Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
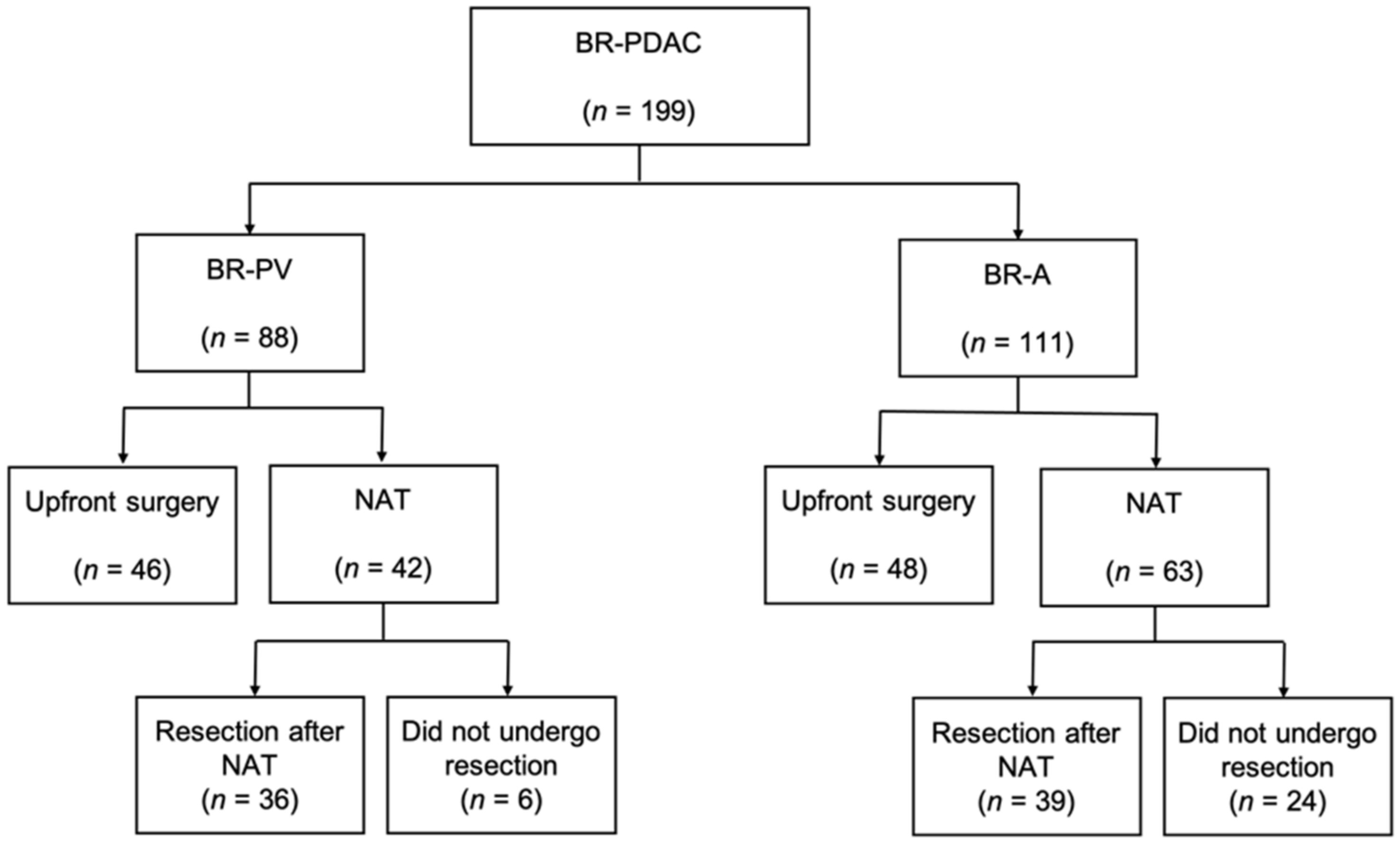
2.1. Cohort Outline
2.2. The Clinical Characteristics of BR-PDAC Patients
2.3. Comparison of Prognosis of Upfront Surgery vs. Neoadjuvant Treatment by Intention to Treat Analysis
2.4. Comparison of Regimens in Neoadjuvant Treatment Induction Cases
2.5. Prognostic Factors in Patients Who Underwent Resection after NAT
2.5.1. Definition of Cutoff Values for PNI
2.5.2. Univariate and Multivariate Analyses of Prognostic Factors in BR-PDAC Patients Who Underwent Resection after Neoadjuvant Treatment
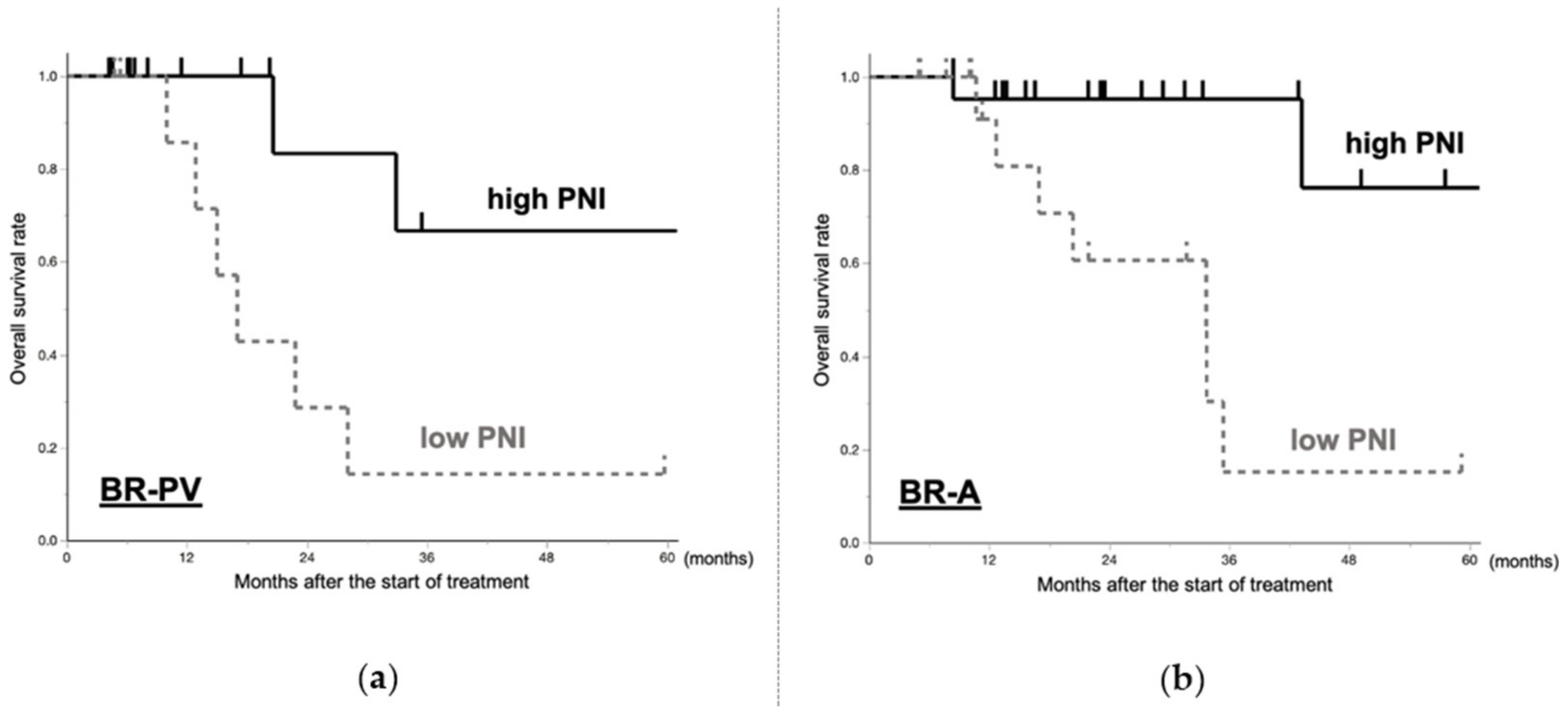
2.5.3. Prognosis of BR-PDAC Patients Who Underwent Resection after Neoadjuvant Treatment Based on PNI
3. Discussion
3.1. BR-PV
3.2. BR-A
3.3. BR-PV and BR-A
4. Materials and Methods
4.1. Study Design
- Comparison of prognosis of upfront surgery vs. NAT by intention to treat analysis;
- Comparison of regimens in patients who underwent NAT;
- Prognostic factors in patients who underwent resection after NAT.
4.2. Definitions of BR-PV PDAC and BR-A PDAC Patients
4.3. Neoadjuvant Treatment
4.4. Postoperative Adjuvant Therapy
4.5. Data Collection
4.6. Nutritional Status
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Traverso, L.W. Pancreatic cancer: Surgery alone is not sufficient. Surg. Endosc. 2006, 20, S446–S449. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines): NCCN, Pancreatic Adenocarcinoma, Version 1. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 27 September 2020).
- Japan Pancreas Society. Classification of Pancreatic Carcinoma, 4th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2017. [Google Scholar]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.; Evans, D.B.; Wolff, R.A. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Satoi, S.; Yamada, S.; Murotani, K.; Yanagimoto, H.; Takami, H.; Yamamoto, T.; Kanda, M.; Yamaki, S.; Hirooka, S.; et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J. Gastroenterol. 2016, 52, 81–93. [Google Scholar] [CrossRef]
- Takahashi, S.; Kinoshita, T.; Konishi, M.; Gotohda, N.; Kato, Y.; Kinoshita, T.; Kobayashi, T.; Mitsunaga, S.; Nakachi, K.; Ikeda, M. Borderline resectable pancreatic cancer: Rationale for multidisciplinary treatment. J. Hepatobiliary Pancreat. Sci. 2011, 18, 567–574. [Google Scholar] [CrossRef]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G.; et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Ohtsuka, T.; Kimura, R.; Matsuda, R.; Mori, Y.; Nakata, K.; Kakihara, D.; Fujimori, N.; Ohno, T.; Oda, Y.; et al. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann. Surg. Oncol. 2019, 26, 1528–1534. [Google Scholar] [CrossRef]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef]
- Motoi, F.; Satoi, S.; Honda, G.; Wada, K.; Shinchi, H.; Matsumoto, I.; Sho, M.; Tsuchida, A.; Unno, M.; Study Group of Preoperative Therapy for Pancreatic Cancer (PREP). A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J. Gastroenterol. 2019, 54, 194–203. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yamada, S.; Murotani, K.; Kanda, M.; Sugimoto, H.; Nakao, A.; Kodera, Y. Inverse Probability of Treatment Weighting Analysis of Upfront Surgery Versus Neoadjuvant Chemoradiotherapy Followed by Surgery for Pancreatic Adenocarcinoma with Arterial Abutment. Medicine (Baltimore) 2015, 94, e1647. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Satoi, S.; Sho, M.; Motoi, F.; Matsumoto, I.; Kawai, M.; Honda, G.; Uemura, K.; Yanagimoto, H.; Shinzeki, M.; et al. National Comprehensive Cancer Network Resectability Status for Pancreatic Carcinoma Predicts Overall Survival. World J. Surg. 2015, 39, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, Y.; Hosokawa, Y.; Nakayama, H.; Sahara, Y.; Takishita, C.; Nakajima, T.; Hijikata, Y.; Kasuya, K.; Katsumata, K.; Tokuuye, K.; et al. A phase II trial of neoadjuvant chemoradiotherapy with intensity-modulated radiotherapy combined with gemcitabine and S-1 for borderline-resectable pancreatic cancer with arterial involvement. Cancer Chemother. Pharmacol. 2017, 79, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Sahai, V.; Griffith, K.A.; Nathan, H.; Kaza, R.; Cuneo, K.C.; Shi, J.; Kim, E.; Sonnenday, C.J.; Cho, C.S.; et al. Phase 2 Trial of Neoadjuvant FOLFIRINOX and Intensity Modulated Radiation Therapy Concurrent With Fixed-Dose Rate-Gemcitabine in Patients With Borderline Resectable Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 124–133. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, S.; Suenaga, M.; Takami, H.; Niwa, Y.; Hayashi, M.; Iwata, N.; Kanda, M.; Tanaka, C.; Nakayama, G.; et al. Impact of the Controlling Nutritional Status Score on the Prognosis After Curative Resection of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 823–829. [Google Scholar] [CrossRef]
- Liang, R.-F.; Li, J.-H.; Li, M.; Yang, Y.; Liu, Y. The prognostic role of controlling nutritional status scores in patients with solid tumors. Clin. Chim. Acta 2017, 474, 155–158. [Google Scholar] [CrossRef]
- Baba, H.; Tokai, R.; Hirano, K.; Watanabe, T.; Shibuya, K.; Hashimoto, I.; Hojo, S.; Yoshioka, I.; Okumura, T.; Nagata, T.; et al. Risk factors for postoperative pneumonia after general and digestive surgery: A retrospective single-center study. Surg. Today 2020, 50, 460–468. [Google Scholar] [CrossRef]
- Kanda, M.; Fujii, T.; Kodera, Y.; Nagai, S.; Takeda, S.; Nakao, A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011, 98, 268–274. [Google Scholar] [CrossRef]
- Kawai, M.; Hirono, S.; Okada, K.-I.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Kobayashi, R.; Ueno, M.; Hayami, S.; Tanioka, K.; et al. Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery 2019, 165, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Yamada, S.; Sonohara, F.; Takami, H.; Suenaga, M.; Hayashi, M.; Niwa, Y.; Tanaka, C.; Kobayashi, D.; Nakayama, G.; et al. Clinical Impact of Neoadjuvant Therapy on Nutritional Status in Pancreatic Cancer. Ann. Surg. Oncol. 2018, 25, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Ziętarska, M.; Krawczyk-Lipiec, J.; Kraj, L.; Zaucha, R.; Małgorzewicz, S. Nutritional status assessment in colorectal cancer patients qualified to systemic treatment. Contemp. Oncol. (Pozn.) 2017, 21, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Devarajan, K.; Milestone, B.N.; Cooper, H.S.; Denlinger, C.; Cohen, S.J.; Meyer, J.E.; Hoffman, J.P. Neoadjuvant Chemoradiation and Duration of Chemotherapy Before Surgical Resection for Pancreatic Cancer: Does Time Interval Between Radiotherapy and Surgery Matter? Ann. Surg. Oncol. 2014, 21, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Satoi, S.; Yamaue, H.; Kato, K.; Takahashi, S.; Hirono, S.; Takeda, S.; Eguchi, H.; Sho, M.; Wada, K.; Shinchi, H.; et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: Results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J. Hepatobiliary Pancreat. Sci. 2013, 20, 590–600. [Google Scholar] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Liu, Q.; Zhang, R.; Cui, M.; Zhang, X.; Gao, X.; Guo, J.; Dai, M.; Zhang, T.; Liao, Q.; et al. Tumor size classification of the 8th edition of TNM staging system is superior to that of the 7th edition in predicting the survival outcome of pancreatic cancer patients after radical resection and adjuvant chemotherapy. Sci. Rep. 2018, 8, 10383. [Google Scholar] [CrossRef]
- Staley, C.A.; Cleary, K.R.; Abbruzzese, J.L.; Lee, J.E.; Ames, F.C.; Fenoglio, C.J.; Evans, D.B. The Need for Standardized Pathologic Staging of Pancreaticoduodenectomy Specimens. Pancreas 1996, 12, 373–380. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, Z.; Shao, Z.; Chen, W.; Xie, H.; Qin, G.; Zhao, N. Prognostic value of postdiagnostic inflammation-based scores in short-term overall survival of advanced pancreatic ductal adenocarcinoma patients. Medicine 2017, 96, e9247. [Google Scholar] [CrossRef] [PubMed]
- Sierzega, M.; Lenart, M.; Rutkowska, M.; Surman, M.; Mytar, B.; Matyja, A.; Siedlar, M.; Kulig, J. Preoperative Neutrophil-Lymphocyte and Lymphocyte-Monocyte Ratios Reflect Immune Cell Population Rearrangement in Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Jomrich, G.; Gruber, E.S.; Winkler, D.; Hollenstein, M.; Gnant, M.; Sahora, K.; Schindl, M. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J. Gastrointest. Surg. 2020, 24, 610–618. [Google Scholar] [CrossRef] [PubMed]


| Variable | BR-PV (n = 88) | BR-A (n = 111) | Variable | BR-PV (n = 88) | BR-A (n = 111) |
|---|---|---|---|---|---|
| Sex (male/female) | 51/37 | 55/56 | Surgical procedures | ||
| Age, years * | 66 (39–83) | 67 (42–83) | Pancreatoduodenectomy | 74 (84%) | 67 (60%) |
| Body mass index * | 21.1 (15.4–43.6) | 21.2 (11.6–30.7) | Distal pancreatectomy | 1 (1%) | 13 (12%) |
| Tumor location | Total pancreatectomy | 7 (8%) | 6 (5%) | ||
| Head/Uncinate | 84 (95%) | 83 (75%) | Operative time, min * | 508 (308–960) | 501 (193–808) |
| Body/Tail | 4 (5%) | 28 (25%) | Blood loss volume, mL * | 1075 (258–6000) | 1090 (80–9845) |
| CA19-9 at diagnosis, U/mL * | 179 (1–2900) | 150 (1–6340) | Operative PRBC transfusion | 34 (41%) | 30 (34%) |
| Chemotherapy | Vascular resection | ||||
| no | 46 (52%) | 48 (43%) | Any venous resection | 72 (82%) | 62 (56%) |
| FFX/GnP | 26 (30%) | 36 (32%) | Any arterial resection | 5 (6%) | 12 (14%) |
| GS | 2 (2%) | 27 (24%) | Celiac axis | 0 | 5 |
| GS + Radiation | 14 (16%) | 14 (13%) | Hepatic artery | 4 | 8 |
| Length of therapy, mo * | 2.1 (1.1–6.6) | 2.7 (0.2–12.9) | Splenic artery | 1 | 0 |
| Tumor size at operation, mm * | 30 (9–100) | 30 (10–100) | Both venous and arterial | 5 (6%) | 8 (9%) |
| CA19-9 at operation, U/mL * | 93 (1–9869) | 102 (1–7316) | Positive lymph nodes | 54 (66%) | 58 (67%) |
| in upfront surgery group | 196 (1–9869) | 321.5 (1–7316) | R0 margin status | 59 (72%) | 49 (56%) |
| in resection after NAT group | 41 (1–1500) | 34 (1–2690) | 90-day operative mortality | 1 (1%) | 1 (1%) |
| CA19-9 normalized | 17 (47%) | 17 (27%) | Adjuvant chemotherapy | 56 (68%) | 75 (86%) |
| CA19-9 decrease rate ≥90% | 7 (19%) | 9 (19%) | Recurrent disease | 42 (51%) | 58 (67%) |
| Nutrition at operation | Vital status at last follow-up | ||||
| CONUT * | 2 (0–11) | 2 (0–11) | Alive, no evidence of recurrence | 32 (36%) | 34 (31%) |
| GPS * | 0 (0–2) | 0 (0–2) | Alive, with recurrence | 7 (8%) | 19 (17%) |
| mGPS * | 0 (0–2) | 0 (0–2) | Not alive | 49 (56%) | 58 (52%) |
| NLR * | 2.4 (0.8–20.4) | 2.5 (0.7–15) | |||
| PLR * | 129.2 (0.1–416.5) | 83.0 (0.05–522.5) | |||
| PNI * | 46.0 (28.5–56.2) | 44.5 (26.3–55.5) | |||
| LMR * | 3.6 (1.0–40.4) | 3.8 (1.0–10.9) | |||
| SII * | 380.1 (0.2–2180.5) | 482.8 (0.1–3669.2) | |||
| CRP/Alb * | 0.07 (0–2.0) | 0.03 (0.002–2.2) |
| Variable | BR-PV (n = 82) | BR-A (n = 87) |
|---|---|---|
| Sex (male/female) | 47/35 | 44/43 |
| Age, years * | 65 (39–83) | 67 (42–83) |
| Chemotherapy | ||
| no | 46 (56%) | 48 (55%) |
| FFX/GnP | 26 (32%) | 36 (30%) |
| GS | 2 (2%) | 27 (31%) |
| GS + Radiation | 14 (17%) | 14 (16%) |
| Tumor size at operation, mm * | 30 (9–100) | 30 (10–100) |
| CA19-9 at operation, U/mL * | 93 (1–9869) | 102 (1–7316) |
| in upfront surgery group | 196 (1–9869) | 321.5 (1–7316) |
| in resection after NAT group | 41 (1–1500) | 34 (1–2690) |
| CA19-9 normalized | 17 (21%) | 17 (20%) |
| Surgical procedures | ||
| Pancreatoduodenectomy | 74 (90%) | 67 (77%) |
| Distal pancreatectomy | 1 (1%) | 13 (15%) |
| Total pancreatectomy | 7 (10%) | 6 (7%) |
| Operative time, min * | 508 (308–960) | 501 (193–808) |
| Blood loss volume, mL * | 1075 (258–6000) | 1090 (80–9845) |
| Operative PRBC transfusion | 34 (41%) | 30 (34%) |
| Vascular resection | ||
| Any venous resection | 72 (88%) | 62 (71%) |
| Any arterial resection | 5 (6%) | 12 (14%) |
| Celiac axis | 0 | 5 |
| Hepatic artery | 4 | 8 |
| Splenic artery | 1 | 0 |
| Both venous and arterial | 5 (6%) | 8 (9%) |
| Positive lymph nodes | 54 (66%) | 58 (67%) |
| R0 margin status | 59 (72%) | 49 (56%) |
| 90-day operative mortality | 1 (1%) | 1 (1%) |
| Adjuvant chemotherapy | 56 (68%) | 75 (86%) |
| Variable | BR-PV (n = 88) | BR-A (n = 111) | ||
|---|---|---|---|---|
| UFS (n = 46) | NAT (n = 42) | UFS (n = 48) | NAT (n = 63) | |
| Sex (male/female) | 27/19 | 24/18 | 27/22 | 28/35 |
| Age, years * | 64 (39–83) | 66 (40–81) | 66 (42–83) | 68 (45–82) |
| Body mass index * | 20.1 (15.5–32.1) | 21.4 (15.4–43.6) | 20.9 (17.1–27.5) | 21.4 (11.6–30.7) |
| Tumor location | ||||
| Head/Uncinate | 44 (96%) | 40 (95%) | 38 (79%) | 45 (71%) |
| Body/Tail | 2 (4%) | 2 (5%) | 10 (21%) | 18 (29%) |
| CA19-9 at diagnosis, U/mL * | N/A | 178.5 (1–2900) | N/A | 150 (1–6340) |
| CA19-9 at operation, U/mL * | 196 (1–9869) | 41 (1–5661) | 321 (1–7316) | 65 (1–5870) |
| Comorbidity (yes/no) | ||||
| Diabetes | 20/26 | 11/26 | 23/25 | 13/34 |
| History of other cancers | 6/40 | 1/36 | 5/43 | 8/40 |
| Pancreatitis | 5/41 | 0/37 | 11/37 | 1/47 |
| Hepatitis | 2/44 | 1/36 | 4/44 | 3/45 |
| Hypertension | 11/35 | 12/25 | 13/35 | 19/29 |
| Renal dysfunction | 1/45 | 0/37 | 0/48 | 0/48 |
| Nutrition at operation | ||||
| CONUT * | 1.5 (0–10) | 3 (0–11) | 2 (0–11) | 2 (0–10) |
| GPS * | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0–1) |
| mGPS * | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| NLR * | 2.2 (1.1–20.4) | 2.9 (0.8–8.6) | 2.6 (1.0–9.7) | 2.5 (0.7–15) |
| PLR * | 97.7 (0.1–325.5) | 166 (67.4–416.5) | 104.2 (0.05–290) | 184.9 (57.9–522.5) |
| PNI * | 46.3 (29.5–56.2) | 44.8 (28.5–52.5) | 44.5 (26.3–55.5) | 43 (32–51.5) |
| LMR * | 4.1 (2.2–6.5) | 3.1 (1.0–40.4) | 5.4 (1.4–10.9) | 3.5 (1–6.1) |
| SII * | 300 (0.2–2180.5) | 600 (168.4–1786.9) | 376.9 (0.1–1944.4) | 530.8 (95.1–3669.2) |
| CRP/Alb * | 0.07 (0–2.0) | 0.07 (0–3.3) | 0.02 (0.002–1.0) | 0.03 (0.002–2.2) |
| BR-PV (n = 42) | BR-A (n = 63) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | MST (Months) | CA19-9 Normalized | Resection | R0 Rate | Evans Grade ≥IIb | n | MST (Months) | CA19-9 Normalized | Resection | R0 Rate | Evans Grade ≥IIb | |
| FFX/GnP | 26 | 32.9 | 47% | 22 (85%) | 86% | 24% | 29 | 35.4 | 40% | 17 (59%) | 71% | 21% |
| FFX/GnP with RT | 0 | 7 | 18.7 | 75% | 4 (57%) | 100% | 0% | |||||
| Old NAC | 2 | 10 | 0% | 2 (100%) | 50% | 0% | 10 | 43.2 | 38% | 9 (90%) | 67% | 0% |
| Old NAC with RT | 14 | 20.6 | 50% | 12 (86%) | 100% | 36% | 17 | 19.7 | 20% | 9 (53%) | 100% | 33% |
| BR-PV Univariate | BR-PV Multivariate | BR-A Univariate | BR-A Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Factor | No. Patients (n = 36) | HR (95% CI) | p | HR (95% CI) | p | No. Patients (n = 39) | HR (95% CI) | p | HR (95% CI) | p |
| Radiation in NAT | 12 | 0.96 (0.23–4.04) | 0.955 | 12 | 1.37 (0.38–4.87) | 0.63 | ||||
| CA19-9 Before NAT >192 U/mL | 17 | 2.37 (0.58–9.64) | 0.23 | 19 | 0.41 (0.22–1.48) | 0.175 | ||||
| Preoperative CA19-9 >34 U/mL | 17 | 1.05 (0.26–4.27) | 0.945 | 19 | 3.90 (0.97–15.72) | 0.056 | ||||
| Tumor marker normalization | 20 | 0.16 (0.031–0.82) | 0.028 * | 0.28 (0.05–1.71) | 0.168 | 16 | 0.10 (0.01–0.83) | 0.033 * | 0.15 (0.01–1.57) | 0.064 |
| Preoperative Alb >3.8 g/dL | 14 | 0.55 (0.14–2.24) | 0.404 | 17 | 0.30 (0.06–1.46) | 0.137 | ||||
| Preoperative CONUT score, >4 | 5 | 3.78 (0.92–15.56) | 0.065 | 9 | 2.48 (0.66–9.34) | 0.179 | ||||
| Preoperative GPS 0 | 16 | 0.15 (0.03–0.79) | 0.025 * | 0.50 (0.05–4.68) | 0.547 | 10 | 0.52 (0.07–3.78) | 0.52 | ||
| Preoperative mGPS 0 | 14 | 0.25 (0.05–1.28) | 0.095 | 22 | 0.26 (0.06–1.08) | 0.064 | ||||
| Preoperative NLR >2.52 | 16 | 3.17 (0.39–25.86) | 0.281 | 18 | 1.47 (0.39–5.51) | 0.571 | ||||
| Preoperative PLR >184 | 11 | 0.97 (0.23–4.10) | 0.972 | 19 | 4.15 (0.85–20.26) | 0.079 | ||||
| Preoperative PNI >42.5 | 16 | 0.15 (0.03–0.76) | 0.022 * | 0.32 (0.03–2.98) | 0.316 | 21 | 0.13 (0.03–0.65) | 0.013 * | 0.15 (0.02–0.85) | 0.014 * |
| Preoperative LMR >3.50 | 9 | 0.19 (0.02–1.72) | 0.141 | 14 | 0.42 (0.11–1.64) | 0.214 | ||||
| Preoperative SII >512 | 15 | 0.89 (0.21–3.74) | 0.873 | 19 | 4.34 (0.84–22.49) | 0.08 | ||||
| Preoperative CRP/Alb >0.062 | 12 | 2.20 (0.43–11.16) | 0.341 | 10 | 1.75 (0.43–7.08) | 0.431 | ||||
| Preoperative diabetes | 11 | 0.78 (0.18–3.33) | 0.739 | 13 | 0.91 (0.23–3.55) | 0.9 | ||||
| Preoperative treatment period >60 day | 19 | 1.23 (0.15–10.09) | 0.844 | 29 | 0.46 (0.12–1.67) | 0.237 | ||||
| Preoperative treatment period >90 day | 7 | 1.72 (0.34–8.67) | 0.509 | 14 | 1.07 (0.28–4.18) | 0.918 | ||||
| Operative time >560 min | 7 | 3.12 (0.74–13.14) | 0.121 | 20 | 1.73 (0.49–6.09) | 0.396 | ||||
| Intraoperative blood loss >830 ml | 21 | 1.06 (0.25–4.45) | 0.941 | 20 | 7.42 (1.53–36.1) | 0.013 * | 2.23 (0.37–13.35) | 0.358 | ||
| Resectability | SubClass | Detail |
|---|---|---|
| 1. Resectable (R) | No contact of the tumor with the SMV/PV. | |
| Abutment/encasement of the SMV/PV of <180° circumference without occlusion (termed R-PV). | ||
| No contact with any major artery (CA, SMA, or CHA). | ||
| 2. Borderline resectable (BR) | BR-PV | |
| Tumor abutment/encasement or occlusion of the SMV/PV of ≥180°. | ||
| No arterial tumor abutment/encasement (CA, SMA, or CHA). | ||
| BR-A | ||
| Tumor abutment/encasement of the SMA or CA of <180° without irregularity in the contour of the artery. | ||
| Tumor abutment/encasement of the CHA without irregularity in the contour of the PHA or CA. | ||
| 3. Unresectable (UR) | UR-LA (Locally advance) | |
| Tumor abutment/encasement of the SMA or CA of ≥180°. | ||
| Tumor abutment/encasement of the CHA and extension of abutment/encasement to the PHA or CA. | ||
| Tumor abutment/encasement of the aorta. | ||
| UR-M (Metastasis) | ||
| Distant metastases, including metastases to lymph nodes beyond regional lymph nodes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, N.; Yamada, S.; Takami, H.; Murotani, K.; Yoshioka, I.; Shibuya, K.; Sonohara, F.; Hoshino, Y.; Hirano, K.; Watanabe, T.; et al. Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer. Cancers 2021, 13, 36. https://doi.org/10.3390/cancers13010036
Kimura N, Yamada S, Takami H, Murotani K, Yoshioka I, Shibuya K, Sonohara F, Hoshino Y, Hirano K, Watanabe T, et al. Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer. Cancers. 2021; 13(1):36. https://doi.org/10.3390/cancers13010036
Chicago/Turabian StyleKimura, Nana, Suguru Yamada, Hideki Takami, Kenta Murotani, Isaku Yoshioka, Kazuto Shibuya, Fuminori Sonohara, Yui Hoshino, Katsuhisa Hirano, Toru Watanabe, and et al. 2021. "Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer" Cancers 13, no. 1: 36. https://doi.org/10.3390/cancers13010036
APA StyleKimura, N., Yamada, S., Takami, H., Murotani, K., Yoshioka, I., Shibuya, K., Sonohara, F., Hoshino, Y., Hirano, K., Watanabe, T., Baba, H., Mori, K., Miwa, T., Kanda, M., Hayashi, M., Matsui, K., Okumura, T., Kodera, Y., & Fujii, T. (2021). Optimal Preoperative Multidisciplinary Treatment in Borderline Resectable Pancreatic Cancer. Cancers, 13(1), 36. https://doi.org/10.3390/cancers13010036






