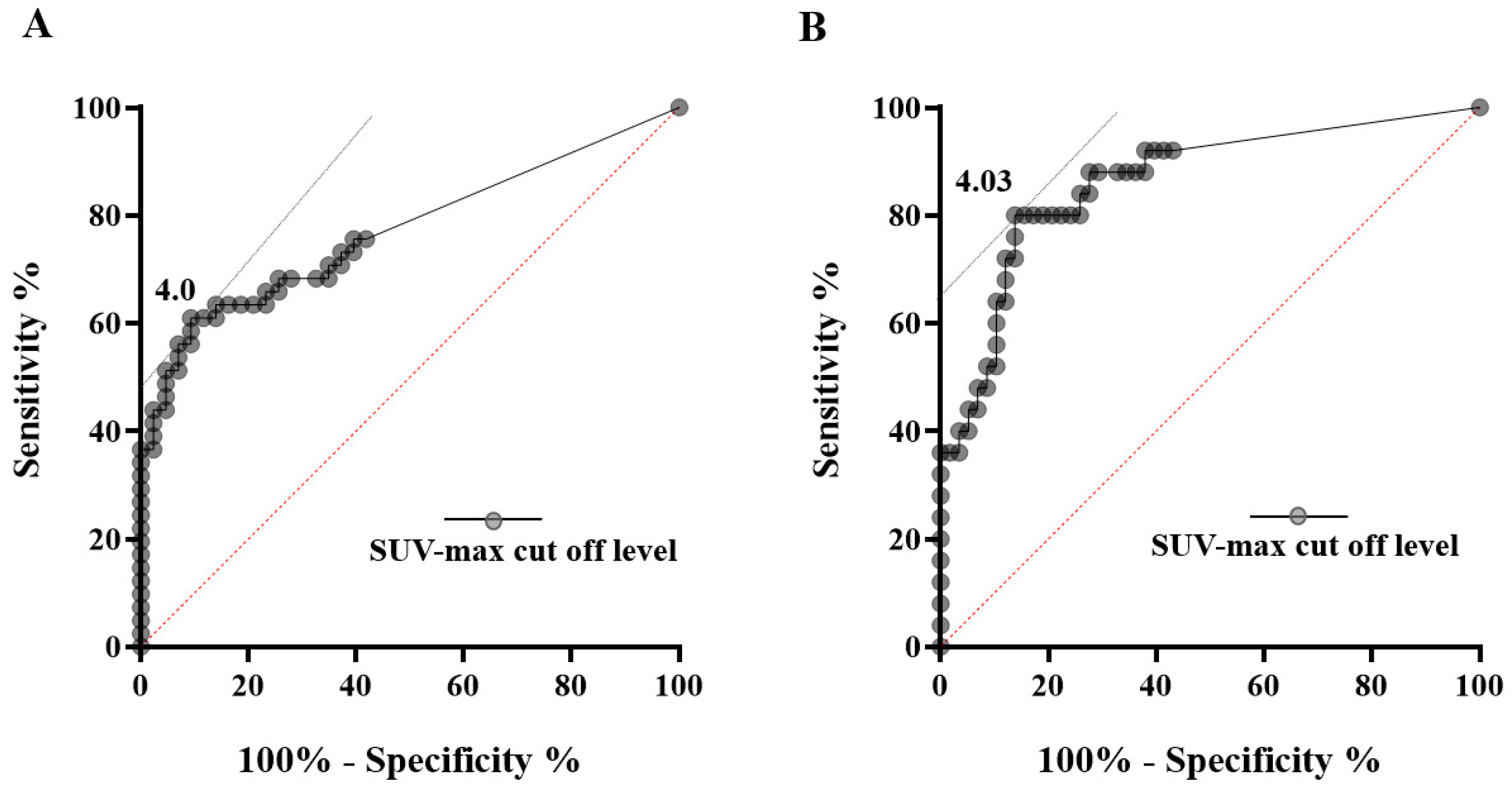
3.2. Malignancy Predictive Ability of 18F-Fluorodeoxyglucose-PET/CT and Mural Nodule Height for Malignant IPMN
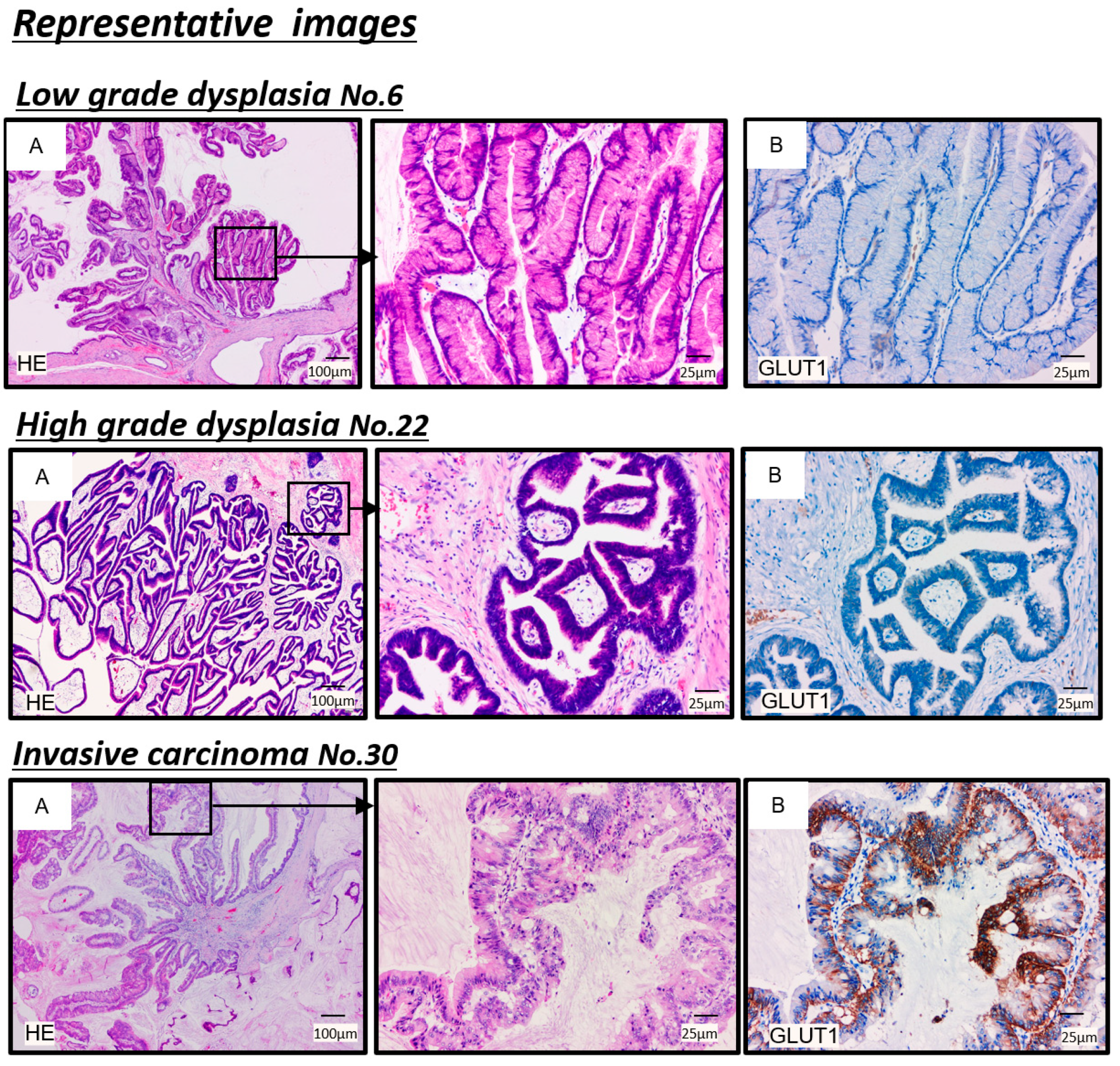
3.3. Relationship between 18F-Fluorodeoxyglucose Uptake and Glucose Transporter 1 (GLUT-1) Expression in Immunohistochemistry for IPMNs
3.4. Relationship between Mural Nodules and Preoperative Imaging Modality including FDG-PET/CT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Takaori, K.; Klimstra, D.S.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Biankin, S.A.; Compton, C.; Fukushima, N.; Furukawa, T.; et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 2004, 28, 977–987. [Google Scholar] [CrossRef]
- Maitra, A.; Fukushima, N.; Takaori, K.; Hruban, R.H. Precursors to invasive pancreatic cancer. Adv. Anat. Pathol. 2005, 12, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Nakaizumi, A.; Ishikawa, O.; Iishi, H.; Tatsumi, K.; Takakura, R.; Ishida, T.; Takano, Y.; Tanaka, S.; Takenaka, A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008, 57, 1561–1565. [Google Scholar] [CrossRef]
- Maire, F.; Hammel, P.; Terris, B.; Paye, F.; Scoazec, J.Y.; Cellier, C.; Barthet, M.; O’Toole, D.; Rufat, P.; Partensky, C.; et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut 2002, 51, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Hirakawa, A.; Takami, H.; Suenaga, M.; Hayashi, M.; Niwa, Y.; Hattori, N.; Iwata, N.; Kanda, M.; et al. Comparison of the Survival Outcomes of Pancreatic Cancer and Intraductal Papillary Mucinous Neoplasms. Pancreas 2018, 47, 974–979. [Google Scholar] [CrossRef]
- Izumo, W.; Higuchi, R.; Furukawa, T.; Yazawa, T.; Uemura, S.; Matsunaga, Y.; Shiihara, M.; Yamamoto, M. Comparison of patients with invasive intraductal papillary mucinous carcinoma and invasive ductal carcinoma of the pancreas: A pathological type- and stage-matched analysis. Scand. J. Gastroenterol. 2019, 54, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Riall, T.S.; Coleman, J.; Belcher, K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006, 244, 10–15. [Google Scholar] [CrossRef]
- Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Arnold, M.A.; Chang, D.C.; Coleman, J.; Hodgin, M.B.; Sauter, P.K.; Hruban, R.H.; Riall, T.S.; et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006, 10, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Wilson, C.B. PET scanning in oncology. Eur. J. Cancer 1992, 28, 508–510. [Google Scholar] [CrossRef]
- Pakzad, F.; Groves, A.M.; Ell, P.J. The role of positron emission tomography in the management of pancreatic cancer. Semin. Nucl. Med. 2006, 36, 248–256. [Google Scholar] [CrossRef]
- Engledow, A.H.; Skipworth, J.R.; Pakzad, F.; Imber, C.; Ell, P.J.; Groves, A.M. The role of 18FDG PET/CT in the management of colorectal liver metastases. HPB 2012, 14, 20–25. [Google Scholar] [CrossRef][Green Version]
- Kitajima, K.; Kono, A.; Konishi, J.; Suenaga, Y.; Takahashi, S.; Sugimura, K. ¹⁸F-FDG-PET/CT findings of retroperitoneal tumors: A pictorial essay. Jpn. J. Radiol. 2013, 31, 301–309. [Google Scholar] [CrossRef]
- Kurahara, H.; Maemura, K.; Mataki, Y.; Sakoda, M.; Iino, S.; Kawasaki, Y.; Arigami, T.; Mori, S.; Kijima, Y.; Ueno, S.; et al. Significance of (18)F-Fluorodeoxyglucose (FDG) Uptake in Response to Chemoradiotherapy for Pancreatic Cancer. Ann. Surg. Oncol. 2019, 26, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; McCook, B.M.; Torok, F.S. An introduction to PET-CT imaging. Radiographics 2004, 24, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Koh, Y.X.; Goh, B.K.P. Systematic review of the utility of 18-FDG PET in the preoperative evaluation of IPMNs and cystic lesions of the pancreas. Surgery 2019, 165, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Bertagna, F.; Gheza, F.; Grazioli, L.; Calanducci, D.; Giubbini, R.; Portolani, N.; Giulini, S.M. Searching for indicators of malignancy in pancreatic intraductal papillary mucinous neoplasms: The value of 18FDG-PET confirmed. Ann. Surg. Oncol. 2012, 19, 3574–3580. [Google Scholar] [CrossRef]
- Pedrazzoli, S.; Sperti, C.; Pasquali, C.; Bissoli, S.; Chierichetti, F. Comparison of International Consensus Guidelines versus 18-FDG PET in detecting malignancy of intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. 2011, 254, 971–976. [Google Scholar] [CrossRef]
- Hata, T.; Mizuma, M.; Motoi, F.; Ishida, M.; Morikawa, T.; Nakagawa, K.; Hayashi, H.; Kanno, A.; Masamune, A.; Kamei, T.; et al. An integrated analysis of host- and tumor-derived markers for predicting high-grade dysplasia and associated invasive carcinoma of intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 2020, 50, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Tanada, M.; Sugawara, Y.; Teramoto, N.; Iguchi, H. Usefulness of positron emission tomography (PET)/contrast-enhanced computed tomography (CE-CT) in discriminating between malignant and benign intraductal papillary mucinous neoplasms (IPMNs). Pancreatology 2017, 17, 911–919. [Google Scholar] [CrossRef]
- Tomimaru, Y.; Takeda, Y.; Tatsumi, M.; Kim, T.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Kitagawa, T.; Nagano, H.; et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol. Rep. 2010, 24, 613–620. [Google Scholar] [PubMed]
- Saito, M.; Ishihara, T.; Tada, M.; Tsuyuguchi, T.; Mikata, R.; Sakai, Y.; Tawada, K.; Sugiyama, H.; Kurosawa, J.; Otsuka, M.; et al. Use of F-18 fluorodeoxyglucose positron emission tomography with dual-phase imaging to identify intraductal papillary mucinous neoplasm. Clin. Gastroenterol. Hepatol. 2013, 11, 181–186. [Google Scholar] [CrossRef]
- Kita, Y.; Okumura, H.; Uchikado, Y.; Sasaki, K.; Omoto, I.; Matsumoto, M.; Setoyama, T.; Tanoue, K.; Mori, S.; Owaki, T.; et al. Clinical significance of ¹⁸F-fluorodeoxyglucose positron emission tomography in superficial esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2013, 20, 1646–1652. [Google Scholar] [CrossRef]
- Izumo, W.; Higuchi, R.; Furukawa, T.; Yazawa, T.; Uemura, S.; Shiihara, M.; Yamamoto, M. Importance of each high-risk stigmata and worrisome features as a predictor of high-grade dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2020, 20, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Sugimori, K.; Shimamura, T.; Hosono, K.; Watanabe, S.; Kato, S.; Ueda, M.; Endo, I.; Inayama, Y.; Maeda, S.; et al. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 2012, 12, 141–145. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Kono, H.; Nagayoshi, Y.; Mori, Y.; Tsutsumi, K.; Sadakari, Y.; Takahata, S.; Morimatsu, K.; Aishima, S.; Igarashi, H.; et al. An increase in the number of predictive factors augments the likelihood of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas. Surgery 2012, 151, 76–83. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Do, R.; Lafemina, J.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Kingham, T.P.; Brennan, M.F.; Jarnagin, W.R.; et al. Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: Development of a preoperative nomogram. Ann. Surg. Oncol. 2013, 20, 4348–4355. [Google Scholar] [CrossRef]
- Jang, J.Y.; Park, T.; Lee, S.; Kim, Y.; Lee, S.Y.; Kim, S.W.; Kim, S.C.; Song, K.B.; Yamamoto, M.; Hatori, T.; et al. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2017, 266, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237.e225. [Google Scholar] [CrossRef]
- Serafini, S.; Sperti, C.; Brazzale, A.R.; Cecchin, D.; Zucchetta, P.; Pierobon, E.S.; Ponzoni, A.; Valmasoni, M.; Moletta, L. The Role of Positron Emission Tomography in Clinical Management of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Cancers 2020, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. [(18)F]Fluoro-2-deoxy-2-D-glucose. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Kaida, H.; Azuma, K.; Kawahara, A.; Yasunaga, M.; Kitasato, Y.; Hattori, S.; Taira, T.; Ureshino, H.; Kage, M.; Ishii, K.; et al. The correlation between FDG uptake and biological molecular markers in pancreatic cancer patients. Eur. J. Radiol. 2016, 85, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Yoo, I.R.; Kim, T.J.; Lee, K.Y.; Kim, Y.K. Is the Glut expression related to FDG uptake in PET/CT of non-small cell lung cancer patients? Technol. Health Care. 2015, 23 (Suppl. 2), S311–S318. [Google Scholar] [CrossRef]
- Hirashita, T.; Hirashita, Y.; Iwashita, Y.; Endo, Y.; Kiyonaga, M.; Matsumoto, S.; Hijiya, N.; Moriyama, M.; Murakami, K.; Inomata, M. S6 ribosomal protein phosphorylation is associated with malignancy of intraductal papillary mucinous neoplasm of the pancreas. Ann. Gastroenterol. Surg. 2020, 4, 571–579. [Google Scholar] [CrossRef]
- Oda, Y.; Aishima, S.; Shindo, K.; Fujino, M.; Mizuuchi, Y.; Hattori, M.; Miyazaki, T.; Tanaka, M.; Oda, Y. SLC2A1/GLUT1 expression in mural nodules of intraductal papillary mucinous neoplasm of the pancreas. Hum. Pathol. 2017, 65, 71–78. [Google Scholar] [CrossRef]
- Schmidt, C.M.; White, P.B.; Waters, J.A.; Yiannoutsos, C.T.; Cummings, O.W.; Baker, M.; Howard, T.J.; Zyromski, N.J.; Nakeeb, A.; DeWitt, J.M.; et al. Intraductal papillary mucinous neoplasms: Predictors of malignant and invasive pathology. Ann. Surg. 2007, 246, 644–651. [Google Scholar] [CrossRef]
- Uehara, H.; Ishikawa, O.; Katayama, K.; Kawada, N.; Ikezawa, K.; Fukutake, N.; Takakura, R.; Takano, Y.; Tanaka, S.; Takenaka, A. Size of mural nodule as an indicator of surgery for branch duct intraductal papillary mucinous neoplasm of the pancreas during follow-up. J. Gastroenterol. 2011, 46, 657–663. [Google Scholar] [CrossRef]
- Akita, H.; Takeda, Y.; Hoshino, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Tanemura, M.; Mori, M.; Doki, Y.; et al. Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am. J. Surg. 2011, 202, 214–219. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Toste, P.A.; Farrell, J.J.; Clerkin, B.M.; Williams, J.; Muthusamy, V.R.; Watson, R.R.; Tomlinson, J.S.; Hines, O.J.; Reber, H.A.; et al. Current recommendations for surveillance and surgery of intraductal papillary mucinous neoplasms may overlook some patients with cancer. J. Gastrointest. Surg. 2015, 19, 258–265. [Google Scholar] [CrossRef]
- Soret, M.; Bacharach, S.L.; Buvat, I. Partial-volume effect in PET tumor imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef]
- Takanami, K.; Hiraide, T.; Tsuda, M.; Nakamura, Y.; Kaneta, T.; Takase, K.; Fukuda, H.; Takahashi, S. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann. Nucl. Med. 2011, 25, 501–510. [Google Scholar] [CrossRef]
- Kawada, N.; Uehara, H.; Nagata, S.; Tsuchishima, M.; Tsutsumi, M.; Tomita, Y. Mural nodule of 10 mm or larger as predictor of malignancy for intraductal papillary mucinous neoplasm of the pancreas: Pathological and radiological evaluations. Pancreatology 2016, 16, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Hijioka, S.; Hirono, S.; Kin, T.; Ohtsuka, T.; Kanno, A.; Koshita, S.; Hanada, K.; Kitano, M.; Inoue, H.; et al. New Model for Predicting Malignancy in Patients With Intraductal Papillary Mucinous Neoplasm. Ann. Surg. 2020, 272, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, H.; Hijioka, S.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Usefulness of septal thickness measurement on endoscopic ultrasound as a predictor of malignancy of branched-duct and mixed-type intraductal papillary mucinous neoplasm of the pancreas. Dig. Endosc. 2019, 31, 672–681. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Value |
|---|---|
| Age, y, median (range) | 70 (35–87) |
| Sex, male, n (%) | 56 (66.6) |
| Symptoms, presence, n (%) | 28 (33.3) |
| History of pancreatitis, presence, n (%) | 8 (9.5) |
| Family history of pancreatic cancer, n (%) | 8 (9.5) |
| Coexistence of diabetes mellitus | 23 (27.4) |
| Morphological subtype, n (%) | |
| Main duct type | 9 (10.7) |
| Branch type | 33 (39.3) |
| Mixed type | 42 (50.0) |
| Location of IPMN, n (%) | |
| Head (including uncus) | 49 (58.3) |
| Distal (left from SMV) | 23 (27.4) |
| Multifocal | 12 (14.3) |
| Histopathological diagnosis, n (%) | |
| Low grade dysplasia | 43 (51.2) |
| High grade dysplasia | 16 (19.0) |
| Invasive carcinoma | 25 (29.8) |
| Characteristic | Benign * (n = 43) | Malignant † (n = 41) | p-Value |
|---|---|---|---|
| Clinical factor | |||
| Age, year, median (range) | 70 (44–80) | 70 (35–87) | 0.4439 |
| Sex, male, n (%) | 34 (79.0) | 22 (53.6) | 0.0128 |
| Symptoms, presence, n (%) | 7 (16.2) | 21 (51.2) | 0.0006 |
| Coexistence of diabetes mellitus, n (%) | 8 (18.6) | 15 (36.6) | 0.0633 |
| Concomitant pancreatitis, n (%) | 4 (9.3) | 4 (9.8) | 0.9449 |
| Family history of pancreatic cancer, n (%) | 4 (9.3) | 3 (7.3) | 0.7416 |
| Laboratory factor | |||
| Serum CEA, high (≥ 3.2 IU/L) | 10 (23.2) | 18 (43.9) | 0.0438 |
| Serum CA 19-9, high (≥ 37 IU/L) | 2 (4.6) | 10 (24.3) | 0.0074 |
| Serum P-AMY, high (≥ 50 IU/L) | 11 (25.5) | 8 (19.5) | 0.5055 |
| Imaging factor | |||
| Morphological subtype, (MD/BD/mixed), n | 1/16/24 | 8/15/18 | 0.0559 |
| Main pancreatic duct size, mean ± SD | 6.51 ± 3.90 | 8.63 ± 5.74 | 0.0484 |
| Main pancreatic duct size, (≥ 10 mm), n (%) | 7 (16.3) | 17 (41.4) | 0.0210 |
| Cystic size, mean ± SD | 34.7 ± 14.1 | 39.8 ± 23.6 | 0.2194 |
| Enhancing mural nodule height ‡, mean ± SD | 3.67 ± 6.01 | 14.6 ± 12.4 | <0.0001 |
| Enhancing mural nodule height ‡, (≥ 5 mm), n (%) | 12 (27.9) | 29 (70.7) | <0.0001 |
| Cyst growth rate ≥ 5 mm/2 years, n (%) | 3 (7.0) | 2 (4.9) | 0.6833 |
| Abrupt change in caliber of pancreatic duct with distal pancreatic atrophy, n (%) | 1 (2.3) | 1 (2.4) | 0.9728 |
| Thickened/enhancing cyst walls, n (%) | 12 (27.9) | 2 (4.9) | 0.0164 |
| Lymphadenopathy, n (%) | 1 (2.3) | 1 (2.3) | 0.9728 |
| FDG uptake, positive, n (%) | 16 (37.2) | 28 (68.2) | 0.0040 |
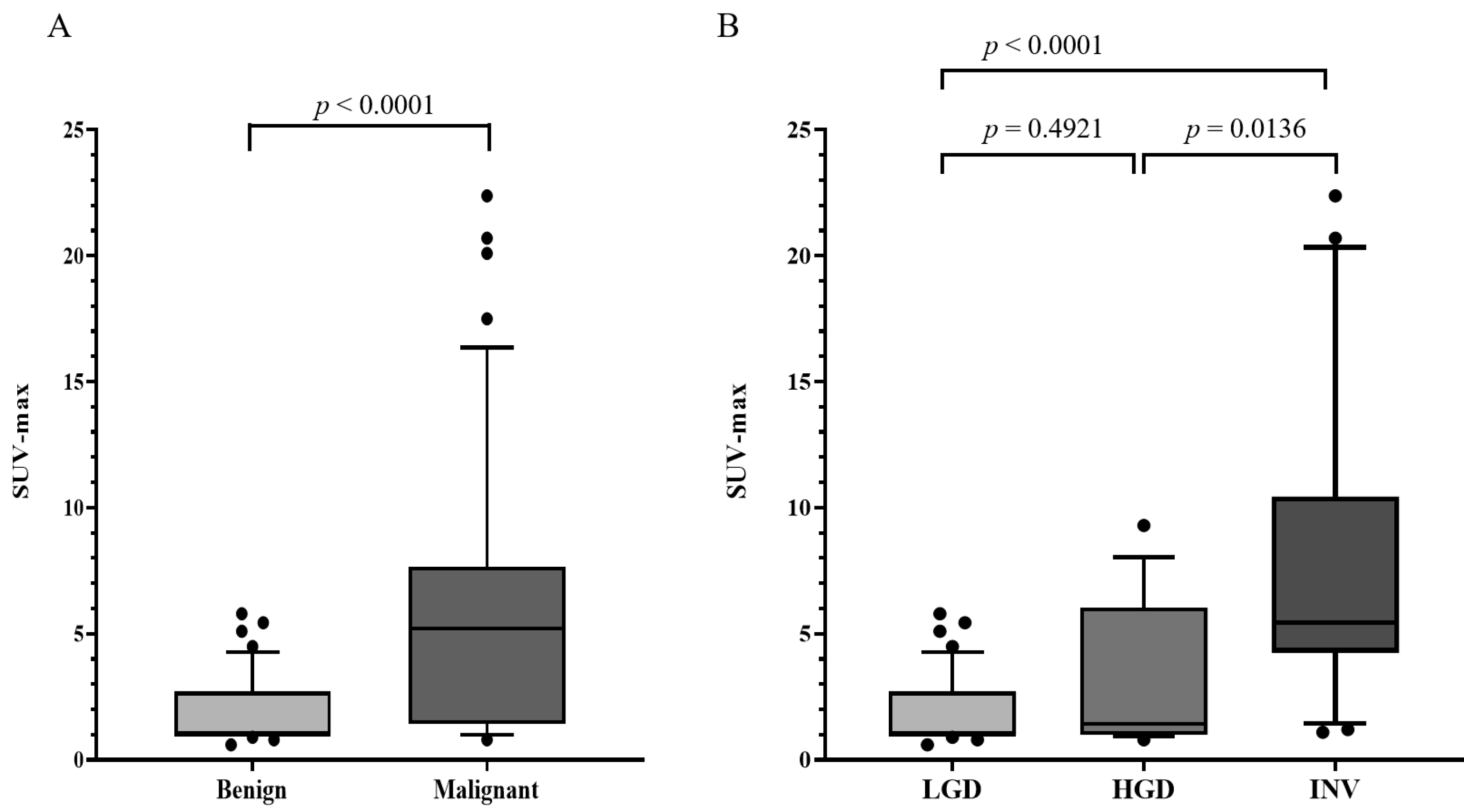
| SUV-max, mean ± SD | 1.95 ± 1.39 | 5.96 ± 5.60 | <0.0001 |
| Other indicators | |||
| High-risk stigmata, positive, n (%) | 17 (39.5) | 34 (82.9) | <0.0001 |
| Worrisome feature, positive, n (%) | 41 (95.3) | 40 (97.6) | 0.5811 |
| Characteristic | LGD (n = 43) | HGD (n = 16) | INV (n = 25) | p-Value (LGD vs. HGD) | p-Value (LGD vs. INV) | p-Value (HGD vs. INV) |
|---|---|---|---|---|---|---|
| Clinical factor | ||||||
| Age, median (range), y | 70 (44–80) | 70 (49–86) | 70 (35–87) | 0.5400 | 0.9126 | 0.7659 |
| Sex, male, n (%) | 34 (79.0) | 9 (56.3) | 13 (52.0) | 0.1951 | 0.0552 | 0.9668 |
| Symptoms, presence, n (%) | 7 (16.2) | 7 (43.8) | 14 (56.0) | 0.0755 | 0.0021 | 0.7393 |
| Coexistence of diabetes mellitus | 8 (18.6) | 7 (43.8) | 8 (32.0) | 0.1265 | 0.4309 | 0.7417 |
| Concomitant pancreatitis, presence, n (%) | 4 (9.3) | 2 (12.5) | 2 (8.0) | 0.5933 | 0.2701 | 0.0726 |
| Family history of pancreatic cancer | 4 (9.3) | 1 (6.3) | 2 (9.1) | 0.9335 | 0.9845 | 0.9827 |
| Laboratory findings | ||||||
| Serum CEA, high (≥3.2 IU/L) | 10 (23.2) | 4 (25.0) | 14 (56.0) | 0.8737 | 0.0709 | 0.0928 |
| Serum CA 19-9, high (≥37 IU/L) | 2 (4.6) | 1 (6.3) | 9 (36.0) | 0.7282 | 0.0043 | 0.0957 |
| Serum P-AMY, high (≥50 IU/L) | 11 (25.5) | 2 (12.5) | 6 (24.0) | 0.1904 | 0.0495 | 0.6175 |
| Imaging findings | ||||||
| Main pancreatic duct size, mean ± SD | 6.51 ± 3.90 | 9.05 ± 4.93 | 8.36 ± 6.19 | 0.0900 | 0.7057 | 0.7815 |
| Main pancreatic duct size, (≥ 10 mm), n (%) | 7 (16.3) | 8 (50.0) | 10 (40.0) | 0.0456 | 0.2556 | 0.6649 |
| Cystic size, mean ± SD | 34.7 ± 14.1 | 38.1 ± 28.9 | 40.9 ± 19.3 | 0.9885 | 0.5333 | 0.7344 |
| Enhancing mural nodule height *, mean ± SD | 3.67 ± 6.01 | 6.56 ± 8.36 | 19.7 ± 11.9 | 0.3411 | < 0.0001 | 0.0045 |
| Enhancing mural nodule height *, (≥ 5 mm), n (%) | 12 (27.9) | 8 (50.0) | 29 (84.0) | 0.2588 | < 0.0001 | 0.0574 |
| Cyst growth rate ≥ 5 mm/2 years, presence, n (%) | 3 (7.0) | 0 (0) | 2 (8.0) | 0.5435 | 0.9892 | 0.5079 |
| Abrupt change in caliber of pancreatic duct with distal pancreatic atrophy | 1 (2.3) | 1 (6.3) | 0 (0) | 0.7593 | 0.7445 | 0.4531 |
| Thickened/enhancing cyst walls, n (%) | 12 (27.9) | 1 (6.3) | 1 (4.0) | 0.1842 | 0.0444 | 0.9558 |
| Lymphadenopathy, n (%) | 1 (2.3) | 1 (6.3) | 0 (0) | 0.7593 | 0.7445 | 0.4531 |
| SUV-max, mean ± SD | 1.95 ± 1.39 | 3.17 ± 2.82 | 7.91 ± 6.06 | 0.4921 | < 0.0001 | 0.0136 |
| Other indicators | ||||||
| High-risk stigmata, positive, n (%) | 17 (39.5) | 12 (75.0) | 22 (88.0) | 0.0442 | 0.0003 | 0.5482 |
| Worrisome feature, positive, n (%) | 41 (95.3) | 16 (100.0) | 24 (96.0) | 0.6762 | 0.9937 | 0.7336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hozaka, Y.; Kurahara, H.; Oi, H.; Idichi, T.; Yamasaki, Y.; Kawasaki, Y.; Tanoue, K.; Jinguji, M.; Nakajo, M.; Tani, A.; et al. Clinical Utility and Limitation of Diagnostic Ability for Different Degrees of Dysplasia of Intraductal Papillary Mucinous Neoplasms of the Pancreas Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography. Cancers 2021, 13, 4633. https://doi.org/10.3390/cancers13184633
Hozaka Y, Kurahara H, Oi H, Idichi T, Yamasaki Y, Kawasaki Y, Tanoue K, Jinguji M, Nakajo M, Tani A, et al. Clinical Utility and Limitation of Diagnostic Ability for Different Degrees of Dysplasia of Intraductal Papillary Mucinous Neoplasms of the Pancreas Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography. Cancers. 2021; 13(18):4633. https://doi.org/10.3390/cancers13184633
Chicago/Turabian StyleHozaka, Yuto, Hiroshi Kurahara, Hideyuki Oi, Tetsuya Idichi, Yoichi Yamasaki, Yota Kawasaki, Kiyonori Tanoue, Megumi Jinguji, Masatoyo Nakajo, Atsushi Tani, and et al. 2021. "Clinical Utility and Limitation of Diagnostic Ability for Different Degrees of Dysplasia of Intraductal Papillary Mucinous Neoplasms of the Pancreas Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography" Cancers 13, no. 18: 4633. https://doi.org/10.3390/cancers13184633
APA StyleHozaka, Y., Kurahara, H., Oi, H., Idichi, T., Yamasaki, Y., Kawasaki, Y., Tanoue, K., Jinguji, M., Nakajo, M., Tani, A., Nakajo, A., Mataki, Y., Fukukura, Y., Noguchi, H., Higashi, M., Yoshiura, T., Tanimoto, A., & Ohtsuka, T. (2021). Clinical Utility and Limitation of Diagnostic Ability for Different Degrees of Dysplasia of Intraductal Papillary Mucinous Neoplasms of the Pancreas Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography. Cancers, 13(18), 4633. https://doi.org/10.3390/cancers13184633







