NRG-HN003: Phase I and Expansion Cohort Study of Adjuvant Pembrolizumab, Cisplatin and Radiation Therapy in Pathologically High-Risk Head and Neck Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects Considerations
2.2. Study Design and Statistical Considerations
2.3. Treatment Plan
3. Results
3.1. Patient and Tumor Characteristics
3.2. Toxicity
3.3. Protocol Treatment
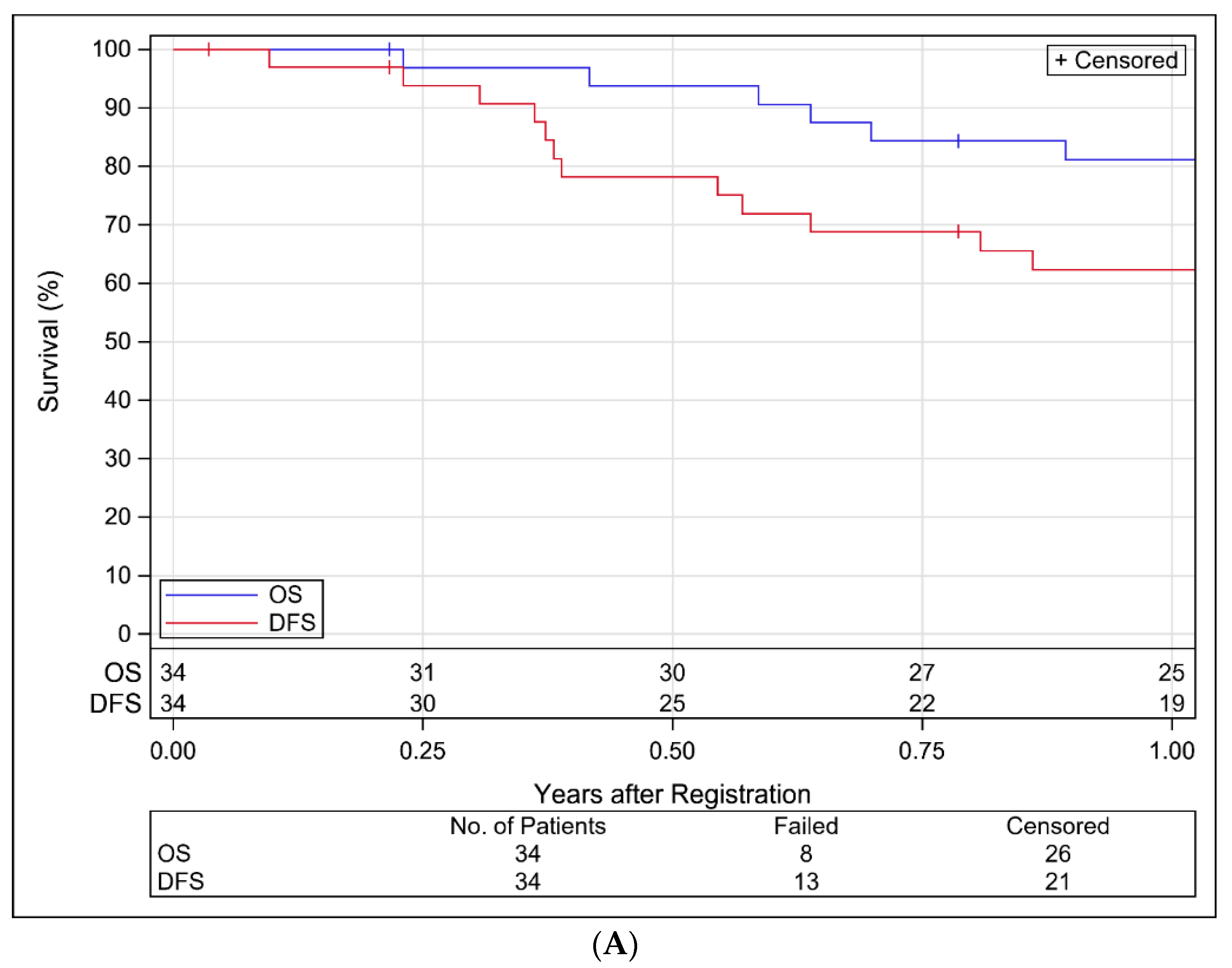
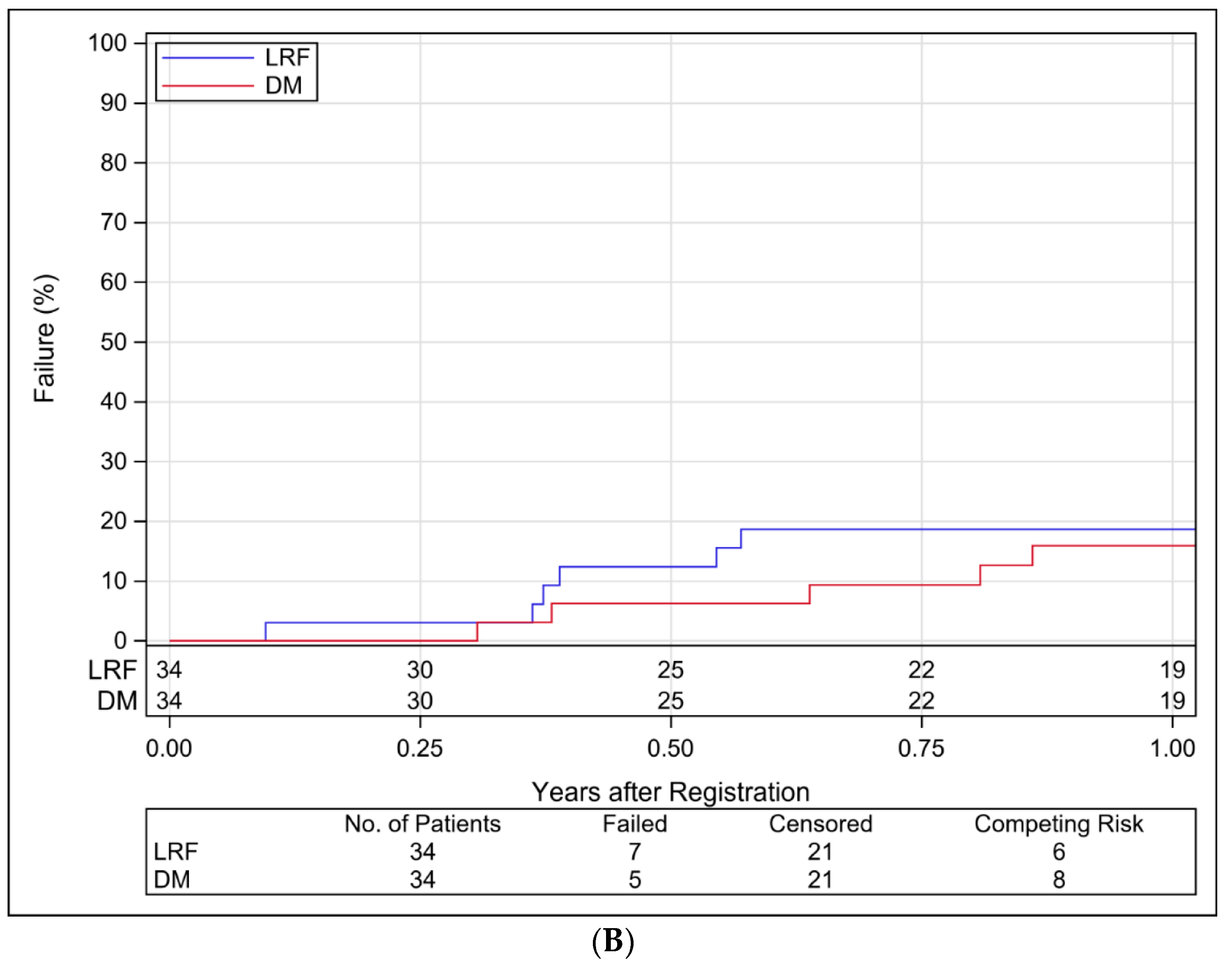
3.4. Study Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Brizel, D.M.; Albers, M.E.; Fisher, S.R.; Scher, R.L.; Richtsmeier, W.J.; Hars, V.; George, S.L.; Huang, A.T.; Prosnitz, L.R. Hyperfractionated Irradiation with or without Concurrent Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 1998, 338, 1798–1804. [Google Scholar] [CrossRef]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.; Harari, P.M.; Giralt, J.; Bell, D.; Raben, D.; Liu, J.; Schulten, J.; Ang, K.K.; Bonner, J.A. Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J. Clin. Oncol. 2016, 34, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Geiger, J.L.; Trivedi, S.; Schmitt, N.C.; Heron, D.E.; Johnson, J.T.; Kim, S.; Duvvuri, U.; Clump, D.A.; Bauman, J.E.; et al. Phase II trial of post-operative radiotherapy with concurrent cisplatin plus panitumumab in patients with high-risk, resected head and neck cancer. Ann. Oncol. 2016, 27, 2257–2262. [Google Scholar] [CrossRef]
- Huguenin, P.; Beer, K.T.; Allal, A.; Rufibach, K.; Friedli, C.; Davis, J.B.; Pestalozzi, B.; Schmid, S.; Thöni, A.; Ozsahin, M.; et al. Concomitant Cisplatin Significantly Improves Locoregional Control in Advanced Head and Neck Cancers Treated with Hyperfractionated Radiotherapy. J. Clin. Oncol. 2004, 22, 4665–4673. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Ir, M.V.G.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef]
- Cooper, J.S.; Zhang, Q.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Long-term Follow-up of the RTOG 9501/Intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. 2012, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Harari, P.M.; Harris, J.; Kies, M.S.; Myers, J.N.; Jordan, R.C.; Gillison, M.L.; Foote, R.L.; Machtay, M.; Rotman, M.; Khuntia, D.; et al. Postoperative Chemoradiotherapy and Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck: Radiation Therapy Oncology Group RTOG-0234. J. Clin. Oncol. 2014, 32, 2486–2495. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.T.; Duffy, S.; Teknos, T.N.; Islam, M.; Chen, Z.; Albert, P.S.; Wolf, G.T.; Van Waes, C. Nuclear Factor-κB–Related Serum Factors as Longitudinal Biomarkers of Response and Survival in Advanced Oropharyngeal Carcinoma. Clin. Cancer Res. 2007, 13, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, M.S.; Filho, P.A.A.; Ferrone, S.; Ferris, R.L. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2011, 60, 525–535. [Google Scholar] [CrossRef]
- Kuss, I.; Hathaway, B.; Ferris, R.L.; Gooding, W.; Whiteside, T.L. Decreased Absolute Counts of T Lymphocyte Subsets and Their Relation to Disease in Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2004, 10, 3755–3762. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005, 24, 95–105. [Google Scholar] [CrossRef]
- Hoffmann, T.K.; Dworacki, G.; Tsukihiro, T.; Meidenbauer, N.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin. Cancer Res. 2002, 8, 2553–2562. [Google Scholar] [PubMed]
- Dasgupta, S.; Bhattacharya-Chatterjee, M.; O’Malley, B.W.; Chatterjee, S.K. Inhibition of NK Cell Activity through TGF-β1 by Down-Regulation of NKG2D in a Murine Model of Head and Neck Cancer. J. Immunol. 2005, 175, 5541–5550. [Google Scholar] [CrossRef]
- Bauernhofer, T.; Kuss, I.; Henderson, B.; Baum, A.S.; Whiteside, T.L. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur. J. Immunol. 2003, 33, 119–124. [Google Scholar] [CrossRef]
- Baruah, P.; Lee, M.; Odutoye, T.; Williamson, P.; Hyde, N.; Kaski, J.C.; Dumitriu, I.E. Decreased levels of alternative co-stimulatory receptors OX40 and 4-1BB characterise T cells from head and neck cancer patients. Immunobiology 2012, 217, 669–675. [Google Scholar] [CrossRef]
- López-Albaitero, A.; Nayak, J.V.; Ogino, T.; Machandia, A.; Gooding, W.; DeLeo, A.B.; Ferrone, S.; Ferris, R.L. Role of Antigen-Processing Machinery in the In Vitro Resistance of Squamous Cell Carcinoma of the Head and Neck Cells to Recognition by CTL. J. Immunol. 2006, 176, 3402–3409. [Google Scholar] [CrossRef]
- Ferris, R.L.; Whiteside, T.L.; Ferrone, S. Immune Escape Associated with Functional Defects in Antigen-Processing Machinery in Head and Neck Cancer. Clin. Cancer Res. 2006, 12, 3890–3895. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1–Expressing Tumor-Infiltrating T Cells Are a Favorable Prognostic Biomarker in HPV-Associated Head and Neck Cancer. Cancer Res. 2012, 73, 128–138. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Hodge, J.W.; Guha, C.; Neefjes, J.; Gulley, J.L. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology 2008, 22, 1064–1084. [Google Scholar]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, S.; Formenti, S.C. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front. Oncol. 2012, 2, 153. [Google Scholar] [CrossRef]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.-B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Kiyota, N.; Tahara, M.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; Hanai, N.; Ogawa, T.; et al. Phase II/III trial of post-operative chemoradiotherapy comparing 3-weekly cisplatin with weekly cisplatin in high-risk patients with squamous cell carcinoma of head and neck (JCOG1008). J. Clin. Oncol. 2020, 38, 6502. [Google Scholar] [CrossRef]
- Powell, S.F.; Gitau, M.M.; Sumey, C.J.; Reynolds, J.T.; Lohr, M.; McGraw, S.; Nowak, R.K.; Terrell, A.M.; Jensen, A.W.; Blanchard, M.J.; et al. Safety of pembrolizumab with chemoradiation (CRT) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J. Clin. Oncol. 2017, 35, 6011. [Google Scholar] [CrossRef]
- Gillison, M.L.; Ferris, R.L.; Harris, J.; Colevas, A.D.; Mell, L.K.; Kong, C.; Jordan, R.C.; Moore, K.; Truong, M.T.; Kirsch, C.; et al. Safety and disease control achieved with the addition of nivolumab (Nivo) to chemoradiotherapy (CRT) for intermediate (IR) and high-risk (HR) local-regionally advanced head and neck squamous cell carcinoma (HNSCC): RTOG Foundation. J. Clin. Oncol. 2019, 37, 6073. [Google Scholar] [CrossRef]
- Egloff, A.M.; Lee, J.-W.; Langer, C.J.; Quon, H.; Vaezi, A.; Grandis, J.R.; Seethala, R.R.; Wang, L.; Shin, D.M.; Argiris, A.; et al. Phase II Study of Cetuximab in Combination with Cisplatin and Radiation in Unresectable, Locally Advanced Head and Neck Squamous Cell Carcinoma: Eastern Cooperative Oncology Group Trial E. Clin. Cancer Res. 2014, 20, 5041–5051. [Google Scholar] [CrossRef]
- Harrington, K.; Temam, S.; Mehanna, H.; D’Cruz, A.; Jain, M.; D’Onofrio, I.; Manikhas, G.; Horvath, Z.; Sun, Y.; Dietzsch, S.; et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients With Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Oncol. 2015, 33, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Ferris, R.; Psyrri, A.; Haddad, R.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.-H.; Lin, J.-C.; Razaq, M.; et al. 910O Primary results of the phase III JAVELIN head & neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). Ann. Oncol. 2020, 31, S658. [Google Scholar] [CrossRef]
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase 2 Trial. Clin. Cancer Res. 2020, 26. [Google Scholar] [CrossRef]
| Modality | Week of Adjuvant IMRT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loading | CRT | Maintenance | ||||||||||||
| −1 | 1 | 2 | 3 | 4 | 5 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | |
| IMRT (60 Gy, 2 Gy/Fx)—All | X | X | X | X | X | X | ||||||||
| Cisplatin 40 mg/m2 IV—All | X | X | X | X | X | X | ||||||||
| Pembrolizumab 200 mg IV | ||||||||||||||
| Schedule 3 (Starting) | X | X | X | X | X | X | X | X | ||||||
| Schedule 2 (1st de-escalation) | X | X | X | X | X | X | X | X | ||||||
| Schedule 1 (2nd de-escalation) | X | X | X | X | X | X | X | X | ||||||
| Characteristic | n | % |
|---|---|---|
| Age (years) | ||
| ≤65 | 27 | 79.4 |
| >65 | 7 | 20.6 |
| Gender | ||
| Male | 23 | 67.6 |
| Female | 11 | 32.4 |
| Race | ||
| American Indian/Alaska Native | 1 | 2.9 |
| Asian | 1 | 2.9 |
| Black or African American | 2 | 5.9 |
| White | 29 | 85.3 |
| Unknown or not reported | 1 | 2.9 |
| Ethnicity | ||
| Hispanic or Latino | 1 | 2.9 |
| Not Hispanic or Latino | 32 | 94.1 |
| Unknown or not reported | 1 | 2.9 |
| Zubrod performance status | ||
| 0 | 9 | 26.5 |
| 1 | 25 | 73.5 |
| Smoking history | ||
| Never | 14 | 41.2 |
| Former | 17 | 50.0 |
| Current | 3 | 8.8 |
| Smoking history: pack-years | ||
| ≤10 | 22 | 64.7 |
| >10 | 12 | 35.3 |
| Primary site | ||
| Oral cavity | 29 | 85.3 |
| Oropharynx, p16-negative | 1 | 2.9 |
| Hypopharynx | 2 | 5.9 |
| Larynx | 2 | 5.9 |
| Pathologic T stage | ||
| pT1 | 5 | 14.7 |
| pT2 | 10 | 29.4 |
| pT3 | 8 | 23.5 |
| pT4 | 11 | 32.4 |
| Pathologic N stage | ||
| pN0 | 1 | 2.9 |
| pN1 | 5 | 14.7 |
| pN2b | 14 | 41.2 |
| pN2c | 8 | 23.5 |
| pN3 | 6 | 17.6 |
| Extracapsular nodal extension (per institution) | ||
| No | 4 | 11.8 |
| Yes | 30 | 88.2 |
| Extracapsular nodal extension (central review) | ||
| No | 3 | 8.8 |
| Yes | 31 | 91.2 |
| Positive margin (per institution) | ||
| No | 27 | 79.4 |
| Yes | 7 | 20.6 |
| Positive margin (central review) | ||
| No | 27 | 79.4 |
| Yes | 7 | 20.6 |
| Any Relationship | Related to Treatment | Related to Pembrolizumab | ||||
|---|---|---|---|---|---|---|
| Term | n (%) by Grade | n (%) by Grade | n (%) by Grade | |||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Overall highest grade | 19 | 13 | 20 | 11 | 12 | 7 |
| (55.9) | (38.2) | (58.8) | (32.4) | (35.3) | (20.6) | |
| Lymphocyte count decreased | 10 | 8 | 11 | 6 | 6 | 4 |
| (29.4) | (23.5) | (32.4) | (17.6) | (17.6) | (11.8) | |
| Mucositis oral | 15 | 1 | 13 | 1 | 4 | 0 |
| (44.1) | (2.9) | (38.2) | (2.9) | (11.8) | (0.0) | |
| White blood cell decreased | 8 | 4 | 7 | 4 | 0 | 3 |
| (23.5) | (11.8) | (20.6) | (11.8) | (0.0) | (8.8) | |
| Dysphagia | 12 | 0 | 8 | 0 | 3 | 0 |
| (35.3) | (0.0) | (23.5) | (0.0) | (8.8) | (0.0) | |
| Neutrophil count decreased | 4 | 6 | 3 | 4 | 1 | 1 |
| (11.8) | (17.6) | (8.8) | (11.8) | (2.9) | (2.9) | |
| Hyponatremia | 9 | 1 | 7 | 1 | 1 | 1 |
| (26.5) | (2.9) | (20.6) | (2.9) | (2.9) | (2.9) | |
| Weight loss | 7 | 0 | 5 | 0 | 2 | 0 |
| (20.6) | (0.0) | (14.7) | (0.0) | (5.9) | (0.0) | |
| Anorexia | 6 | 0 | 6 | 0 | 1 | 0 |
| (17.6) | (0.0) | (17.6) | (0.0) | (2.9) | (0.0) | |
| Platelet count decreased | 6 | 0 | 4 | 0 | 1 | 0 |
| (17.6) | (0.0) | (11.8) | (0.0) | (2.9) | (0.0) | |
| Oral pain | 4 | 0 | 3 | 0 | 0 | 0 |
| (11.8) | (0.0) | (8.8) | (0.0) | (0.0) | (0.0) | |
| Dehydration | 3 | 0 | 2 | 0 | 0 | 0 |
| (8.8) | (0.0) | (5.9) | (0.0) | (0.0) | (0.0) | |
| Anemia | 3 | 0 | 1 | 0 | 1 | 0 |
| (8.8) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Lung infection | 1 | 1 | 1 | 1 | 0 | 0 |
| (2.9) | (2.9) | (2.9) | (2.9) | (0.0) | (0.0) | |
| Fatigue | 2 | 0 | 2 | 0 | 2 | 0 |
| (5.9) | (0.0) | (5.9) | (0.0) | (5.9) | (0.0) | |
| Nausea | 2 | 0 | 2 | 0 | 1 | 0 |
| (5.9) | (0.0) | (5.9) | (0.0) | (2.9) | (0.0) | |
| Febrile neutropenia | 2 | 0 | 2 | 0 | 0 | 0 |
| (5.9) | (0.0) | (5.9) | (0.0) | (0.0) | (0.0) | |
| Infections and infestations—Other | 2 | 0 | 1 | 0 | 1 | 0 |
| (5.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Dermatitis radiation | 2 | 0 | 1 | 0 | 0 | 0 |
| (5.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Sore throat | 2 | 0 | 1 | 0 | 0 | 0 |
| (5.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Soft tissue infection | 2 | 0 | 0 | 0 | 0 | 0 |
| (5.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Pleural effusion | 0 | 1 | 0 | 0 | 0 | 0 |
| (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Fever | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Lymphocyte count increased | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Pain | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Pharyngeal mucositis | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Rash maculo-papular | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Wound infection | 1 | 0 | 1 | 0 | 1 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (2.9) | (0.0) | |
| Dry mouth | 1 | 0 | 1 | 0 | 0 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Gastrointestinal disorders—Other | 1 | 0 | 1 | 0 | 0 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Metabolism and nutrition disorders—Other | 1 | 0 | 1 | 0 | 0 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Pharyngolaryngeal pain | 1 | 0 | 1 | 0 | 0 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Vomiting | 1 | 0 | 1 | 0 | 0 | 0 |
| (2.9) | (0.0) | (2.9) | (0.0) | (0.0) | (0.0) | |
| Aphonia | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Back pain | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Catheter-related infection | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Colitis | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Diarrhea | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Generalized muscle weakness | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Hepatobiliary disorders—Other | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Hypertension | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| INR increased | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Mucosal infection | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Musculoskeletal and connective tissue disorder—Other | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Skin and subcutaneous tissue disorders—Other | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Trismus | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Upper respiratory infection | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Vasovagal reaction | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Weight gain | 1 | 0 | 0 | 0 | 0 | 0 |
| (2.9) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Any Relationship | Related to Treatment | Related to Pembrolizumab | ||||
|---|---|---|---|---|---|---|
| Term | n (%) by Grade | n (%) by Grade | n (%) by Grade | |||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Overall highest grade | 6 | 2 | 5 | 1 | 3 | 0 |
| (21.4) | (7.1) | (17.9) | (3.6) | (10.7) | (0.0) | |
| Weight loss | 3 | 0 | 2 | 0 | 0 | 0 |
| (10.7) | (0.0) | (7.1) | (0.0) | (0.0) | (0.0) | |
| Dysphagia | 2 | 0 | 1 | 0 | 1 | 0 |
| (7.1) | (0.0) | (3.6) | (0.0) | (3.6) | (0.0) | |
| Anorexia | 2 | 0 | 1 | 0 | 0 | 0 |
| (7.1) | (0.0) | (3.6) | (0.0) | (0.0) | (0.0) | |
| Hyponatremia | 0 | 1 | 0 | 1 | 0 | 0 |
| (0.0) | (3.6) | (0.0) | (3.6) | (0.0) | (0.0) | |
| Hypercalcemia | 0 | 1 | 0 | 0 | 0 | 0 |
| (0.0) | (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Hypophosphatemia | 0 | 1 | 0 | 0 | 0 | 0 |
| (0.0) | (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Esophageal stenosis | 1 | 0 | 1 | 0 | 1 | 0 |
| (3.6) | (0.0) | (3.6) | (0.0) | (3.6) | (0.0) | |
| Pharyngeal mucositis | 1 | 0 | 1 | 0 | 1 | 0 |
| (3.6) | (0.0) | (3.6) | (0.0) | (3.6) | (0.0) | |
| Fracture | 1 | 0 | 1 | 0 | 0 | 0 |
| (3.6) | (0.0) | (3.6) | (0.0) | (0.0) | (0.0) | |
| Mucositis oral | 1 | 0 | 1 | 0 | 0 | 0 |
| (3.6) | (0.0) | (3.6) | (0.0) | (0.0) | (0.0) | |
| Infections and infestations—Other | 1 | 0 | 0 | 0 | 1 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (3.6) | (0.0) | |
| Abdominal pain | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Anemia | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Aspiration | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Dehydration | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Dermatitis radiation | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Fall | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Hypertension | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Ileus | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Vomiting | 1 | 0 | 0 | 0 | 0 | 0 |
| (3.6) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauman, J.E.; Harris, J.; Uppaluri, R.; Yao, M.; Ferris, R.L.; Chen, J.; Jordan, R.C.; Joshi, N.P.; Jujjuvaparu, S.; Blakaj, D.M.; et al. NRG-HN003: Phase I and Expansion Cohort Study of Adjuvant Pembrolizumab, Cisplatin and Radiation Therapy in Pathologically High-Risk Head and Neck Cancer. Cancers 2021, 13, 2882. https://doi.org/10.3390/cancers13122882
Bauman JE, Harris J, Uppaluri R, Yao M, Ferris RL, Chen J, Jordan RC, Joshi NP, Jujjuvaparu S, Blakaj DM, et al. NRG-HN003: Phase I and Expansion Cohort Study of Adjuvant Pembrolizumab, Cisplatin and Radiation Therapy in Pathologically High-Risk Head and Neck Cancer. Cancers. 2021; 13(12):2882. https://doi.org/10.3390/cancers13122882
Chicago/Turabian StyleBauman, Julie E., Jonathan Harris, Ravindra Uppaluri, Min Yao, Robert L. Ferris, Josephine Chen, Richard C. Jordan, Nikhil P. Joshi, Srinivas Jujjuvaparu, Dukagjin M. Blakaj, and et al. 2021. "NRG-HN003: Phase I and Expansion Cohort Study of Adjuvant Pembrolizumab, Cisplatin and Radiation Therapy in Pathologically High-Risk Head and Neck Cancer" Cancers 13, no. 12: 2882. https://doi.org/10.3390/cancers13122882
APA StyleBauman, J. E., Harris, J., Uppaluri, R., Yao, M., Ferris, R. L., Chen, J., Jordan, R. C., Joshi, N. P., Jujjuvaparu, S., Blakaj, D. M., Henson, C., Sheqwara, J., Mell, L. K., Sen, N., Clump, D. A., Garg, M. K., Yilmaz, E., Torres-Saavedra, P., & Le, Q.-T. (2021). NRG-HN003: Phase I and Expansion Cohort Study of Adjuvant Pembrolizumab, Cisplatin and Radiation Therapy in Pathologically High-Risk Head and Neck Cancer. Cancers, 13(12), 2882. https://doi.org/10.3390/cancers13122882






