Metastatic Risk Factors Associated with Class 1A Uveal Melanoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Statistics
3. Results
3.1. Patient Characteristics
3.2. Outcomes
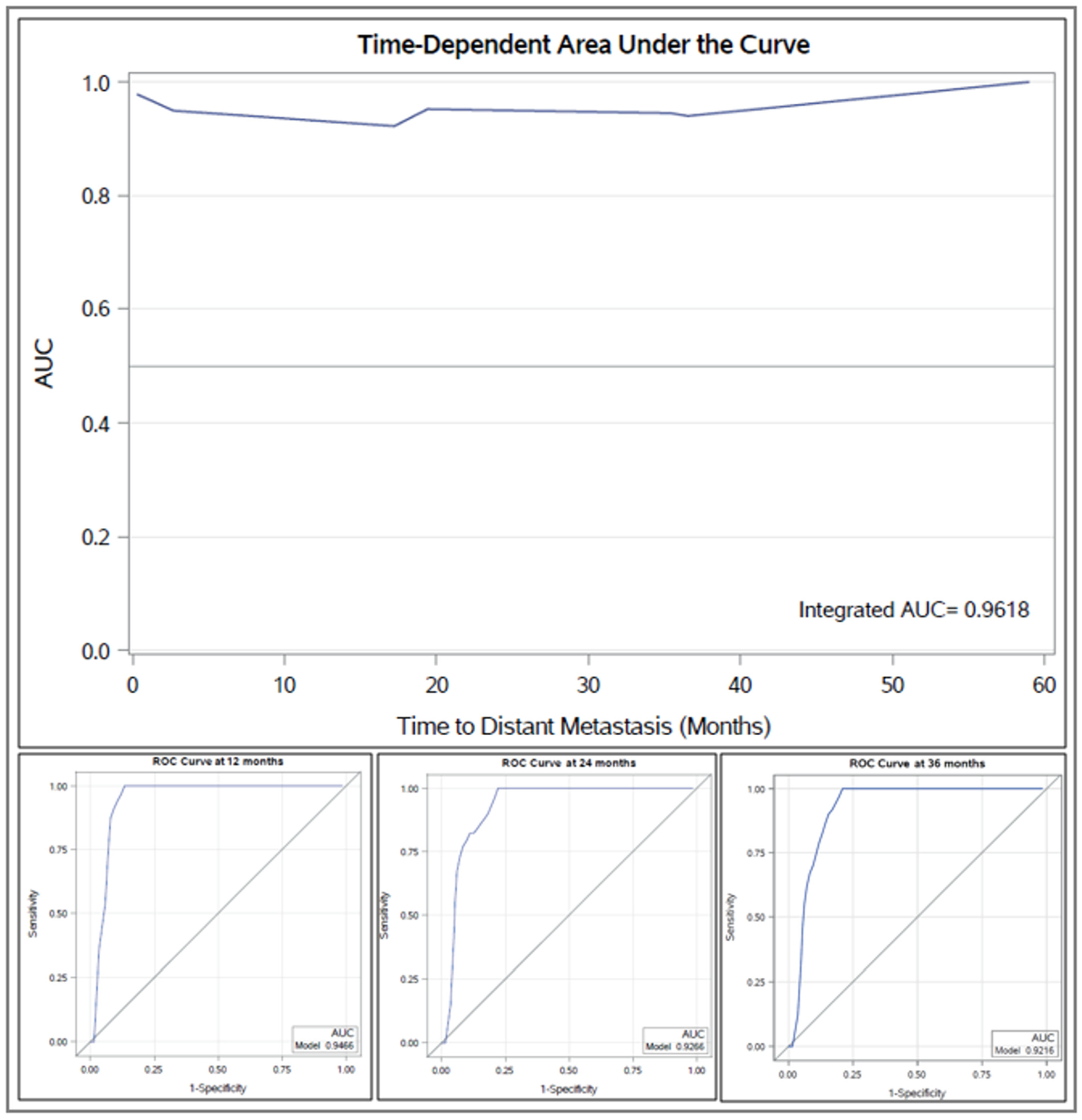
3.3. Predictors of Distant Metastasis Development and OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M., Jr.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onken, M.D.; Worley, L.A.; Harbour, J.W. Association between gene expression profile, proliferation and metastasis in uveal melanoma. Curr. Eye Res. 2010, 35, 857–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onken, M.D.; Worley, L.A.; Tuscan, M.D.; Harbour, J.W. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J. Mol. Diagn. 2010, 12, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Masshofer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Harbour, J.W.; Roberson, E.D.; Anbunathan, H.; Onken, M.D.; Worley, L.A.; Bowcock, A.M. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat. Genet. 2013, 45, 133–135. [Google Scholar] [CrossRef]
- Afshar, A.R.; Damato, B.E.; Stewart, J.M.; Zablotska, L.B.; Roy, R.; Olshen, A.B.; Joseph, N.M.; Bastian, B.C. Next-Generation Sequencing of Uveal Melanoma for Detection of Genetic Alterations Predicting Metastasis. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Field, M.G.; Decatur, C.L.; Kurtenbach, S.; Gezgin, G.; van der Velden, P.A.; Jager, M.J.; Kozak, K.N.; Harbour, J.W. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin. Cancer Res. 2016, 22, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- Field, M.G.; Durante, M.A.; Decatur, C.L.; Tarlan, B.; Oelschlager, K.M.; Stone, J.F.; Kuznetsov, J.; Bowcock, A.M.; Kurtenbach, S.; Harbour, J.W. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget 2016, 7, 59209–59219. [Google Scholar] [CrossRef] [Green Version]
- Schefler, A.C.; Koca, E.; Bernicker, E.H.; Correa, Z.M. Relationship between clinical features, GEP class, and PRAME expression in uveal melanoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 1541–1545. [Google Scholar] [CrossRef]
- Binkley, E.M.; Bena, J.F.; Davanzo, J.M.; Hinz, C.; Boldt, H.C.; Singh, A.D. Gene Expression Profiling Prognostication of Posterior Uveal Melanoma: Does Size Matter? Ophthalmol. Retina 2020, 4, 620–629. [Google Scholar] [CrossRef]
- Demirci, H.; Niziol, L.M.; Ozkurt, Z.; Slimani, N.; Ozgonul, C.; Liu, T.; Musch, D.C.; Materin, M. Do Largest Basal Tumor Diameter and the American Joint Committee on Cancer’s Cancer Staging Influence Prognostication by Gene Expression Profiling in Choroidal Melanoma. Am. J. Ophthalmol. 2018, 195, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Kim, R.S.; Bretana, M.E.; Kegley, E.; Schefler, A.C. Association between traditional clinical high-risk features and gene expression profile classification in uveal melanoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 421–427. [Google Scholar] [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Collaborative Ocular Melanoma Study, G. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study, G. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Diener-West, M.; Hawkins, B.S.; Markowitz, J.A.; Schachat, A.P. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch. Ophthalmol. 1992, 110, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [Green Version]
- Mahendraraj, K.; Lau, C.S.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7,516 patients from the Surveillance, Epidemiology, and End Results database (1973-2012). Clin. Ophthalmol. 2016, 10, 2113–2119. [Google Scholar] [CrossRef] [Green Version]
- Decatur, C.L.; Ong, E.; Garg, N.; Anbunathan, H.; Bowcock, A.M.; Field, M.G.; Harbour, J.W. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA Ophthalmol. 2016, 134, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study, G. Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Salama, A.K. New NCCN Guidelines for Uveal Melanoma and Treatment of Recurrent or Progressive Distant Metastatic Melanoma. J. Natl. Compr. Canc. Netw. 2018, 16, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.K.; Barker, C.; Coit, D.G.; Joseph, R.W.; Materin, M.; Rengan, R.; Sosman, J.; Thompson, J.A.; Albertini, M.R.; Boland, G.; et al. NCCN Guidelines Insights: Uveal Melanoma, Version 1.2019. J. Natl. Compr. Canc. Netw. 2020, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.A.; Grewal, P.; Lapere, S.; Larocque, M.; Murtha, A.; Weis, E. Optimising prediction of early metastasis-free survival in uveal melanoma using a four-category model incorporating gene expression profile and tumour size. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
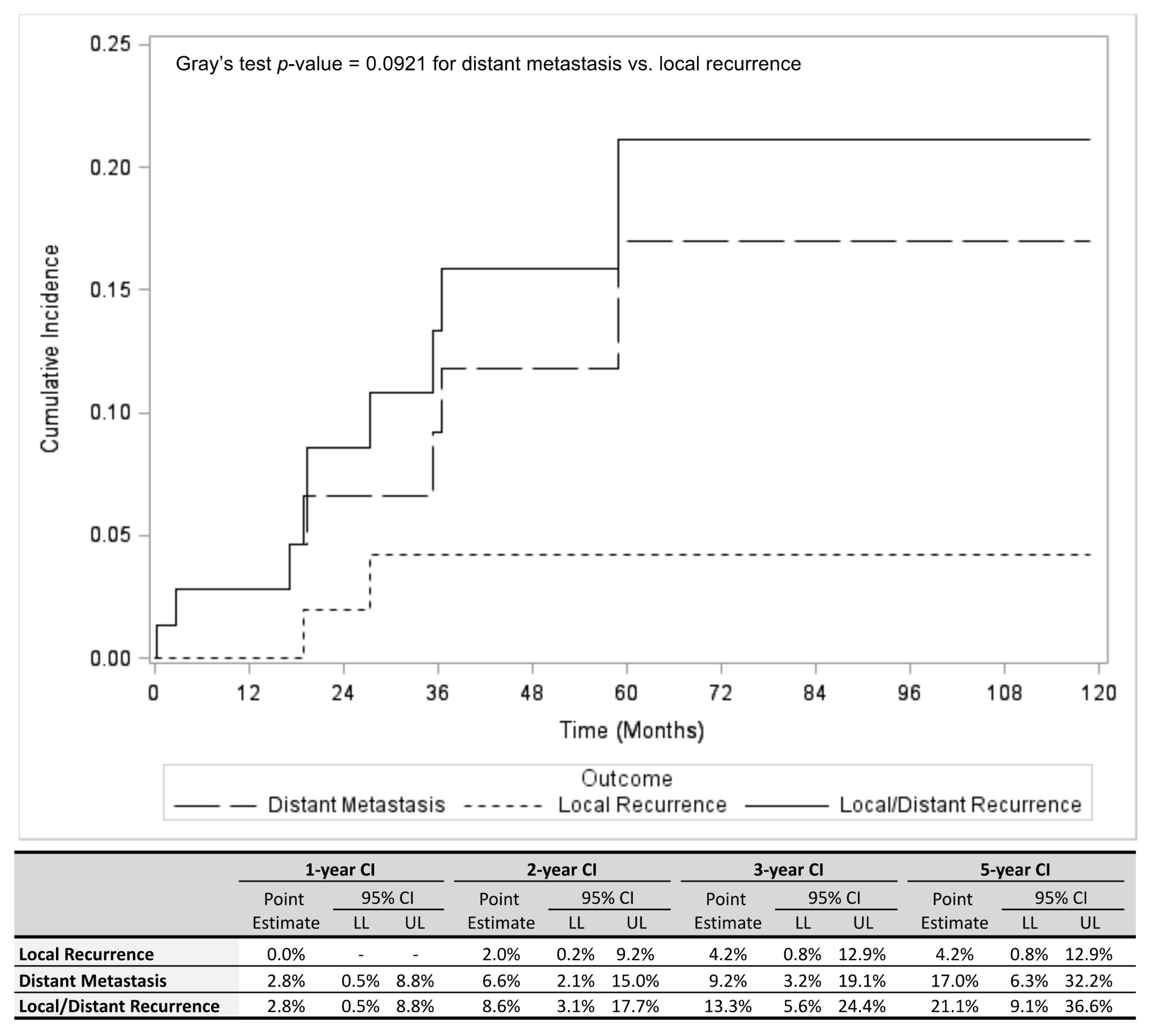
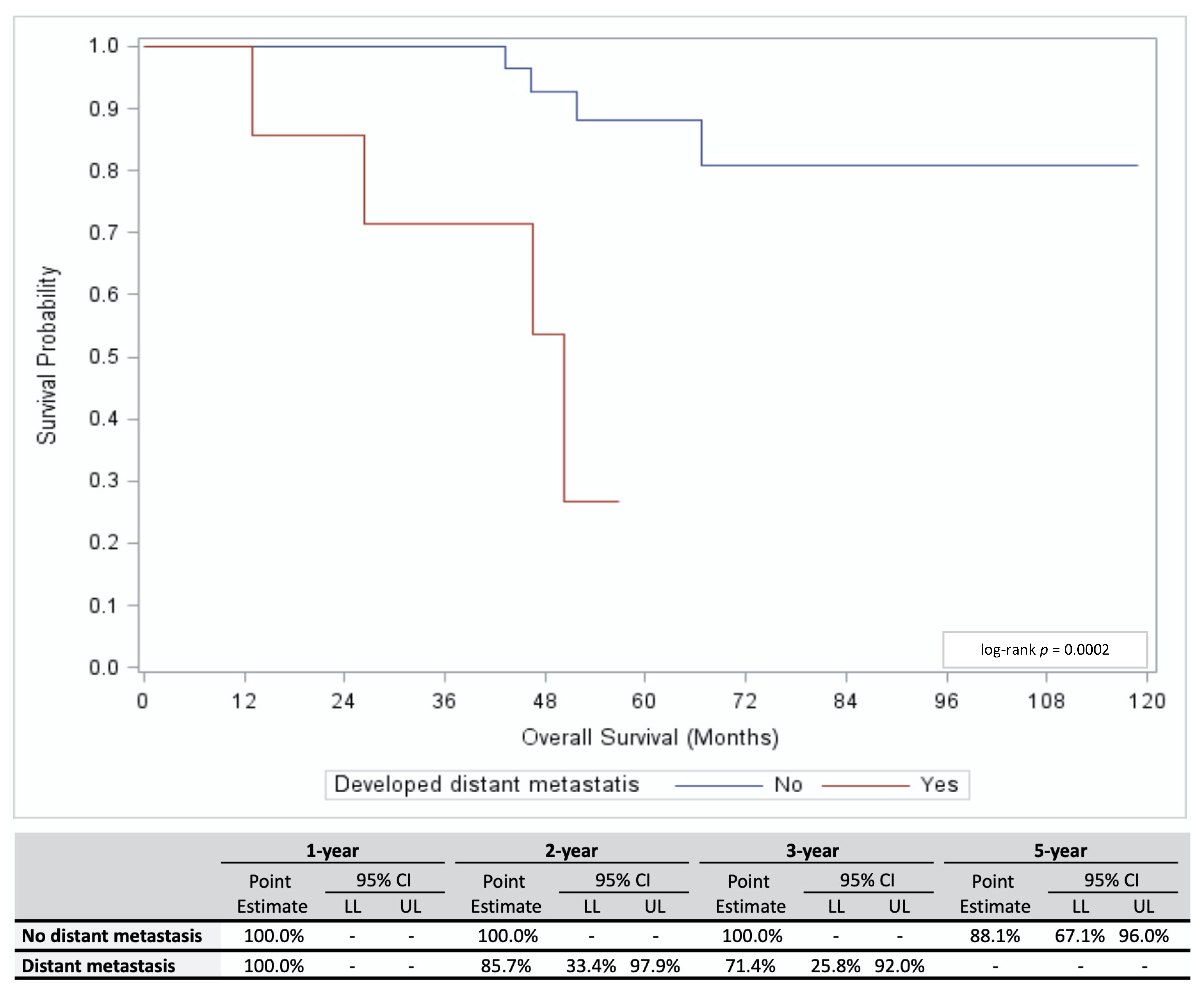
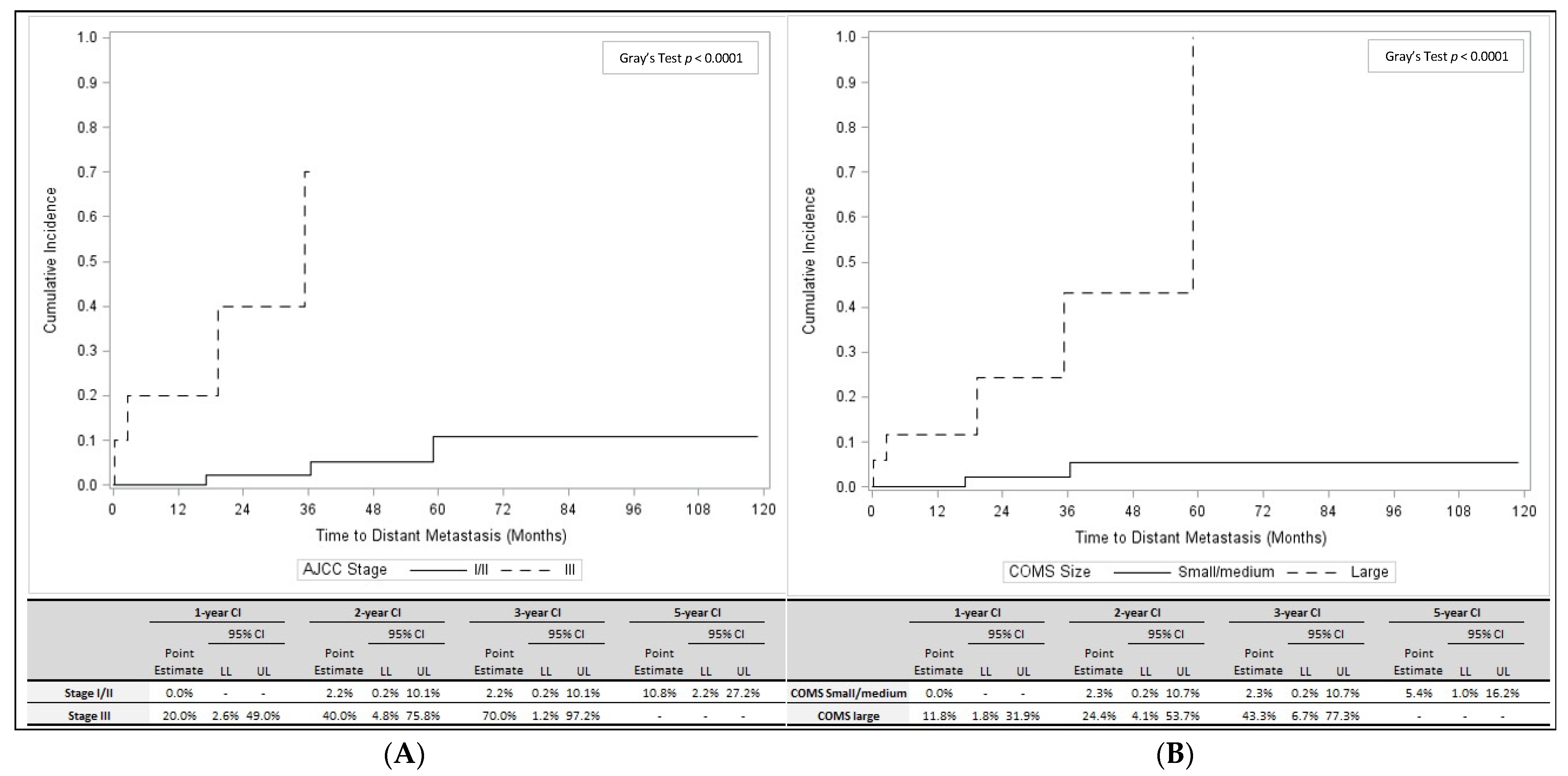
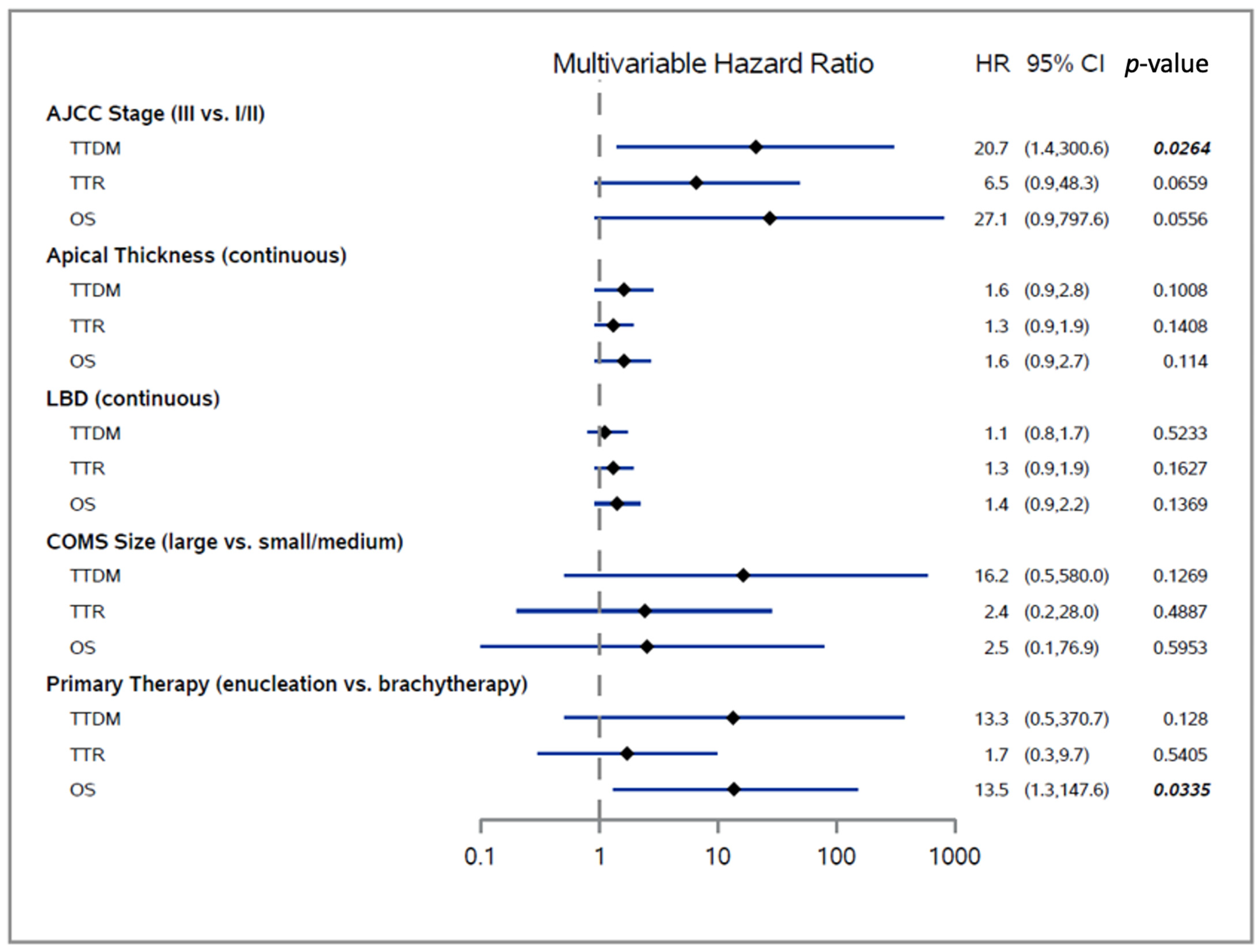
| Variable | All Patients (n = 73) | No Local Recurrence or Distant Metastases (n = 64) | Local Recurrence (n = 2) | Distant Metastasis (n = 7) | p-Value * (Distant Metastasis Yes vs. No) | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age at Initial Diagnosis | 0.0622 | ||||||||
| Mean (Standard Deviation) | 59.6 (11.3) | 58.9 (11.6) | 70.9 (18.2) | 62.5 (2.3) | |||||
| Median (Minimum, Maximum) | 61 (32, 86) | 59 (32, 86) | 71 (58, 84) | 62 (59, 65) | |||||
| Gender | 0.4484 | ||||||||
| Female | 42 | 57.5% | 38 | 59.4% | 1 | 50.0% | 3 | 42.9% | |
| Male | 31 | 42.5% | 26 | 40.6% | 1 | 50.0% | 4 | 57.1% | |
| AJCC Stage | 0.0116 | ||||||||
| I | 14 | 20.0% | 13 | 21.3% | 0 | 0.0% | 1 | 14.3% | |
| IIA | 27 | 38.6% | 24 | 39.3% | 2 | 100.0% | 1 | 14.3% | |
| IIB | 19 | 27.1% | 18 | 29.5% | 0 | 0.0% | 1 | 14.3% | |
| IIIA | 7 | 10.0% | 5 | 8.2% | 0 | 0.0% | 2 | 28.6% | |
| IIIB | 3 | 4.3% | 1 | 1.6% | 0 | 0.0% | 2 | 28.6% | |
| Apical Thickness (mm) | 0.2446 | ||||||||
| Mean (Standard Deviation) | 5.9 (3.2) | 5.7 (2.9) | 4.3 (1.3) | 8.2 (5.2) | |||||
| Median (Minimum, Maximum) | 5 (2.0, 15.7) | 4.6 (2.3, 14.6) | 4.3 (3.3, 5.2) | 8.5 (2.0, 15.7) | |||||
| LBD (mm) | 0.0048 | ||||||||
| Mean (Standard Deviation) | 12.6 (3.6) | 12.2 (3.3) | 13.3 (0.9) | 16.2 (4.9) | |||||
| Median (Minimum, Maximum) | 12.3 (4.5, 21.0) | 11.9 (4.5, 21.0) | 13.3 (12.6, 13.9) | 17.6 (7.0, 21.0) | |||||
| COMS Size | 0.0018 | ||||||||
| Small | 3 | 4.1% | 2 | 3.0% | 0 | 0.0% | 1 | 14.3% | |
| Medium | 53 | 72.6% | 52 | 78.8% | 2 | 100.0% | 1 | 14.3% | |
| Large | 17 | 23.3% | 12 | 18.2% | 0 | 0.0% | 5 | 71.4% | |
| PRAME | 0.3182 | ||||||||
| Positive | 7 | 9.6% | 6 | 9.4% | 0 | 0.0% | 1 | 14.3% | |
| Negative | 15 | 20.5% | 15 | 23.4% | 0 | 0.0% | 0 | 0.0% | |
| Not tested | 51 | 69.9% | 43 | 67.2% | 2 | 100.0% | 6 | 85.7% | |
| Mutation | 1.0000 | ||||||||
| GNAQ | 2 | 2.7% | 1 | 1.6% | 0 | 0.0% | 1 | 14.3% | |
| GNA11 | 2 | 2.7% | 0 | 0.0% | 0 | 0.0% | 2 | 28.6% | |
| Not Tested | 69 | 94.5% | 63 | 98.4% | 2 | 100.% | 4 | 57.1% | |
| Primary Site | 0.405 | ||||||||
| Choroidal | 68 | 93.2% | 60 | 93.8% | 2 | 100.0% | 6 | 85.7% | |
| Ciliary body | 5 | 6.8% | 4 | 6.3% | 0 | 0.0% | 1 | 14.3% | |
| Primary Therapy | 0.0497 | ||||||||
| Brachytherapy | 38 | 52.1% | 35 | 54.7% | 2 | 100.0% | 1 | 14.3% | |
| Enucleation | 35 | 47.9% | 29 | 45.3% | 0 | 0.0% | 6 | 85.7% | |
| Variable | Distant Metastasis | Local/Distant Recurrence | Overall Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||
| HR | LL | UL | p-Value | HR | LL | UL | p-Value | HR | LL | UL | p-Value | |
| Diagnosis Age (continuous) | 1.02 | 0.96 | 1.09 | 0.5101 | 1.04 | 0.98 | 1.11 | 0.1952 | 1.04 | 0.98 | 1.11 | 0.2151 |
| Gender (male vs. female) | 2.07 | 0.46 | 9.27 | 0.3419 | 1.92 | 0.52 | 7.16 | 0.3315 | 3.20 | 0.76 | 13.52 | 0.1129 |
| AJCC Stage (III vs. I/II) | 24.26 | 4.17 | 141.15 | 0.0004 | 11.99 | 2.87 | 50.04 | 0.0007 | 7.64 | 1.25 | 46.83 | 0.0280 |
| Apical Thickness (continuous) | 1.30 | 1.08 | 1.57 | 0.0057 | 1.22 | 1.03 | 1.45 | 0.0203 | 1.15 | 0.95 | 1.39 | 0.1506 |
| LBD (continuous) | 1.49 | 1.15 | 1.94 | 0.0026 | 1.39 | 1.12 | 1.72 | 0.0026 | 1.21 | 0.97 | 1.50 | 0.0889 |
| COMS Size (large vs. small/medium) | 16.95 | 3.12 | 92.02 | 0.0010 | 7.97 | 2.07 | 30.71 | 0.0026 | 2.76 | 0.52 | 14.67 | 0.2329 |
| Primary Site (ciliary body vs. choroidal) | 2.54 | 0.31 | 21.13 | 0.3885 | 1.92 | 0.24 | 15.40 | 0.5377 | 0.00 | 0.00 | - | 0.9949 |
| Primary Therapy (enucleation vs. brachytherapy) | 7.59 | 0.91 | 63.34 | 0.0610 | 2.44 | 0.61 | 9.79 | 0.2076 | 9.16 | 1.12 | 74.58 | 0.0385 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballhausen, A.; Urias, E.; Gruschkus, S.K.; Williams, M.; Glover, M.S.; Qin, Y.; Gombos, D.S.; Patel, S.P. Metastatic Risk Factors Associated with Class 1A Uveal Melanoma Patients. Cancers 2021, 13, 3292. https://doi.org/10.3390/cancers13133292
Ballhausen A, Urias E, Gruschkus SK, Williams M, Glover MS, Qin Y, Gombos DS, Patel SP. Metastatic Risk Factors Associated with Class 1A Uveal Melanoma Patients. Cancers. 2021; 13(13):3292. https://doi.org/10.3390/cancers13133292
Chicago/Turabian StyleBallhausen, Alexej, Elizabeth Urias, Stephen K. Gruschkus, Michelle Williams, Maura S. Glover, Yong Qin, Dan S. Gombos, and Sapna P. Patel. 2021. "Metastatic Risk Factors Associated with Class 1A Uveal Melanoma Patients" Cancers 13, no. 13: 3292. https://doi.org/10.3390/cancers13133292
APA StyleBallhausen, A., Urias, E., Gruschkus, S. K., Williams, M., Glover, M. S., Qin, Y., Gombos, D. S., & Patel, S. P. (2021). Metastatic Risk Factors Associated with Class 1A Uveal Melanoma Patients. Cancers, 13(13), 3292. https://doi.org/10.3390/cancers13133292






