Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
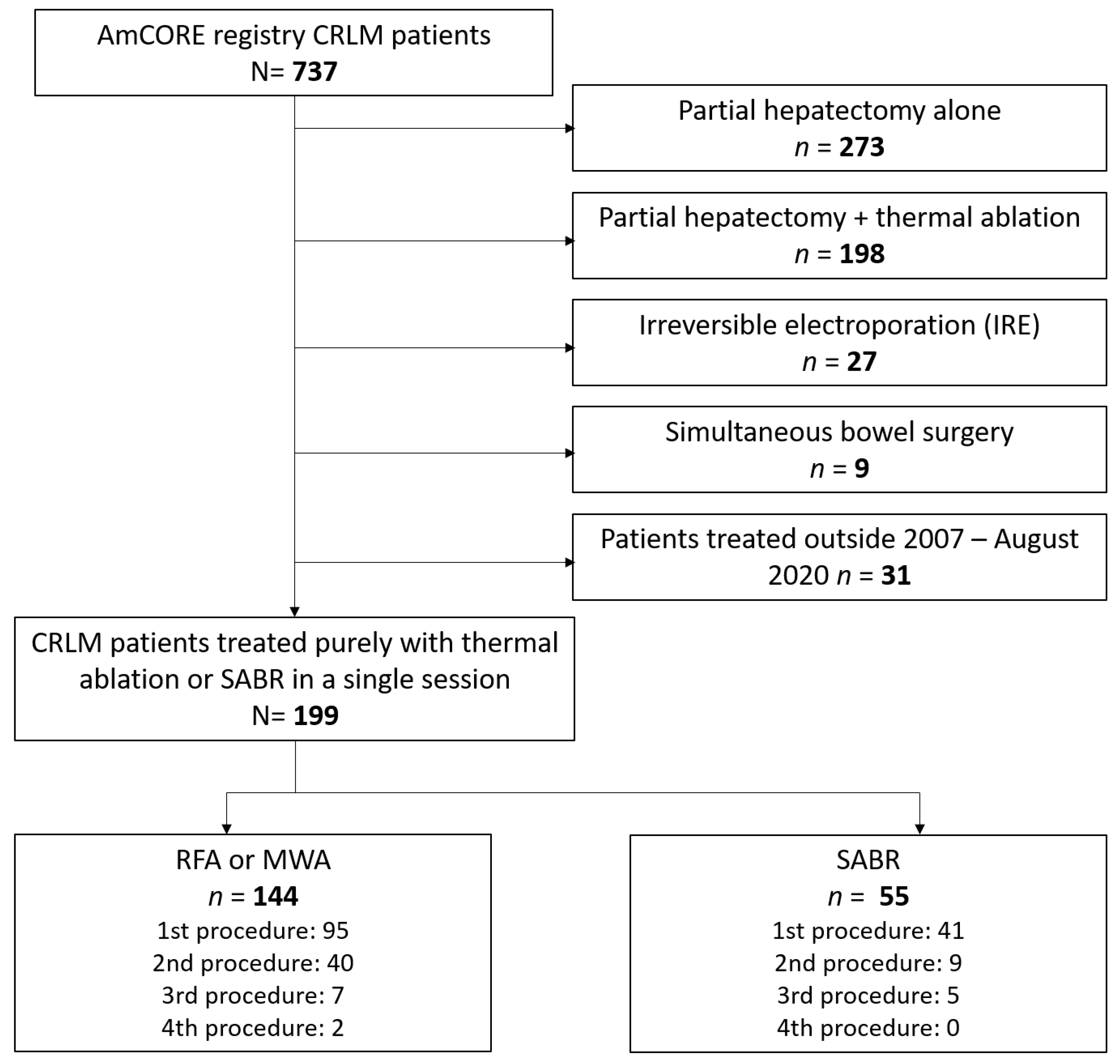
2.1. Study Design and Population
2.2. Radiofrequency Ablation, Microwave Ablation and Stereotactic Ablative Radiotherapy
2.3. Thermal Ablation
2.4. Stereotactic Ablative Radiotherapy
2.5. Follow-Up
2.6. Statistical Analyses
3. Results
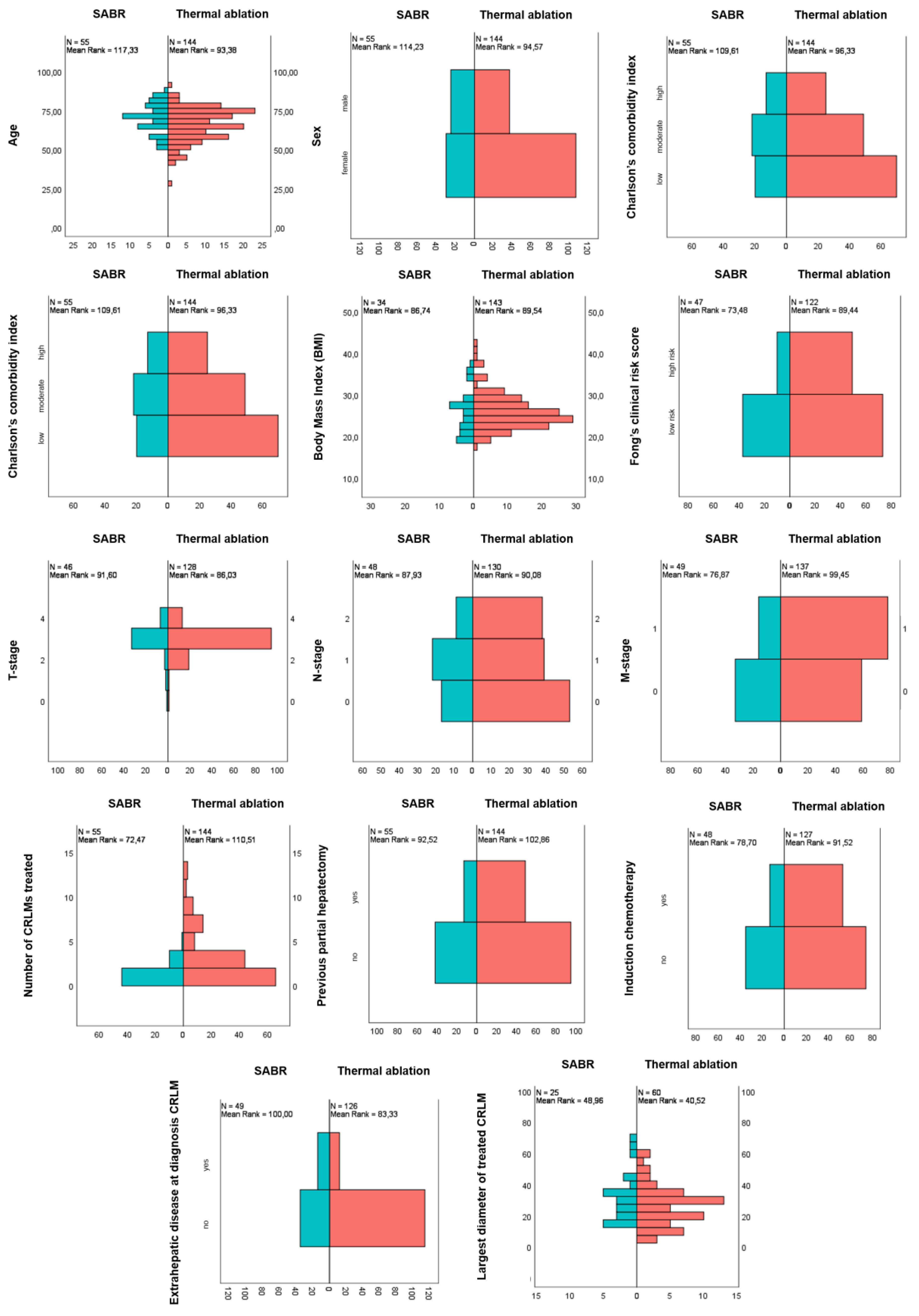
3.1. Baseline and Treatment Characteristics
3.2. Adverse Events and Hospital Stay
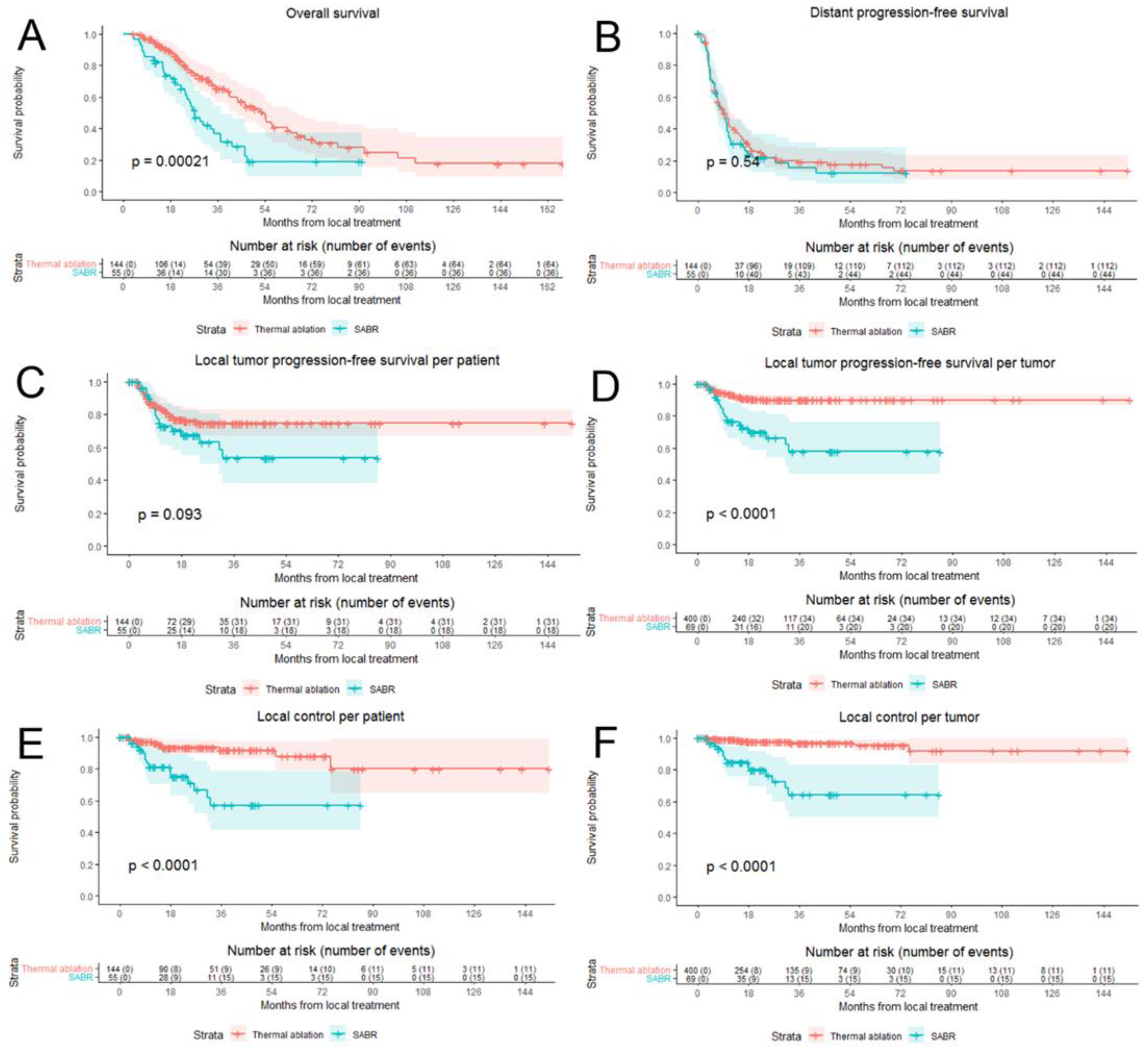
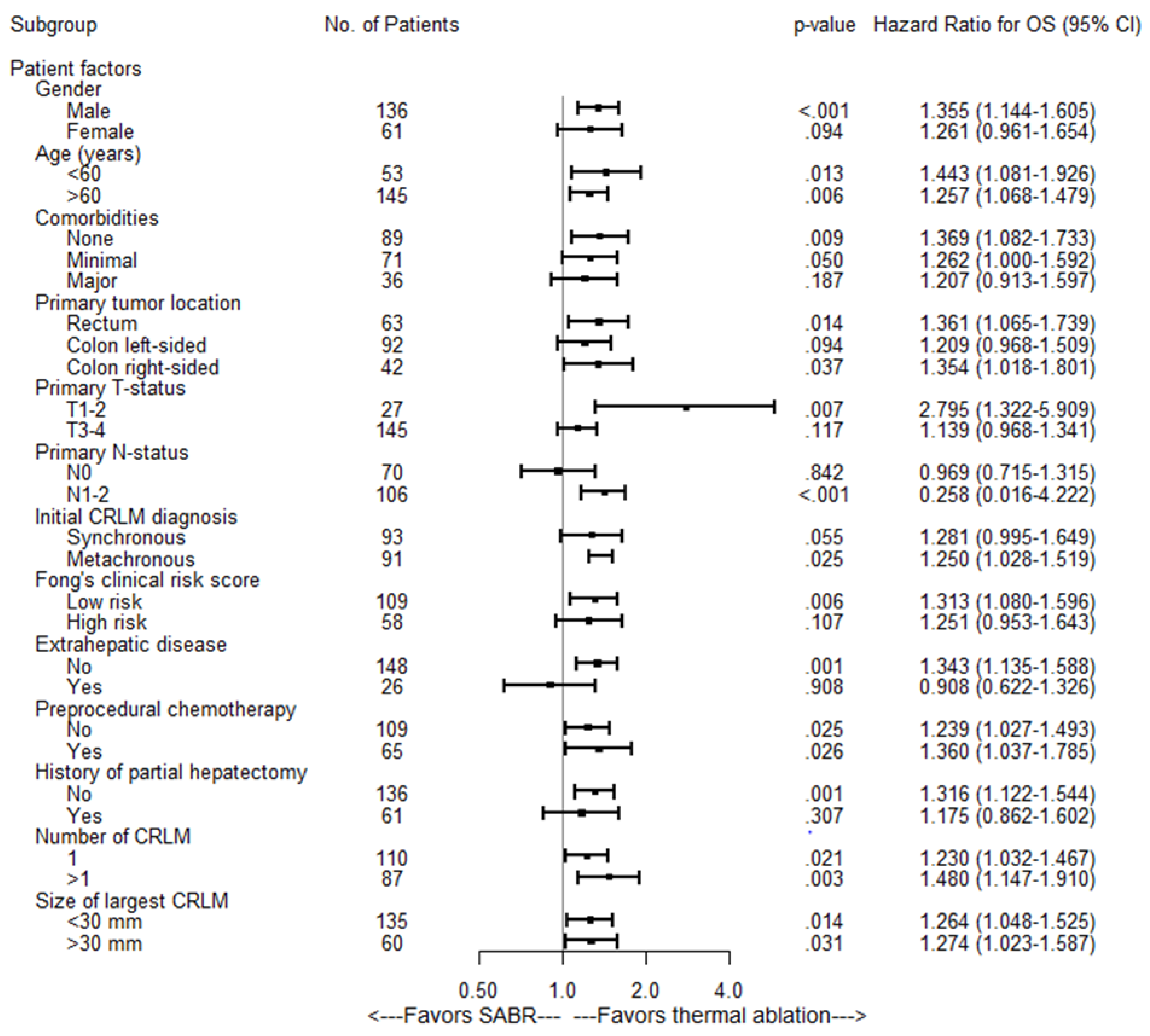
3.3. Overall Survival and Distant Progression-Free Survival
3.4. Local Tumor Progression-Free Survival and Eventual Local Control
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Estimated Age-Standardized Incidence Rates (World) in 2018, All Cancers, Both Sexes, All Ages. 2018. Available online: http://gco.iarc.fr/today/online-analysis-map (accessed on 1 April 2021).
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Leporrier, J.; Maurel, J.; Chiche, L.; Bara, S.; Segol, P.; Launoy, G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. BJS 2006, 93, 465–474. [Google Scholar] [CrossRef]
- Taylor, A.; Primrose, J.N.; Langeberg, W.; Kelsh, M.; Mowat, F.; Alexander, D.; Choti, M.; Poston, G.; Kanas, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012, 4, 283–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated With Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- House, M.G.; Ito, H.; Gönen, M.; Fong, Y.; Allen, P.J.; DeMatteo, R.P.; Brennan, M.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Survival after Hepatic Resection for Metastatic Colorectal Cancer: Trends in Outcomes for 1,600 Patients during Two Decades at a Single Institution. J. Am. Coll. Surg. 2010, 210, 744–752. [Google Scholar] [CrossRef]
- Adam, R.; Avisar, E.; Ariche, A.; Giachetti, S.; Azoulay, D.; Castaing, D.; Kunstlinger, F.; Levi, F.; Bismuth, F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann. Surg. Oncol. 2001, 8, 347–353. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; Van Tilborg, A.A.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; De Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef] [Green Version]
- van Amerongen, M.J.; Jenniskens, S.F.M.; van den Boezem, P.B.; Fütterer, J.J.; de Wilt, J.H.W. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases—A meta-analysis. HPB 2017, 19, 749–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuizen, S.; Puijk, R.S.; van den Bemd, B.; Aldrighetti, L.; Arntz, M.; van den Boezem, P.B.; Bruynzeel, A.M.; Burgmans, M.C.; De Cobelli, F.; Coolsen, M.M.; et al. Resectability and Ablatability Criteria for the Treatment of Liver Only Colorectal Metastases: Multidisciplinary Consensus Document from the COLLISION Trial Group. Cancers 2020, 12, 1779. [Google Scholar] [CrossRef]
- Mohamed, F.; Kallioinen, M.; Braun, M.; Fenwick, S.; Shackcloth, M.; Davies, R.J.; Bradbury, J.; Burgess, G.; Chew, C.; Dawson, C.; et al. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: Summary of NICE guidance. Br. J. Surg. 2020, 107, 943–945. [Google Scholar] [CrossRef]
- Vera, R.; González-Flores, E.; Rubio, C.; Urbano, J.; Camps, M.V.; Ciampi-Dopazo, J.J.; Rincón, J.O.; Macías, V.M.; Braco, M.A.G.; Suarez-Artacho, G. Multidisciplinary management of liver metastases in patients with colorectal cancer: A consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin. Transl. Oncol. 2020, 22, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.; van Tilborg, A.A.; Meijerink, M.R.; Macintosh, M.O.; Zonderhuis, B.M.; de Lange, E.S.; Comans, E.F.I.; Meijer, S.; van den Tol, M.P. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J. Surg. 2013, 37, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, A.A.; Scheffer, H.J.; de Jong, M.C.; Vroomen, L.G.; Nielsen, K.; van Kuijk, C.; van den Tol, P.M.P.; Meijerink, M.R. MWA Versus RFA for Perivascular and Peribiliary CRLM: A Retrospective Patient- and Lesion-Based Analysis of Two Historical Cohorts. Cardiovasc. Intervent. Radiol. 2016, 39, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Tanis, E.; Nordlinger, B.; Mauer, M.; Sorbye, H.; van Coevorden, F.; Gruenberger, T.; Schlag, P.; Punt, C.; Ledermann, J.; Ruers, T. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur. J. Cancer 2014, 50, 912–919. [Google Scholar] [PubMed]
- Solbiati, L.; Livraghi, T.; Goldberg, S.N.; Ierace, T.; Meloni, F.; Dellanoce, M.; Cova, L.; Halpern, E.F.; Gazelle, G.S. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001, 221, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Berber, E.; Siperstein, A. Local Recurrence after Laparoscopic Radiofrequency Ablation of Liver Tumors: An Analysis of 1032 Tumors. Ann. Surg. Oncol. 2008, 15, 2757–2764. [Google Scholar] [CrossRef]
- Ayav, A.; Germain, A.; Marchal, F.; Tierris, I.; Laurent, V.; Bazin, C.; Yuan, Y.; Robert, L.; Brunaud, L.; Bresler, L. Radiofrequency ablation of unresectable liver tumors: Factors associated with incomplete ablation or local recurrence. Am. J. Surg. 2010, 200, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Comito, T.; Tozzi, A.; Navarria, P.; Fogliata, A.; Clerici, E.; Mancosu, P.; Reggiori, G.; Rimassa, L.; Torzilli, G.; et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Puijk RSA, M.; Adam, A.; Arai, Y.; Arellano, R. Consensus guidelines for the definition of time-to-event endpoints in image guided tumor ablation: Results of the Society for Interventional Oncology (SIO) and DATECAN (Definition for the Assessment of Time-to-event End-points in CANcer trials) initiative. Radiology 2021. not yet published. [Google Scholar]
- Franzese, C.; Comito, T.; Clerici, E.; Di Brina, L.; Tomatis, S.; Navarria, P.; Reggiori, G.; Viganò, L.; Poretti, D.; Pedicini, V.; et al. Liver metastases from colorectal cancer: Propensity score-based comparison of stereotactic body radiation therapy vs. microwave ablation. J. Cancer Res. Clin. Oncol. 2018, 144, 1777–1783. [Google Scholar] [CrossRef]
- Stintzing, S.; Grothe, A.; Hendrich, S.; Hoffmann, R.-T.; Heinemann, V.; Rentsch, M.; Fuerweger, C.; Muacevic, A.; Trumm, C.G. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncol. 2013, 52, 971–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.T.; Kim, J.J.; Dinniwell, R.; Brierley, J.; Lockwood, G.; Wong, R.; Cummings, B.; Ringash, J.; Tse, R.V.; Knox, J.J.; et al. Phase I Study of Individualized Stereotactic Body Radiotherapy of Liver Metastases. J. Clin. Oncol. 2009, 27, 1585–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andratschke, N.H.; Nieder, C.; Heppt, F.; Molls, M.; Zimmermann, F. Stereotactic radiation therapy for liver metastases: Factors affecting local control and survival. Radiat. Oncol. 2015, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Goodman, B.D.; Mannina, E.M.; Althouse, S.K.; Maluccio, M.A.; Cárdenes, H.R. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pr. Radiat. Oncol. 2016, 6, 86–95. [Google Scholar] [CrossRef]
- Joo, J.H.; Park, J.-H.; Kim, J.C.; Yu, C.S.; Lim, S.-B.; Park, I.J.; Kim, T.W.; Hong, Y.S.; Kim, K.-P.; Yoon, S.M.; et al. Local Control Outcomes Using Stereotactic Body Radiation Therapy for Liver Metastases from Colorectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 876–883. [Google Scholar] [CrossRef]
- Mahadevan, A.; Blanck, O.; Lanciano, R.; Peddada, A.; Sundararaman, S.; D’Ambrosio, D.; Sharma, S.; Perry, D.; Kolker, J.; Davis, J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis—Clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat. Oncol. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andratschke, N.; Alheid, H.; Allgäuer, M.; Becker, G.; Blanck, O.; Boda-Heggemann, J.; Brunner, T.; Duma, M.; Gerum, S.; Guckenberger, M.; et al. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): Patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer 2018, 18, 283. [Google Scholar] [CrossRef] [Green Version]
- Clerici, E.; Comito, T.; Franzese, C.; Di Brina, L.; Tozzi, A.; Iftode, C.; Navarria, P.; Mancosu, P.; Reggiori, G.; Tomatis, S.; et al. Role of stereotactic body radiation therapy in the treatment of liver metastases: Clinical results and prognostic factors. Strahlenther. Onkol. 2020, 196, 325–333. [Google Scholar] [CrossRef]
- Mendez Romero, A.; Schillemans, W.; van Os, R.; Koppe, F.; Haasbeek, C.J.; Hendriksen, E.M. The Dutch-Belgian Registry of Stereotactic Body Radiation Therapy for Liver Metastases: Clinical Outcomes of 515 Patients and 668 Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, R.; Llacer, C.; Riou, O.; Deshayes, E. Evaluation of response after SBRT for liver tumors. Reports of practical oncology and radiotherapy. J. Greatpoland Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2017, 22, 170–175. [Google Scholar]
- Jarraya, H.; Borde, P.; Mirabel, X.; Ernst, O.; Boulanger, T.; Lartigau, E.; Ceugnart, L.; Kramar, A.; Taieb, S. Lobulated Enhancement Evaluation in the Follow-Up of Liver Metastases Treated by Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. 2015, 92, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.; Cook, G.J.R.; Owczarczyk, K.; Goh, V. Challenges in imaging assessment following liver stereotactic body radiotherapy: Pitfalls to avoid in clinical practice. Chin. Clin Oncol. 2017, 6 (Suppl. 2), S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
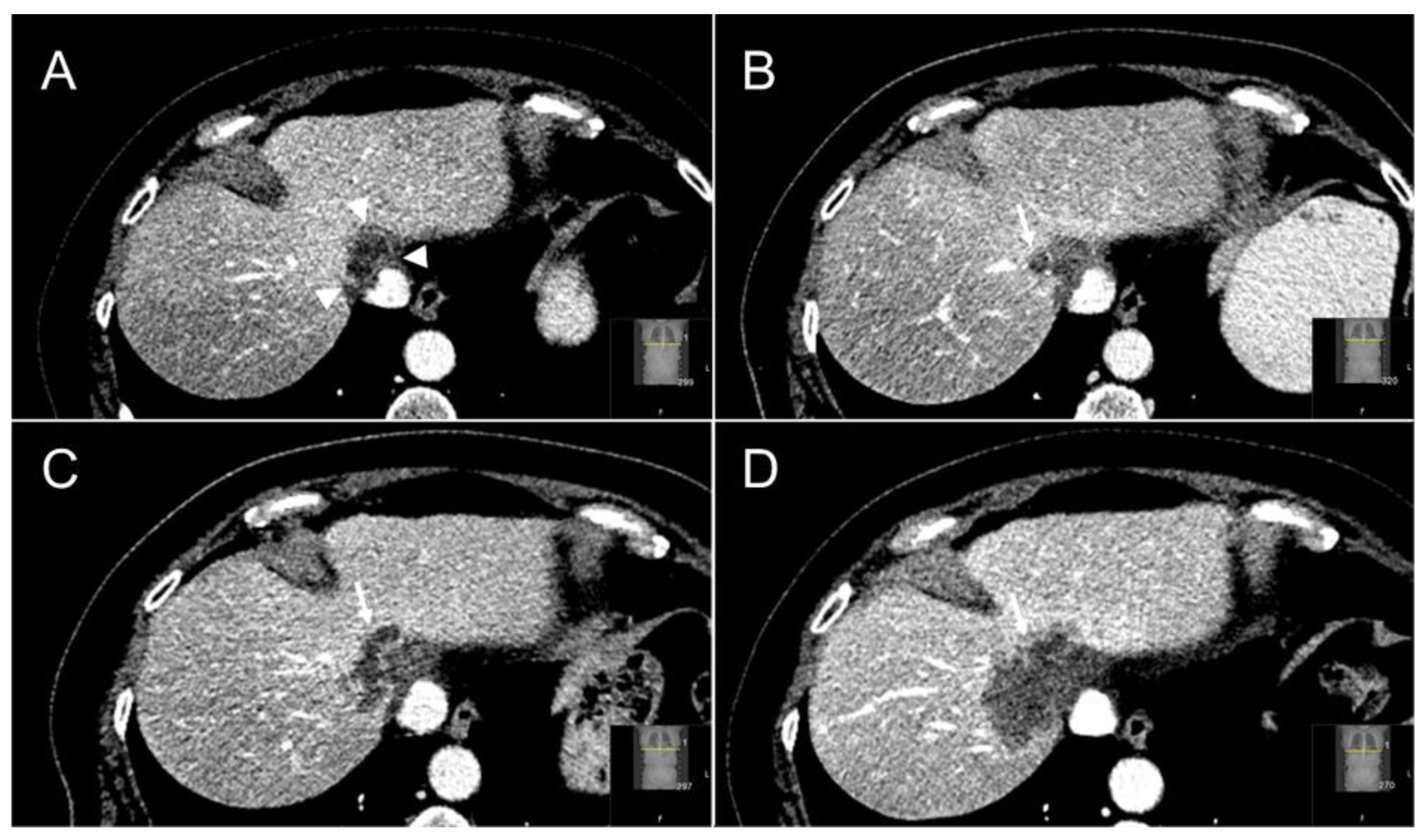
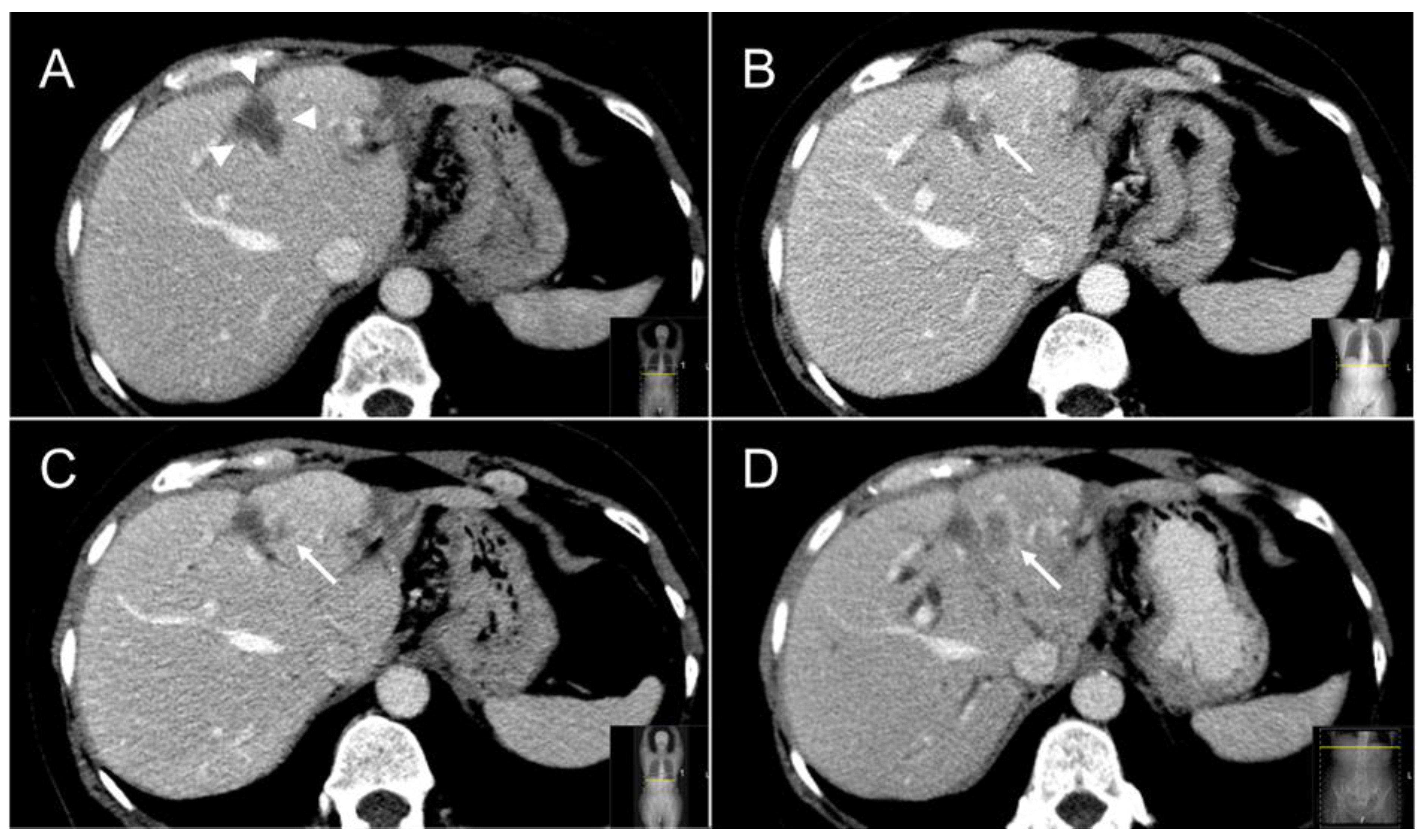

| Factor | Total Cohort | Thermal Ablation | SABR | p-Value | |
|---|---|---|---|---|---|
| N | N | N | |||
| Total | 199 | 144 | 55 | ||
| Patient characteristics | |||||
| Sex | 0.010 | ||||
| Male | 137 (68.8%) | 107 (74.3%) | 30 (54.5%) | ||
| Female | 62 (31.2%) | 37 (25.7%) | 25 (45.5%) | ||
| Age (years) | Median (IQR) | 68.6 (15.9) | 66.8 (16.5) | 71.5 (13.0) | 0.006 |
| Charlson’s Comorbidity Index (CCI) | 0.281 | ||||
| Low CCI 0–5 | 90 (45.2%) | 70 (48.6%) | 20 (36.4%) | ||
| Moderate CCI 5–8 | 71 (35.7%) | 49 (34%) | 22 (40%) | ||
| High CCI >8 | 38 (19.1%) | 25 (17.4%) | 13 (23.6%) | ||
| Location primary tumor | 0.857 | ||||
| Rectum | 64 (32.2%) | 45 (31.3%) | 19 (34.5%) | ||
| Left-sided colon | 93 (46.7%) | 69 (47.9%) | 24 (43.6%) | ||
| Right-sided colon | 42 (21.1%) | 30 (20.8%) | 12 (21.8%) | ||
| Primary T-status | 0.813 | ||||
| T1-2 | 27 (13.6%) | 21 (16.4%) | 6 (13%) | ||
| T3-4 | 147 (73.9%) | 107 (83.6%) | 40 (87%) | ||
| Unknown | 25 (12.6%) | 16 | 9 | ||
| Primary N-status | 0.605 | ||||
| N0 | 70 (35.2%) | 53 (40.8%) | 17 (35.4%) | ||
| N1-2 | 108 (54.3%) | 77 (59.2%) | 31 (64.6%) | ||
| Unknown | 21 (10.6%) | 14 | 7 | ||
| Primary M-status | 0.005 | ||||
| synchronous | 94 (47.2%) | 78 (56.9%) | 16 (32.7%) | ||
| metachronous | 92 (46.2%) | 59 (43.1%) | 33 (67.3%) | ||
| Unknown | 13 (6.5%) | 7 | 6 | ||
| Fong’s clinical risk score | 0.030 | ||||
| Low risk | 110 (55.3%) | 73 (59.8%) | 37 (78.8%) | ||
| High risk | 59 (29.6%) | 49 (40.2%) | 10 (21.3%) | ||
| Extrahepatic disease at diagnosis | 0.004 | ||||
| No | 147 (73.9%) | 112 (90.3%) | 35 (71.4%) | ||
| Yes | 26 (13.1%) | 12 (9.7%) | 14 (28.6%) | ||
| History of preprocedural chemotherapy | 0.083 | ||||
| No | 109 (54.8%) | 74 (58.7%) | 35 (72.9%) | ||
| Yes | 66 (33.2%) | 53 (41.7%) | 13 (27.1%) | ||
| History of partial hepatectomy | 0.174 | ||||
| No | 137 (68.8%) | 95 (66%) | 42 (76.4%) | ||
| Yes | 62 (31.2%) | 49 (34%) | 13 (23.6%) | ||
| Number of CRLM treated within procedure | |||||
| Median | 2 (1–18) | 2 (1–18) | 1 (1–5) | 0.0004 | |
| Diameter of CRLM at procedure (mm) | <0.0005 | ||||
| Median | 21 (2–68) | 14 (2–65) | 29 (6–68) |
| Univariate Analysis OS | Multivariate Analysis OS | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p Value | Hazard Ratio | p Value |
| Treatment | ||||
| Thermal ablation | 1 [reference] | <0.001 | 1 [reference] | 0.001 |
| SABR | 1.29 (1.12–1.49) | 1.27 (1.10–1.46) | ||
| Sex | ||||
| Male | 1 [reference] | 0.374 | ||
| Female | 0.83 (0.53–1.29) | |||
| Age in years | ||||
| <68 years | 1 [reference] | 0.150 | ||
| >68 years | 1.35 (0.90–2.01) | |||
| Comorbidities | ||||
| None | 1 [reference] | 0.034 | 1 [reference] | 0.204 |
| Minor | 1.08 (0.68–1.70) | 0.98 (0.62–1.56) | ||
| Major | 1.92 (1.16–3.17) | 1.54 (0.90–2.64) | ||
| Location primary tumor | ||||
| Rectal | 1 [reference] | 0.282 | ||
| Left sided colon | 0.89 (0.57–1.39) | |||
| Right sided colon | 1.36 (0.80–2.32) | |||
| Primary T category | ||||
| T1-2 | 1 [reference] | 0.469 | ||
| T3-4 | 1.28 (0.66–2.48) | |||
| Primary N category | ||||
| N0 | 1 [reference] | 0.077 | 1 [reference] | 0.068 |
| N1-2 | 1.51 (0.96–2.38) | 1.54 (0.97–2.46) | ||
| Timing of CRLM | ||||
| Metachronous | 1 [reference] | 0.722 | ||
| Synchronous | 1.09 (0.72–1.64) | |||
| Fong CRS | ||||
| Low risk | 1 [reference] | 0.180 | ||
| High risk | 1.38 (0.88–2.16) | |||
| Extrahepatic disease at diagnosis | ||||
| No | 1 [reference] | 0.844 | ||
| Yes | 0.93 (0.51–1.73) | |||
| Preprocedural chemotherapy | ||||
| No | 1 [reference] | 0.315 | ||
| Yes | 0.80 (0.52–1.25) | |||
| History of liver resection | ||||
| No | 1 [reference] | 0.064 | 1 [reference] | 0.193 |
| Yes | 0.64 (0.41–1.02) | 0.73 (0.45–1.17) | ||
| Number of CRLM during procedure | ||||
| 1 CRLM | 1 [reference] | 0.541 | ||
| >1 CRLM | 0.892 (0.60–1.33) | |||
| Diameter CRLM at procedure | ||||
| ≤3 cm | 1 [reference] | 0.004 | 1 [reference] | 0.014 |
| 3 cm | 1.81 (1.21–2.70) | 1.67 (1.11–2.51) | ||
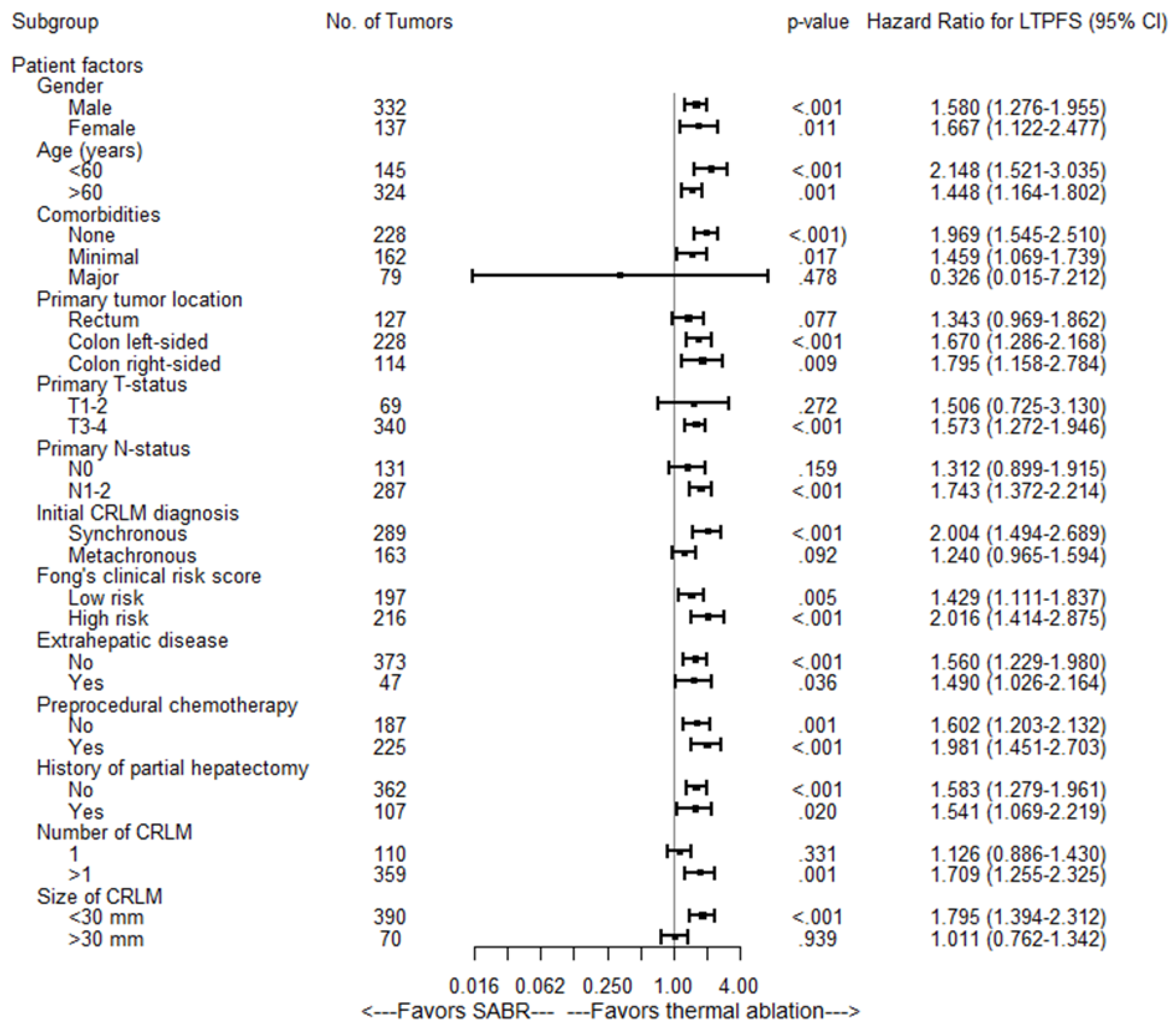
| Univariate Analysis LTPFS | Multivariate Analysis LTPFS | |||
|---|---|---|---|---|
| Per-Tumor | ||||
| Variable | Hazard Ratio (95% CI) | pValue | Hazard Ratio (95% CI) | pValue |
| Treatment | ||||
| Thermal ablation | 1 [reference] | <0.001 | 1 [reference] | 0.003 |
| SABR | 1.58 (1.31–1.90) | 1.35 (1.11–1.65) | ||
| Sex | ||||
| Male | 1 [reference] | 0.398 | ||
| Female | 0.75 (0.39–1.46) | |||
| Age in years | ||||
| <68.6 years | 1 [reference] | 0.363 | ||
| >68.6 years | 1.28 (0,75–2.19) | |||
| Comorbidities | ||||
| None | 1 [reference] | 0.331 | ||
| Minor | 0.78 (0.44–1.39) | |||
| Major | 0.51 (0.20–1.31) | |||
| Location primary tumor | ||||
| Rectal | 1 [reference] | 0.496 | ||
| Left-sided colon | 0.95 (0.53–1.73) | |||
| Right-sided colon | 0.63 (0.28–1.41) | |||
| Primary T category | ||||
| T1-2 | 1 [reference] | 0.220 | ||
| T3-4 | 1.79 (0.71–4.56) | |||
| Primary N category | ||||
| N0 | 1 [reference] | 0.914 | ||
| N1-2 | 0.97 (0.53–1.78) | |||
| Timing of CRLM | ||||
| Metachronous | 1 [reference] | <0.001 | 1 [reference] | 0.103 |
| Synchronous | 0.36 (0.21–0.63) | 0.61 (0.34–1.11) | ||
| Fong CRS | ||||
| Low risk | 1 [reference] | 0.019 | 1 [reference] | 0.141 |
| High risk | 0.46 (0.25–0.88) | 0.61 (0.32–1.18) | ||
| Extrahepatic disease at diagnosis | ||||
| No | 1 [reference] | 0.002 | 1 [reference] | 0.137 |
| Yes | 2.80 (1.48–5.30) | 1.67 (0.85–3.29) | ||
| Preprocedural chemotherapy | ||||
| No | 1 [reference] | 0.234 | ||
| Yes | 0.69 (0.37–1.27) | |||
| History of liver resection | ||||
| No | 1 [reference] | 0.576 | ||
| Yes | 1.19 (0.65–2.19) | |||
| Diameter CRLM at procedure | ||||
| ≤3 cm | 1 [reference] | <0.001 | 1 [reference] | <0.001 |
| >3 cm | 6.30 (3.67–10.83) | 4.73 (2.65–8.45) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieuwenhuizen, S.; Dijkstra, M.; Puijk, R.S.; Timmer, F.E.F.; Nota, I.M.; Opperman, J.; van den Bemd, B.; Geboers, B.; Ruarus, A.H.; Schouten, E.A.C.; et al. Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry. Cancers 2021, 13, 4303. https://doi.org/10.3390/cancers13174303
Nieuwenhuizen S, Dijkstra M, Puijk RS, Timmer FEF, Nota IM, Opperman J, van den Bemd B, Geboers B, Ruarus AH, Schouten EAC, et al. Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry. Cancers. 2021; 13(17):4303. https://doi.org/10.3390/cancers13174303
Chicago/Turabian StyleNieuwenhuizen, Sanne, Madelon Dijkstra, Robbert S. Puijk, Florentine E. F. Timmer, Irene M. Nota, Jip Opperman, Bente van den Bemd, Bart Geboers, Alette H. Ruarus, Evelien A. C. Schouten, and et al. 2021. "Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry" Cancers 13, no. 17: 4303. https://doi.org/10.3390/cancers13174303
APA StyleNieuwenhuizen, S., Dijkstra, M., Puijk, R. S., Timmer, F. E. F., Nota, I. M., Opperman, J., van den Bemd, B., Geboers, B., Ruarus, A. H., Schouten, E. A. C., de Vries, J. J. J., Scheffer, H. J., van Geel, A. M., van Waesberghe, J. H. T. M., Swijnenburg, R.-J., Versteeg, K. S., Lissenberg-Witte, B. I., van den Tol, M. P., Haasbeek, C. J. A., & Meijerink, M. R. (2021). Thermal Ablation versus Stereotactic Ablative Body Radiotherapy to Treat Unresectable Colorectal Liver Metastases: A Comparative Analysis from the Prospective Amsterdam CORE Registry. Cancers, 13(17), 4303. https://doi.org/10.3390/cancers13174303








