Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Outcomes
2.3. Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Procedures
4.3. Outcomes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Shyu, S.; Rajgariah, A.; Saoud, C.; Rogers, N.; Ali, S.Z. Image-guided lymph node fine-needle aspiration: The Johns Hopkins Hospital experience. J. Am. Soc. Cytopathol. 2021, in press. [Google Scholar] [CrossRef]
- Catalano, M.F.; Sivak, M.V., Jr.; Rice, T.; Gragg, L.A.; Van Dam, J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994, 40, 442–446. [Google Scholar] [CrossRef]
- Crinó, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Bellocchi, M.C.C.; Manfrin, E.; Bernardoni, L.; Sina, S.; D’Onofrio, M.; Marchegiani, G.; et al. Endoscopic Ultrasound Features Associated with Malignancy and Aggressiveness of Nonhypovascular Solid Pancreatic Lesions: Results from a Prospective Observational Study. Ultraschall Med. Eur. J. Ultrasound 2021, 42, 167–177. [Google Scholar] [CrossRef]
- Takasaki, Y.; Irisawa, A.; Shibukawa, G.; Sato, A.; Abe, Y.; Yamabe, A.; Arakawa, N.; Maki, T.; Yoshida, Y.; Igarashi, R.; et al. New endoscopic ultrasonography criteria for malignant lymphadenopathy based on inter-rater agreement. PLoS ONE 2019, 14, e0212427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, I.S.; Tziatzios, G.; Karatzas, P.S.; Gkolfakis, P.; Facciorusso, A.; Triantafyllou, K. Quality in pancreatic endoscopic ultrasound: What’s new in 2020? Ann. Gastroenterol. 2020, 33, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Stasi, E.; Di Maso, M.; Serviddio, G.; Hussein, M.S.A.; Muscatiello, N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciorusso, A.; Ramai, D.; Bellocchi, M.C.; Bernardoni, L.; Manfrin, E.; Muscatiello, N.; Crinò, S. Diagnostic Yield of Endoscopic Ultrasound-Guided Liver Biopsy in Comparison to Percutaneous Liver Biopsy: A Two-Center Experience. Cancers 2021, 13, 3062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Gao, X.; Lin, S.; He, L.; Luo, G.; Li, J.; Huang, C.; Wang, G.; Yang, Q.; et al. High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020, 66, 2763–2775. [Google Scholar] [CrossRef]
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic Ultrasound–guided Fine-needle Biopsy with or without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021, 161, 899–909.e5. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Larghi, A.; Bernardoni, L.; Parisi, A.; Frulloni, L.; Gabbrielli, A.; Parcesepe, P.; Scarpa, A.; Manfrin, E. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology 2019, 30, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Wani, S.; Triantafyllou, K.; Tziatzios, G.; Cannizzaro, R.; Muscatiello, N.; Singh, S. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2019, 90, 893–903.e7. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Bellocchi, M.C.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Buccino, V.R.; Purohit, P.; Setia, P.; Muscatiello, N. Diagnostic yield of Franseen and Fork-Tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: A meta-analysis. Endosc. Int. Open 2019, 7, E1221–E1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciorusso, A.; Barresi, L.; Cannizzaro, R.; Antonini, F.; Triantafyllou, K.; Tziatzios, G.; Muscatiello, N.; Hart, P.A.; Wani, S. Diagnostic yield of endoscopic ultrasound-guided tissue acquisition in autoimmune pancreatitis: A systematic review and meta-analysis. Endosc. Int. Open 2021, 9, E66–E75. [Google Scholar] [CrossRef]
- Crinò, S.F.; Le Grazie, M.; Manfrin, E.; Bellocchi, M.C.C.; Bernardoni, L.; Granato, A.; Locatelli, F.; Parisi, A.; Di Stefano, S.; Frulloni, L.; et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest. Endosc. 2020, 92, 648–658.e2. [Google Scholar] [CrossRef]
- Facciorusso, A.; Sunny, S.P.; Del Prete, V.; Antonino, M.; Muscatiello, N. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest. Endosc. 2020, 91, 14–22.e2. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.; Mccarty, T.R.; Jirapinyo, P.; Ribeiro, I.B.; Farias, G.F.A.; Ryou, M.; Lee, L.S.; Thompson, C.C. Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis. Clin. Endosc. 2020, 53, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Hedenström, P.; Chatzikyriakos, V.; Shams, R.; Lewerin, C.; Sadik, R. High Sensitivity of EUS-FNA and EUS-FNB in Lymphadenopathy Caused by Metastatic Disease: A Prospective Comparative Study. Clin. Endosc. 2021. [Google Scholar] [CrossRef]
- Puri, R.; Mangla, R.; Eloubeidi, M.; Vilmann, P.; Thandassery, R.; Sud, R. Diagnostic yield of EUS-guided FNA and cytology in suspected tubercular intra-abdominal lymphadenopathy. Gastrointest. Endosc. 2012, 75, 1005–1010. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, I.; Goto, N.; Tsurumi, H.; Nakashima, M.; Doi, S.; Iwashita, T.; Kanemura, N.; Kasahara, S.; Adachi, S.; Hara, T.; et al. Endoscopic Ultrasound-Guided Fine Needle Aspiration Biopsy for Diagnosis of Lymphoproliferative Disorders: Feasibility of Immunohistological, Flow Cytometric, and Cytogenetic Assessments. Am. J. Gastroenterol. 2012, 107, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Tanisaka, Y.; Mizuide, M.; Fujita, A.; Ogawa, T.; Araki, R.; Suzuki, M.; Katsuda, H.; Saito, Y.; Miyaguchi, K.; Tashima, T.; et al. Comparison of Endoscopic Ultrasound-Guided Fine-Needle Aspiration and Biopsy Device for Lymphadenopathy. Gastroenterol. Res. Pract. 2021, 2021, 6640862. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Kirtane, S.; Krall, K.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig. Endosc. 2019, 31, 197–202. [Google Scholar] [CrossRef]
- Larghi, A.; Rimbaş, M.; Crinó, S.F.; Gasbarrini, A.; Costamagna, G.; Scarpa, A. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc. Ultrasound 2018, 7, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.D.; Abraham, N.S.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fisher, D.A.; Fonkalsrud, L.; et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest. Endosc. 2016, 83, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Turco, A.; Barnabà, C.; Longo, G.; DiPasquale, G.; Muscatiello, N. Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics 2020, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Muthusamy, V.R.; McGrath, C.M.; Sepulveda, A.R.; Das, A.; Messersmith, W.; Kochman, M.L.; Shah, J. AGA White Paper: Optimizing Endoscopic Ultrasound–Guided Tissue Acquisition and Future Directions. Clin. Gastroenterol. Hepatol. 2018, 16, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat. Med. 2007, 27, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciorusso, A.; Di Maso, M.; Antonino, M.; Del Prete, V.; Panella, C.; Barone, M.; Muscatiello, N. Polidocanol injection decreases the bleeding rate after colon polypectomy: A propensity score analysis. Gastrointest. Endosc. 2015, 82, 350–358.e2. [Google Scholar] [CrossRef] [PubMed]
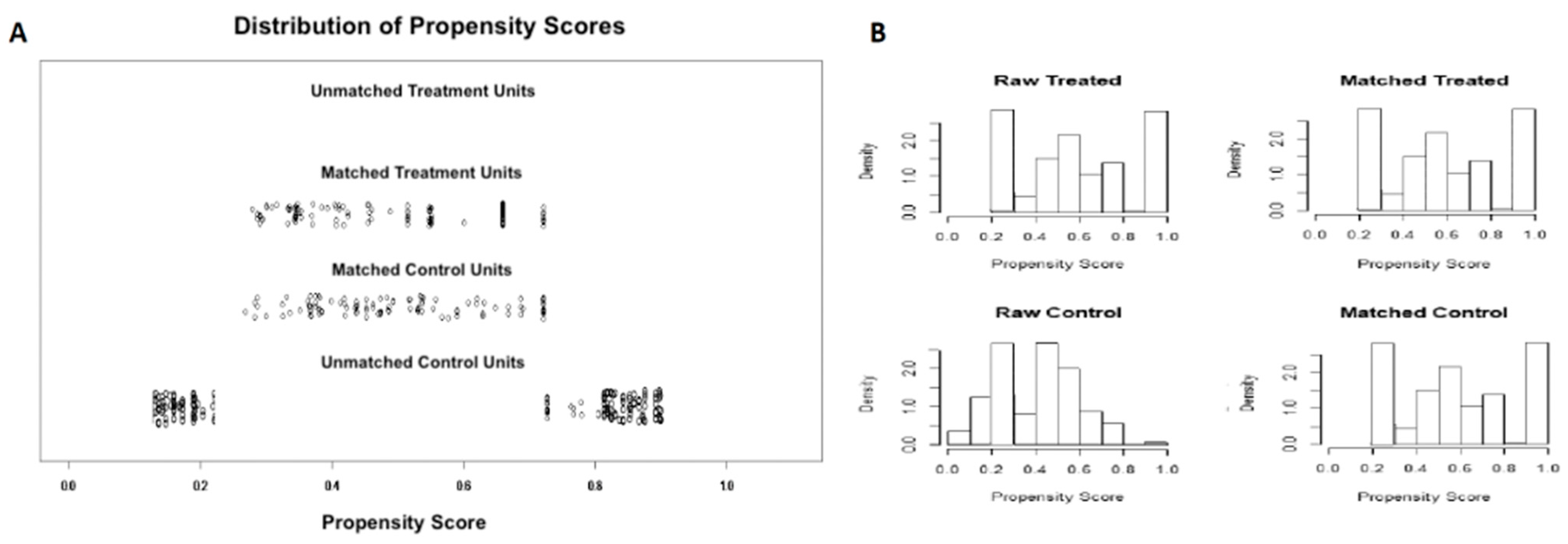
| Variable | EUS-FNB (n = 105) | EUS-FNA (n = 247) | p Value |
|---|---|---|---|
| Age (years) | 64.4 ± 7 | 66.3 ± 5 | 0.6 |
| Gender | |||
| M | 67 (63.8%) | 142 (57.4%) | 0.26 |
| F | 38 (36.2%) | 105 (42.6%) | - |
| Lymph node location | |||
| Peri-gastroduodenal | 12 (11.4%) | 24 (9.7%) | - |
| Peri-hepatic | 48 (45.7%) | 93 (37.6%) | - |
| Peri-pancreatic | 27 (25.7%) | 82 (33.1%) | 0.36 |
| Celiac | 3 (2.8%) | 15 (6%) | - |
| Peri-rectal | 15 (14.4%) | 33 (13.6%) | - |
| Lesion size (mm) | 21.4 ± 2.1 | 32.4 ± 0.8 | 0.04 |
| Diagnostic sample approach | |||
| Trans-gastric | 31 (29.5%) | 94 (38%) | - |
| Trans-duodenal | 59 (56.1%) | 120 (48.4%) | 0.30 |
| Trans-rectal | 15 (14.4%) | 33 (13.6%) | - |
| Needle size | |||
| 22 G | 75 (71.4%) | 155 (62.7%) | 0.11 |
| 25 G | 30 (28.6%) | 92 (37.3%) | - |
| Antithrombotic therapy | 34 (32.3%) | 94 (38%) | 0.31 |
| Variable | EUS-FNB (n = 105) | EUS-FNA (n = 105) | p Value |
|---|---|---|---|
| Age (years) | 64.4 ± 7 | 64.6 ± 5 | 0.9 |
| Gender | |||
| M | 67 (63.8%) | 68 (64.7%) | 0.86 |
| F | 38 (36.2%) | 37 (35.3%) | - |
| Lymph node location | |||
| Peri-gastroduodenal | 12 (11.4%) | 12 (11.4%) | - |
| Peri-hepatic | 48 (45.7%) | 49 (46.6%) | - |
| Peri-pancreatic | 27 (25.7%) | 26 (24.8%) | 0.94 |
| Celiac | 3 (2.8%) | 3 (2.8%) | - |
| Peri-rectal | 15 (14.4%) | 15 (14.4%) | - |
| Lesion size (mm) | 21.4 ± 2.1 | 22.4 ± 1.8 | 0.64 |
| Diagnostic sample approach | |||
| Trans-gastric | 31 (29.5%) | 31 (29.5%) | - |
| Trans-duodenal | 59 (56.1%) | 59 (56.1%) | 1.0 |
| Trans-rectal | 15 (14.4%) | 15 (14.4%) | - |
| Needle size | |||
| 22 G | 75 (71.4%) | 75 (71.4%) | 1.0 |
| 25 G | 30 (28.6%) | 30 (28.6%) | - |
| Number of passes | 2.4 ± 0.9 | 3.2 ± 0.9 | 0.03 |
| Antithrombotic therapy | 34 (32.3%) | 37 (35.3%) | 0.21 |
| Outcome | EUS-FNB | EUS-FNA | p Value |
|---|---|---|---|
| - | (105 pts) | (105 pts) | - |
| Sensitivity | 84.71% (75.2–91.6%) | 70.11% (59.3–79.4%) | 0.01 |
| Specificity | 100% (83.16–100%) | 100% (81.4–100%) | 0.6 |
| Diagnostic adequacy | 101 (96.1%) | 94 (89.5%) | 0.06 |
| Diagnostic accuracy | 92 | 79 | - |
| - | 87.62% (79.7–93.2%) | 75.24% (65.8–83.1%) | 0.02 |
| Final diagnosis | |||
| Metastasis | 74 (70.4%) | 70 (66.6%) | |
| Lymphoma | 20 (19%) | 15 (14.2%) | 0.22 |
| Benign | 7 (6.6%) | 9 (8.5%) | |
| Inconclusive | 4 (4%) | 11 (10.7%) | |
| Histological core procurement | 99 (94.2%) | 54 (54.4%) | <0.001 |
| Procedure-related adverse events | 0 (0%) | 0 (0%) | 1.0 |
| Outcome | EUS-FNB | EUS-FNA | p Value |
|---|---|---|---|
| Peri-Hepatic Location | |||
| EUS-FNB (48 patients) | EUS-FNA (49 patients) | ||
| Sensitivity | 86.49% (71.2–95.4%) | 68.42% (51.3–82.5%) | 0.01 |
| Specificity | 100% (71.5–100%) | 100% (71.5–100%) | 0.7 |
| Diagnostic adequacy | 45 (93.7%) | 42 (85.7%) | 0.19 |
| Diagnostic accuracy | 89.58% (77.3–96.5%) | 75.51% (61.1–86.6%) | 0.01 |
| Histological core procurement | 44 (91.6%) | 24 (48.9%) | <0.001 |
| Peri-pancreatic location | |||
| EUS-FNB (27 patients) | EUS-FNA (26 patients) | ||
| Sensitivity | 90% (68.3–98.7%) | 75% (50.9–91.3%) | 0.02 |
| Specificity | 100% (59–100%) | 100% (54–100%) | 0.5 |
| Diagnostic adequacy | 25 (92.59%) | 23 (88.4%) | 0.6 |
| Diagnostic accuracy | 92.59% (75.7–99%) | 80.77% (60.6–93.4%) | 0.01 |
| Histological core procurement | 23 (85.1%) | 15 (57.7%) | <0.001 |
| Peri-rectal location | |||
| EUS-FNB (15 patients) | EUS-FNA (15 patients) | ||
| Sensitivity | 92.3% (63.9–99.8%) | 71.4% (41.9–91.6%) | 0.03 |
| Specificity | 100% (15.8–100%) | 100% (2.5–100%) | 0.56 |
| Diagnostic adequacy | 15 (100%) | 13 (86.6%) | 0.07 |
| Diagnostic accuracy | 93.33% (68–99.8%) | 73.33% (44.9–92.2%) | 0.01 |
| Histological core procurement | 13 (86.6%) | 6 (40%) | <0.001 |
| 22 G needle | |||
| EUS-FNB (75 patients) | EUS-FNA (75 patients) | ||
| Sensitivity | 91.6% (81.6–97.2%) | 74.6% (62.5–84.4%) | 0.01 |
| Specificity | 100% (78.2–100%) | 100% (63–100%) | 0.6 |
| Diagnostic adequacy | 71 (94.6%) | 63 (84%) | 0.03 |
| Diagnostic accuracy | 93.33% (85.1–97.8%) | 77.33% (66.2–86.2%) | 0.02 |
| Histological core procurement | 67 (89.3%) | 31 (41.3%) | <0.001 |
| 25 G needle | |||
| EUS-FNB (30 patients) | EUS-FNA (30 patients) | ||
| Sensitivity | 88% (68.7–97.4%) | 75% (55.1–89.3%) | 0.04 |
| Specificity | 100% (47.8–100%) | 100% (15.8–100%) | 0.8 |
| Diagnostic adequacy | 28 (93.3%) | 24 (80%) | 0.12 |
| Diagnostic accuracy | 90% (73.4–97.9%) | 76.67% (57.7–90%) | 0.03 |
| Histological core procurement | 25 (83.3%) | 12 (40%) | <0.001 |
| Metastases | |||
| EUS-FNB (74 patients) | EUS-FNA (70 patients) | ||
| Sensitivity | 88.7% (78.1–95.3%) | 74.6% (62.5–84.4%) | 0.05 |
| Specificity | 100% (63–100%) | 100% (29.2–100%) | 0.6 |
| Diagnostic adequacy | 69 (93.2%) | 57 (81.4%) | 0.66 |
| Diagnostic accuracy | 90% (80.4–95.8%) | 75.7% (64–85.7%) | 0.02 |
| Histological core procurement | 63 (85.1%) | 30 (42.8%) | <0.001 |
| Lymphoma | |||
| EUS-FNB (20 patients) | EUS-FNA (15 patients) | ||
| Sensitivity | 88.2% (63.5–98.5%) | 53.8% (25.1–80.8%) | 0.008 |
| Specificity | 100% (29.2–100%) | 100% (15.8–100%) | 0.3 |
| Diagnostic adequacy | 18 (90%) | 12 (80%) | 0.4 |
| Diagnostic accuracy | 90% (68.3–98.7%) | 60% (32.3–83.6%) | 0.006 |
| Histological core procurement | 17 (85%) | 4 (26.6%) | <0.001 |
| Benign disease | |||
| EUS-FNB (7 patients) | EUS-FNA (9 patients) | ||
| Sensitivity | 89.1% (67.5–98.1%) | 73.8% (65.1–82.5%) | 0.04 |
| Specificity | 100% (29.2–100%) | 100% (15.8–100%) | 0.3 |
| Diagnostic adequacy | 7 (100%) | 9 (100%) | 1.0 |
| Diagnostic accuracy | 91% (69.3–97.8%) | 73% (52.3–86.6%) | 0.03 |
| Histological core procurement | 7 (100%) | 2 (22.2%) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, A.; Crinò, S.F.; Muscatiello, N.; Gkolfakis, P.; Samanta, J.; Londoño Castillo, J.; Cotsoglou, C.; Ramai, D. Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study. Cancers 2021, 13, 4298. https://doi.org/10.3390/cancers13174298
Facciorusso A, Crinò SF, Muscatiello N, Gkolfakis P, Samanta J, Londoño Castillo J, Cotsoglou C, Ramai D. Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study. Cancers. 2021; 13(17):4298. https://doi.org/10.3390/cancers13174298
Chicago/Turabian StyleFacciorusso, Antonio, Stefano Francesco Crinò, Nicola Muscatiello, Paraskevas Gkolfakis, Jayanta Samanta, Juliana Londoño Castillo, Christian Cotsoglou, and Daryl Ramai. 2021. "Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study" Cancers 13, no. 17: 4298. https://doi.org/10.3390/cancers13174298
APA StyleFacciorusso, A., Crinò, S. F., Muscatiello, N., Gkolfakis, P., Samanta, J., Londoño Castillo, J., Cotsoglou, C., & Ramai, D. (2021). Endoscopic Ultrasound Fine-Needle Biopsy versus Fine-Needle Aspiration for Tissue Sampling of Abdominal Lymph Nodes: A Propensity Score Matched Multicenter Comparative Study. Cancers, 13(17), 4298. https://doi.org/10.3390/cancers13174298







