Lifestyle Intervention on Body Weight and Physical Activity in Patients with Breast Cancer Can Reduce the Risk of Death in Obese Women: The EMILI Study
Abstract
:1. Introduction
2. Results
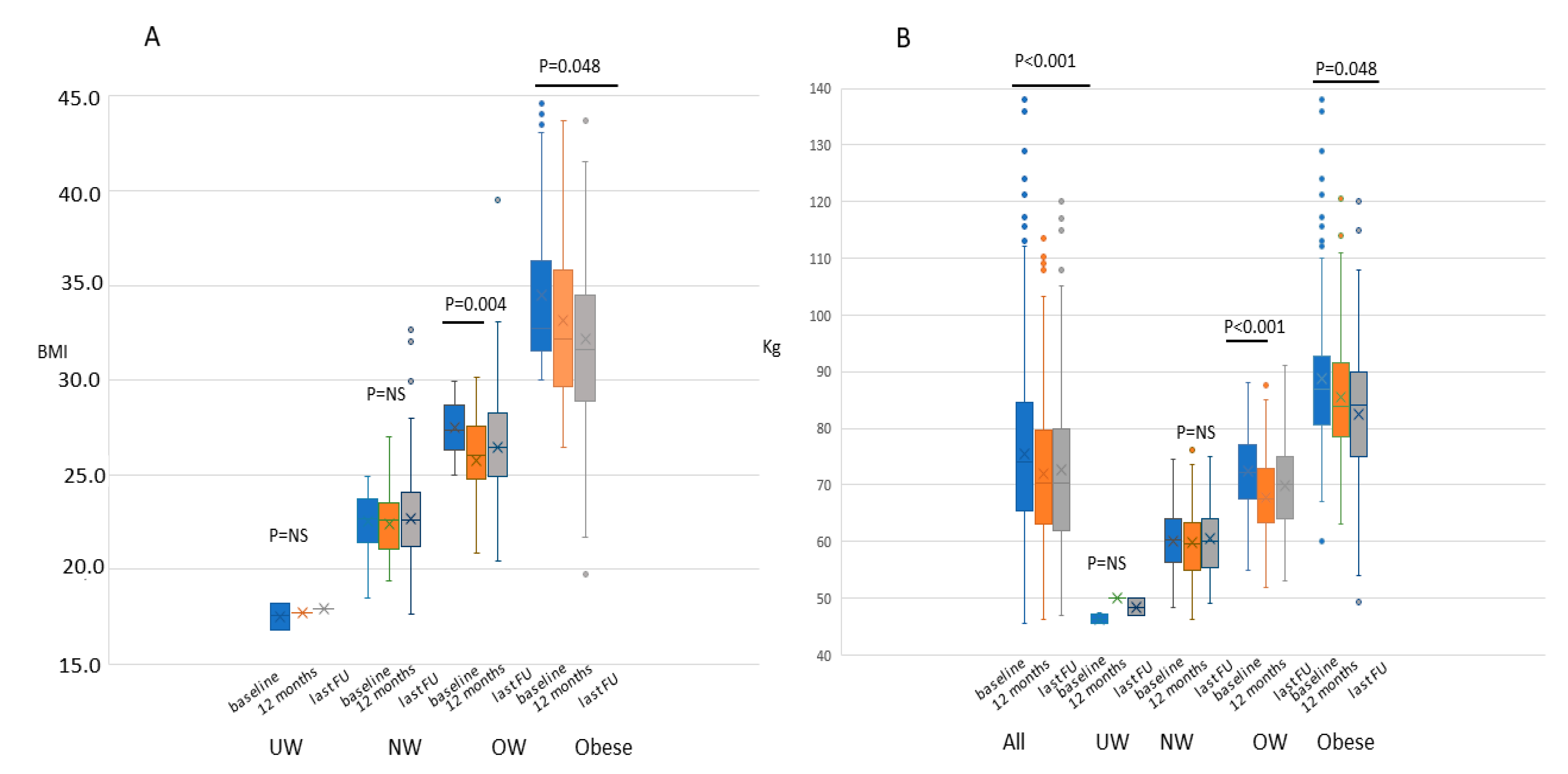
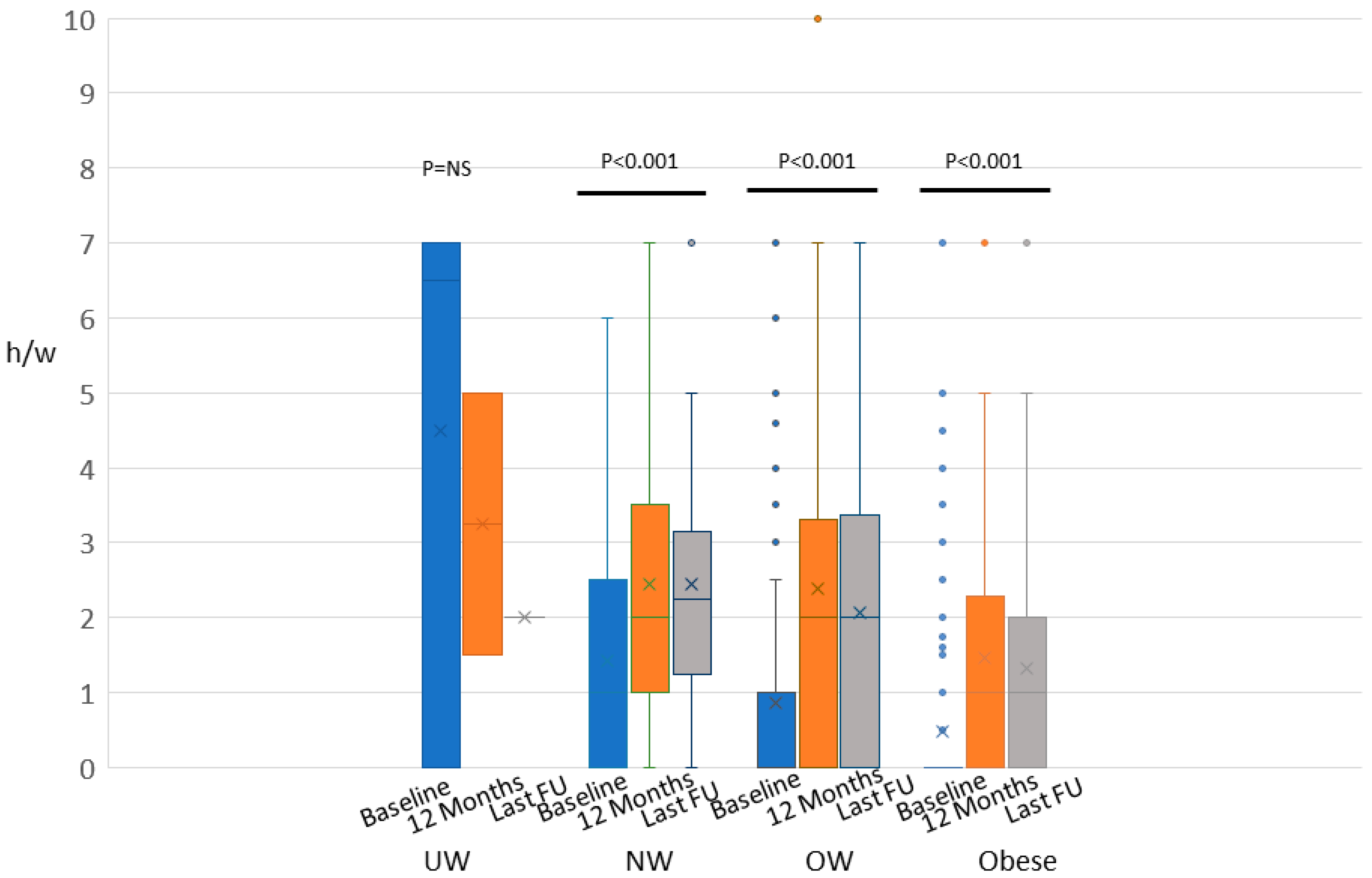
2.1. Weight, BMI, and Physical Activity
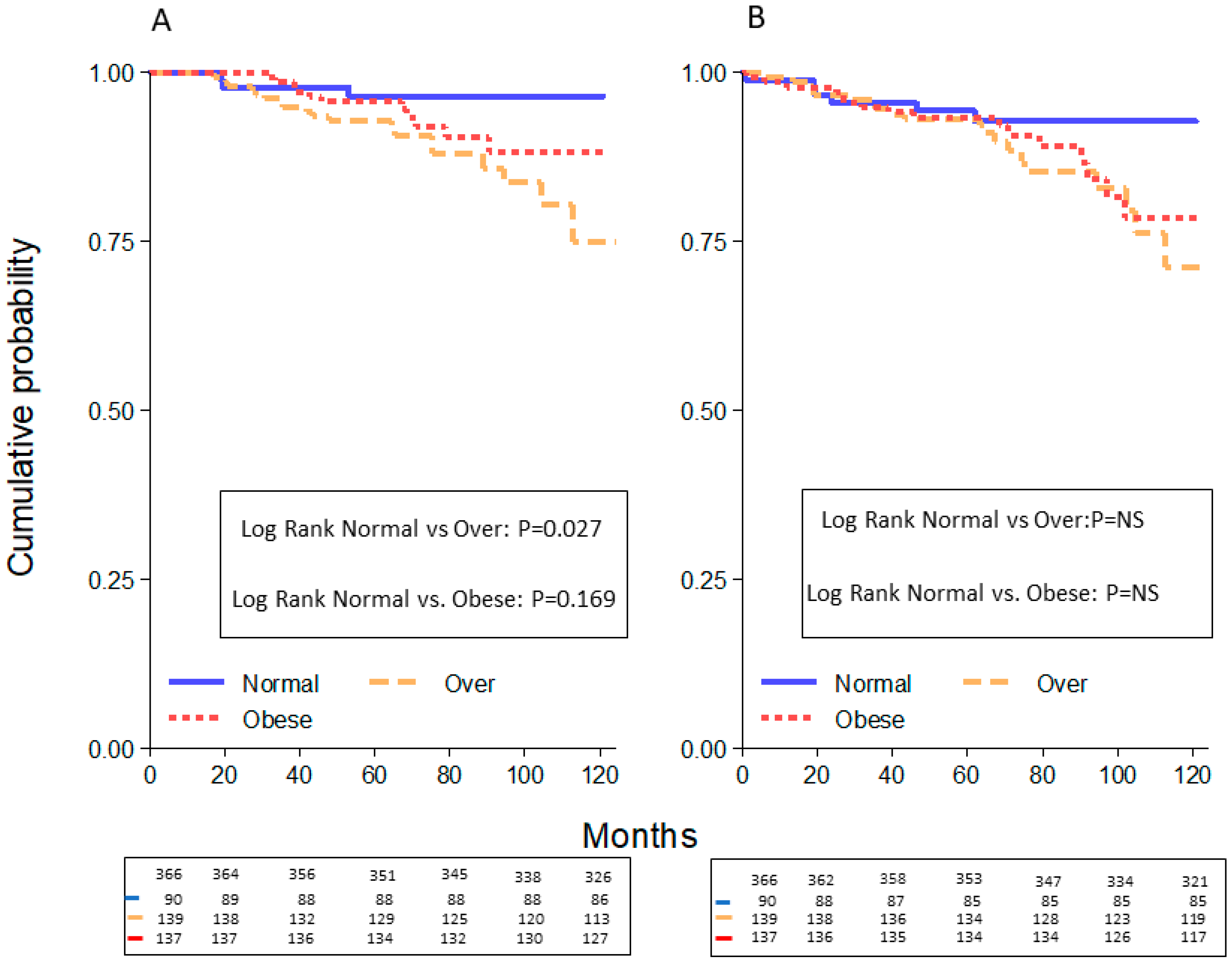
2.2. Survival Curves
3. Discussion
4. Materials and Methods
4.1. Methods
4.2. Eligibility Criteria
4.3. Study Procedures
4.4. Definition of BC Subtypes
4.5. Dietary Intervention
4.6. Physical Activity Program
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AIOM-AIRTUM. I Numeri del Cancro in Italia 2016. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia-2016 (accessed on 25 May 2020).
- AIOM-AIRTUM. I Numeri del Cancro in Italia 2018. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia-2018 (accessed on 25 May 2020).
- Mathews, J.C.; Nadeem, S.; Levine, A.J.; Pouryahya, M.; Deasy, J.O.; Tannenbaum, A. Robust and interpretable PAM50 reclassification exhibits survival advantage for myoepithelial and immune phenotypes. NPJ Breast Cancer 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Jovanovic, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Sebastiani, F.; Cortesi, L.; Sant, M.; Lucarini, V.; Cirilli, C.; De Matteis, E.; Marchi, I.; Negri, R.; Gallo, E.; Federico, M. Increased Incidence of Breast Cancer in Postmenopausal Women with High Body Mass Index at the Modena Screening Program. J. Breast Cancer 2016, 19, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Niraula, S.; Ocana, A.; Ennis, M.; Gododwin, P.J. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res. Treat. 2012, 134, 769–781. [Google Scholar] [CrossRef]
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer: Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Goodwin, P.J. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef]
- Playdon, M.C.; Bracken, M.B.; Sanft, T.B.; Ligibel, J.A.; Harrigan, M.; Irwin, M.L. Weight Gain after Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2015, 107, djv275. [Google Scholar] [CrossRef] [Green Version]
- Ballard-Barbash, R.; Hunsberger, S.; Alciati, M.H.; Blair, S.N.; Goodwin, P.J.; McTiernan, A.; Wing, R.; Schatzkin, A. Physical Activity, Weight Control, and Breast Cancer Risk and Survival: Clinical Trial Rationale and Design Considerations. J. Natl. Cancer Inst. 2009, 101, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.L.; Smith, A.W.; McTiernan, A.; Ballard-Barbash, R.; Cronin, K.; Gilliland, F.D.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L. Influence of Pre- and Postdiagnosis Physical Activity on Mortality in Breast Cancer Survivors: The Health, Eating, Activity, and Lifestyle Study. J. Clin. Oncol. 2008, 26, 3958–3964. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical activity and survival after breast cancer diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [Green Version]
- Holick, C.N.; Newcomb, P.A.; Trentham-Dietz, A.; Titus-Ernstoff, L.; Bersch, A.J.; Stampfer, M.J.; Baron, J.A.; Egan, K.M.; Willett, W.C. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Pierce, J.P.; Stefanick, M.L.; Flatt, S.W.; Natarajan, L.; Sternfeld, B.; Madlensky, L.; Al-Delaimy, W.K.; Thomson, C.A.; Kealey, S.; Hajek, R.; et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J. Clin. Oncol. 2007, 25, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Speck, R.M.; Courneya, K.S.; Mâsse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 87–100. [Google Scholar] [CrossRef]
- Borch, K.B.; Braaten, T.; Lund, E.; Weiderpass, E. Physical activity before and after breast cancer diagnosis and survival—The Norwegian women and cancer cohort study. BMC Cancer 2015, 15, 967. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M.; Lancet Physical Activity Series 2 Executive Committee; Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.A. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef] [Green Version]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer. Continuous Update Project Report 2014. Available online: dietandcancerreport.org (accessed on 25 May 2020).
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity, and Breast Cancer. Continuous Update Project Report: 2017. Available online: dietandcancerreport.org (accessed on 25 May 2020).
- Rock, C.L.; Flatt, S.W.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. J. Clin. Oncol. 2015, 33, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, V.B.; Hicks, J.; Makambi, K.; Hurtado-de-Mendoza, A.; Demark-Wahnefied, W.; Adams-Campbell, L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemp. Clin. Trials 2016, 46, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Van Patten, C.L.; Neil, S.E.; Kirkham, A.A.; Gotay, C.C.; Gelmon, K.A.; McKenzie, D.C. Feasibility of a Lifestyle Intervention on Body Weight and Serum Biomarkers in Breast Cancer Survivors with Overweight and Obesity. J. Acad. Nutr. Diet. 2012, 112, 559–567. [Google Scholar] [CrossRef]
- Travier, N.; Fonseca-Nunes, A.; Javierre, C.; Guillamo, E.; Arribas, L.; Peirò, I.; Buckland, G.; Moreno, F.; Urruticoechea, A.; Oviedo, G.R.; et al. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med. Oncol. 2014, 31, 783. [Google Scholar] [CrossRef]
- Stolley, M.; Sheean, P.; Gerber, B.; Arroyo, C.; Schiffer, L.; Banerjee, A.; Visotcky, A.; Fantuzzi, G.; Strahan, D.; Matthews, L.; et al. Efficacy of a Weight Loss Intervention for African American Breast Cancer Survivors. J. Clin. Oncol. 2017, 35, 2820–2828. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Ligibel, J.A.; Barry, W.T.; Alfano, C.; Hershman, D.L.; Irwin, M.; Neuhouser, M.; Thomson, C.A.; Delahanty, L.; Frank, E.; Spears, P.; et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): Study design. NPJ Breast Cancer 2017, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Ewertz, M.; Jensen, M.B.; Gunnarsdòttir, K.A.; Højris, I.; Jakobsen, E.H.; Stenbygaard, D.N.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef]
- De Azambuja, E.; McCaskill-Stevens, W.; Francis, P.; Quinaux, E.; Crown, J.P.A.; Vecente, M.; Giuliani, R.; Nordenskjold, B.; Gutiérez, J.; Andresson, M.; et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: The experience of the BIG 02-98 trial. Breast Cancer Res. Treat. 2010, 119, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Altomare, R.; Cacciabaudo, F.; Damiano, G.; Palumbo, V.D.; Gioviale, M.C.; Bellavia, M.; Tomasello, G.; Lo Monte, A.I. The mediterranean diet: A history of health. Iran J. Public Health 2013, 42, 449–457. [Google Scholar] [PubMed]
- Kaviani, A.; Neishaboury, M.; Mohammadzadeh, N.; Ansari-Damavandi, M.; Jamei, K. Effects of obesity on presentation of breast cancer, lymph node metastasis and patient survival: A retrospective review. Asian Pac. J. Cancer Prev. 2013, 14, 2225–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekri, J.; Farag, K.; Allithy, A. Obesity and outcome of post-menopausal women receiving adjuvant letrozole for breast cancer. Ecancermedicalscience 2018, 12, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestak, I.; Distler, W.; Forbes, J.F.; Dowsett, M.; Howell, A.; Cuzick, J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J. Clin. Oncol. 2010, 28, 3411–3415. [Google Scholar] [CrossRef] [PubMed]
- Ewertz, M.; Gray, K.P.; Regan, M.M.; Ejlertsen, B.; Price, K.N.; Thurlimann, B.; Bonnefoi, H.; Forbes, J.F.; Paridaens, R.J.; Rabaglio, M.; et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the breast international group 1–98 trial. J. Clin. Oncol. 2012, 30, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; Elledge, R.M. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J. Natl. Cancer Inst. 2005, 97, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Schech, A.; Yu, S.; Goloubeva, O.; McLenithan, J.; Sabnis, G. A nude mouse model of obesity to study the mechanisms of resistance to aromatase inhibitors. Endocr. Relat. Cancer 2015, 22, 645–656. [Google Scholar] [CrossRef]
- Azrad, M.; Demark-Wahnefried, W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr. Nutr. Rep. 2014, 3, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Carsin, A.E.; Keidel, D.; Fuertes, E.; Imboden, M.; Weyler, J.; Nowak, D.; Heinrich, J.; Pascual Erquicia, S.; Martinez-Moratalla, J.; Huerta, I.; et al. Regular Physical Activity Levels and Incidence of Restrictive Spirometry Pattern: A Longitudinal Analysis of Two Population-based Cohorts. Am. J. Epidemiol. 2020, 146. [Google Scholar] [CrossRef]
- Jeong, S.H.; An, Y.; Ahn, C.; Park, B.; Lee, M.H.; Noh, D.; Park, S.K. Body mass index and risk of breast cancer molecular subtypes in Korean women: A case-control study. Breast Cancer Res. Treat. 2020, 179, 459–470. [Google Scholar] [CrossRef]
- Ahern, A.L.; Woolston, J.; Wells, E.; Sharp, S.J.; Islam, N.; Lawlor, E.R.; Duschinsky, R.; Hill, A.J.; Doble, B.; Wilson, E.; et al. Clinical and cost-effectiveness of a diabetes education and behavioural weight management programme versus a diabetes education programme in adults with a recent diagnosis of type 2 diabetes: Study protocol for the Glucose Lowering through Weight management (GLoW) randomised controlled trial. BMJ Open. 2020, 10, e035020. [Google Scholar] [CrossRef] [PubMed]
- Vernaci, G.; Dieci, M.V.; Manfrin, S.; Mantiero, M.; Falci, C.; Faggioni, G.; Mioranza, E.; Menichetti, A.; Tascxa, G.; Griguolo, G.; et al. BMI Is an Independent Prognostic Factor for Late Outcome in Patients Diagnosed With Early Breast Cancer: A Landmark Survival Analysis. Breast 2019, 47, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casinio, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Montesi, L.; Caselli, C.; Centis, E.; Nuccitelli, C.; Moscatiello, S.; Suppini, A.; Marchesini, G. Physical activity support or weight loss counseling for nonalcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 10128–10136. [Google Scholar] [CrossRef]

| All (n = 430) | Underweight (n= 3) | Normal (n = 100) | Overweight (n = 167) | Obese (n = 160) | p-Value * | |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 52.6 ± 23.4 | 56.7 ± 4.9 | 48.5 ± 9.3 | 53.1 ± 9.6 | 54.6 ± 10 | 0.032 |
| Stage at diagnosis N (%) | 0.619 | |||||
| In situ | 18 (4.2) | 0 | 3 (3.0) | 5 (3.0) | 10 (6.2) | |
| I | 221 (51.4) | 1 (33.3) | 56 (56.0) | 91 (54.5) | 73 (45.6) | |
| II | 128 (29.8) | 1 (33.3) | 24 (24.0) | 49 (29.3) | 54 (33.8) | |
| III | 48 (11.1) | 0 | 14 (14.0) | 16 (9.6) | 18 (11.3) | |
| IV | 10 (2.3) | 0 | 0 | 6 (3.6) | 4 (2.5) | |
| nd | 5 (1.2) | 1 (33.4) | 3 (3.0) | 0 | 1 (0.6) | |
| Therapy N (%) | 0.639 | |||||
| CT only | 29 (6.7) | 0 | 7 (7.0) | 11 (6.6) | 11 (6.9) | |
| HT only | 200 (46.5) | 1 (33.3) | 50 (50.0) | 77 (46.1) | 72 (45.0) | |
| CT+HT | 181 (42.1) | 1 (33.3) | 39 (39.0) | 74 (44.3) | 67 (41.9) | |
| None | 14 (3.5) | 0 | 1 (1.0) | 5 (3.0) | 8 (5.0) | |
| nd | 6 (1.6) | 1 (33.4) | 3 (3.0) | 0 | 2 (1.2) | |
| Type of HT N (%) | 0.041 | |||||
| TAM | 130 (34.2) | 0 | 41 (46.6) | 51 (34.0) | 38 (27.1) | |
| AI | 175 (45.9) | 2 (66.7) | 29 (33.0) | 72 (48.0) | 72 (51.4) | |
| TAM+AI | 66 (17.3) | 0 | 15 (17.0) | 25 (16.7) | 26 (18.6) | |
| nd | 10 (2.6) | 1 (33.3) | 3 (3.4) | 2 (1.3) | 4 (2.9) | |
| Menopausal status N (%) | <0.001 | |||||
| Premenopausal | 182 (42.3) | 0 | 58 (58.0) | 71 (42.5) | 53 (33.1) | |
| Post-menopausal | 214 (49.8) | 2 (66.7) | 34 (34.0) | 87 (52.1) | 91 (56.9) | |
| nd | 34 (7.9) | 1 (33.3) | 8 (8.0) | 9 (5.4) | 16 (10.0) | |
| Phenotype N (%) | 0.001 | |||||
| In situ | 18 (4.2) | 0 | 3 (3.0) | 6 (3.6) | 9 (5.6) | |
| Luminal A | 189 (43.9) | 0 | 48 (48.0) | 63 (37.7) | 78 (48.7) | |
| Luminal B | 124 (28.9) | 0 | 24 (24.0) | 60 (35.9) | 40 (25.0) | |
| Luminal/HER2 | 55 (12.8) | 2 (66.7) | 12 (12.0) | 24 (14.4) | 17 (10.6) | |
| HER2 enriched | 11 (2.6) | 0 | 2 (2.0) | 5 (3.0) | 4 (2.5) | |
| TNBC | 29 (6.7) | 0 | 9 (9.0) | 9 (5.4) | 11 (6.9) | |
| nd | 4 (0.9) | 1 (33.3) | 2 (2.0) | 0 | 1 (0.7) | |
| Second BC N (%) | 34 (7.9) | 0 | 8 (23.5) | 17 (50.0) | 9 (26.5) | <0.001 |
| Size invasive T N (%) | 0.001 | |||||
| ≤2 cm | 250 (60.9) | 1 (33.3) | 83 (85.6) | 120 (74.1) | 46 (30.7) | |
| >2 cm and ≤5 cm | 90 (21.8) | 0 | 11 (12.2) | 36 (22.2) | 43 (28.7) | |
| >5 cm | 45 (10.9) | 0 | 0 | 0 | 45 (30.0) | |
| nd | 27 (6.4) | 2 (66.7) | 3 (2.2) | 6 (3.7) | 16 (10.6) | |
| Histology invasive BC N(%) | 0.71 | |||||
| Ductal | 354 (85.9) | 3 (100) | 82 (84.5) | 143 (88.3) | 126 (84.0) | |
| Lobular | 55 (13.4) | 0 | 15 (15.5) | 17 (10.5) | 23 (15.3) | |
| nd | 3 (0.7) | 0 | 0 | 2 (1.2) | 1 (0.7) | |
| Grade invasive BC N (%) | <0.001 | |||||
| I | 74 (18.0) | 0 | 32 (33.0) | 33 (20.4) | 9 (6.0) | |
| II | 156 (37.9) | 1 (33.3) | 34 (35.1) | 69 (42.6) | 52 (34.7) | |
| III | 159 (38.5) | 0 | 25 (24.7) | 54 (33.3) | 80 (60.0) | |
| nd | 23 (5.6) | 2 (66.7) | 7 (7.2) | 6 (3.7) | 8 (5.3) |
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| Characteristics | HR | p value | 95% CI | HR | p value | 95% CI |
| BMI | ||||||
| Normal+UW | 1.00 (ref.) | 1.00 (ref.) | ||||
| Overweight | 2.85 | 0.062 | 0.95–8.61 | 2.15 | 0.085 | 0.90–5.14 |
| Obese | 2.23 | 0.168 | 0.71–6.95 | 1.55 | 0.348 | 0.62–3.87 |
| Menopausal status | ||||||
| Premenopausal | 1.00 (ref.) | 1.00 (ref.) | ||||
| Postmenopausal | 1.58 | 0.399 | 0.55–4.56 | 0.94 | 0.885 | 0.41–2.16 |
| HT | ||||||
| TAM | 1.00 (ref.) | 1.00 (ref.) | ||||
| AI | 1.44 | 0.487 | 0.52–3.99 | 1.16 | 0.749 | 0.47–2.88 |
| TAM+AI | 0.83 | 0.771 | 0.23–2.97 | 0.55 | 0.328 | 0.17–1.82 |
| Phenotype | ||||||
| Luminal A | 1.00 (ref.) | 1.00 (ref.) | ||||
| Luminal B | 1.22 | 0.702 | 0.47–3.42 | 1.19 | 0.720 | 0.46–3.03 |
| Luminal/HER2 | 1.64 | 0.183 | 0.79–3.98 | 1.74 | 0.098 | 0.90–3.33 |
| HER2 enriched | 0.88 | 0.934 | 0.20–3.7 | 0.60 | 0.624 | 0.32–2.4 |
| TNBC | 1.35 | 0.326 | 0.72–2.99 | 1.21 | 0.124 | 0.88–3.5 |
| Previous BC | 2.78 | 0.007 | 1.32–5.86 | 3.10 | 0.002 | 1.54–6.25 |
| Tumor size | ||||||
| T ≤ 2 cm | 1.00 (ref.) | 1.00 (ref.) | ||||
| T > 2 cm | 2.08 | 0.026 | 1.09–3.95 | 1.73 | 0.079 | 0.94–3.21 |
| Stage | ||||||
| 0-I | 1.00 (ref.) | 1.00 (ref.) | ||||
| II | 0.41 | 0.417 | 0.05–3.52 | 1.46 | 0.251 | 0.76–2.81 |
| III-IV | 6.29 | 0.002 | 2.00–19.8 | 3.15 | 0.001 | 1.60–6.22 |
| Grading | ||||||
| I | 1.00 (ref.) | 1.00 (ref.) | ||||
| II-III | 1.78 | 0.258 | 0.81–4.33 | 1.82 | 0.074 | 0.96–4.55 |
| OS | |||
|---|---|---|---|
| Characteristics | HR | p value | 95% CI |
| BMI | |||
| Normal and UW | 1.00 (ref.) | ||
| Overweight | 4.57 | 0.005 | 1.57–13.4 |
| Obese | 2.77 | 0.072 | 0.91–8.39 |
| Stage | |||
| I | 1.00 (ref.) | ||
| II | 1.25 | 0.583 | 0.57–2.74 |
| III–IV | 4.94 | <0.001 | 2.46–9.92 |
| Second Tumor | |||
| No | 1.00 (ref.) | ||
| Yes | 4.49 | <0.001 | 1.98–10.2 |
| T ≤ 2 cm | 1.00 (ref.) | ||
| T > 2 cm | 2.04 | 0.031 | 1.07–3.88 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortesi, L.; Sebastiani, F.; Iannone, A.; Marcheselli, L.; Venturelli, M.; Piombino, C.; Toss, A.; Federico, M. Lifestyle Intervention on Body Weight and Physical Activity in Patients with Breast Cancer Can Reduce the Risk of Death in Obese Women: The EMILI Study. Cancers 2020, 12, 1709. https://doi.org/10.3390/cancers12071709
Cortesi L, Sebastiani F, Iannone A, Marcheselli L, Venturelli M, Piombino C, Toss A, Federico M. Lifestyle Intervention on Body Weight and Physical Activity in Patients with Breast Cancer Can Reduce the Risk of Death in Obese Women: The EMILI Study. Cancers. 2020; 12(7):1709. https://doi.org/10.3390/cancers12071709
Chicago/Turabian StyleCortesi, Laura, Federica Sebastiani, Anna Iannone, Luigi Marcheselli, Marta Venturelli, Claudia Piombino, Angela Toss, and Massimo Federico. 2020. "Lifestyle Intervention on Body Weight and Physical Activity in Patients with Breast Cancer Can Reduce the Risk of Death in Obese Women: The EMILI Study" Cancers 12, no. 7: 1709. https://doi.org/10.3390/cancers12071709
APA StyleCortesi, L., Sebastiani, F., Iannone, A., Marcheselli, L., Venturelli, M., Piombino, C., Toss, A., & Federico, M. (2020). Lifestyle Intervention on Body Weight and Physical Activity in Patients with Breast Cancer Can Reduce the Risk of Death in Obese Women: The EMILI Study. Cancers, 12(7), 1709. https://doi.org/10.3390/cancers12071709





