Abstract
The development of drug resistance is one of the main causes of failure in anti-cancer treatments. Tumor cells adopt many strategies to counteract the action of chemotherapeutic agents, e.g., enhanced DNA damage repair, inactivation of apoptotic pathways, alteration of drug targets, drug inactivation, and overexpression of ABC (Adenosine triphosphate-binding cassette, or ATP-binding cassette) transporters. These are broad substrate-specificity ATP-dependent efflux pumps able to export toxins or drugs out of cells; for instance, ABCB1 (MDR1, or P-glycoprotein 1), overexpressed in most cancer cells, confers them multidrug resistance (MDR). The gene coding for sorcin (SOluble Resistance-related Calcium-binding proteIN) is highly conserved among mammals and is located in the same chromosomal locus and amplicon as the ABC transporters ABCB1 and ABCB4, both in human and rodent genomes (two variants of ABCB1, i.e., ABCB1a and ABCB1b, are in rodent amplicon). Sorcin was initially characterized as a soluble protein overexpressed in multidrug (MD) resistant cells and named “resistance-related” because of its co-amplification with ABCB1. Although for years sorcin overexpression was thought to be only a by-product of the co-amplification with ABC transporter genes, many papers have recently demonstrated that sorcin plays an important part in MDR, indicating a possible role of sorcin as an oncoprotein. The present review illustrates sorcin roles in the generation of MDR via many mechanisms and points to sorcin as a novel potential target of different anticancer molecules.
Keywords:
sorcin; ABCB1; multidrug resistance; cancers; chemotherapeutic drugs; calcium; endoplasmic reticulum 1. Introduction
Sorcin (SOluble Resistance-related Calcium-binding proteIN) is one of the most expressed calcium-binding proteins in many tissues (source Protein Abundance Database, PaxDb, https://pax-db.org/). Although its most characterized function concerns the regulation of cardiac contractile activity, a significant role has emerged in the context of cancer and, especially, in multidrug resistance (MDR). In fact, sorcin is overexpressed in many human cancers, as lymphomas, leukemias, gastric, breast, lung, nasopharyngeal, ovarian tumors, adenocarcinoma, glioblastoma, astrocytoma, oligodendroglioma, and multidrug (MD)-resistant tumors, with respect to normal tissues (for a review, [1,2]).
In leukemia patients, sorcin expression levels inversely correlate with response to chemotherapies and with overall prognosis. Sorcin is overexpressed in cell lines resistant to chemotherapeutic drugs and significantly upregulated in the doxorubicin-induced MD-resistant leukemia K562/A02 cell line with respect to its parent cells. Sorcin overexpression by gene transfection: (i) increased drug resistance to a variety of chemotherapeutic agents (e.g., doxorubicin, etoposide, homoharringtonine, and vincristine) in K562 cells; and (ii) determined drug resistance (to vincristine, adriamycin, taxol, and 5-fluorouracil) in SGC7901 cells, ovarian and breast cancer. On the other hand, several recent studies have demonstrated that inhibition of sorcin expression by RNA interference led to a reversal of drug resistance in a number of cell lines.
Resistance to chemotherapeutic treatments is one of the main challenges in the fight against cancer. Tumor cells can adopt several strategies to evade death induced by chemotherapeutic agents. These include changes in apoptotic pathways, increased DNA damage repair, drug inactivation, alteration of drug targets, and increased expression of ABC (ATP-binding cassette) transporters (Figure 1) [3]. As illustrated in the present review, sorcin participates in many of such strategies (Table 1). Taken together, the above data indicate that sorcin has a significant and general role in MDR so that it can be a useful marker of MDR and may represent a therapeutic target for reversing tumor MDR.
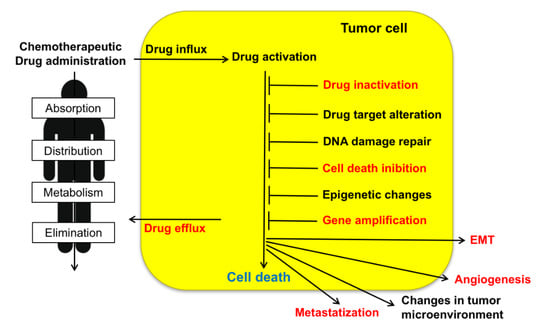
Figure 1.
Upon administration of chemotherapeutic drugs, intrinsic or extrinsic factors determine multidrug resistance (MDR). These include absorption, distribution, metabolism, and elimination (ADME), drug influx, drug efflux, drug activation and inactivation, drug target alteration, DNA damage repair, cell death (in particular apoptosis) inhibition, epigenetic effects, epithelial-to-mesenchymal transition (EMT), changes in tumor environment, angiogenesis, metastasis. Sorcin participates in several of such MDR mechanisms (indicated in red, see text).

Table 1.
Sorcin: Roles in cells, tumors, and multidrug resistance (MDR).
2. Role and Mode of Action of Sorcin in Physiological and Pathological Processes
2.1. Sorcin Structure and Calcium-dependent Activation
Sorcin (SOluble Resistance-related Calcium-binding proteIN) was labeled “resistance-related” since it was found co-amplified with ABCB1 in MD-resistant cells [73]. The gene coding for sorcin (SRI) is about 21.9 kb-long and is located in chromosome 7 (region 7q21). At least four different Sorcin isoforms are transcribed in human, i.e., isoforms A (a 15 kb-transcript, with 8 exons and 7 introns, translated into a 22-kDa, 198-residues long isoform), B, C, and D (translated into shorter, 19-kDa, isoforms, lacking part of the N-terminal domain and/or the last amino acids of the C-terminal domain); the 22-kDa isoform A is the most studied sorcin isoform, although some studies refer to 19-kDa forms of the protein. A sorcin-like pseudogene (SRIL) is located in chromosome 4 [2].
Sorcin is evolutionarily rather recent, being present in vertebrates, and more generally in metazoans. Sorcin sequence is highly conserved among species, e.g., human and mouse sorcin differ only by eight residues (T114S, A140T, I144V, N151S, T178S, A179G, P187S, S197T) (Figure 2, upper panel) among which three (in both human and mouse sorcin) are phosphorylatable serine and threonine residues of the C-terminal domain, possibly indicating species-specific phosphorylation-dependent sorcin regulation.

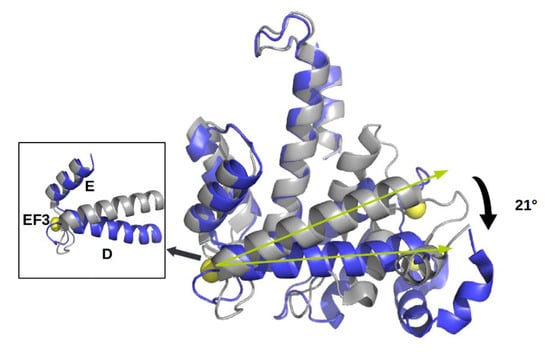
Figure 2.
Upper panel. Alignment between human sorcin (hSor) and mouse sorcin (mSor). The variant residues are indicated in red. The “+” indicates residues with similar characteristics. Lower panel. The X-ray crystal structure of human sorcin in the apo form (gray) and in the calcium-bound form (blue; calcium ions are represented by yellow spheres). Upon calcium binding, sorcin activation occurs, with a transition from a closed to an open structure (see also detail of the EF3 hand), involving a movement of the long D-helix of 21°.
From a structural viewpoint, sorcin belongs to the small penta-EF-hand (PEF) family, which also comprises calpains, grancalcin, PDCD6, and peflin [79]. EF-hands are structural helix–loop–helix motifs, with a 12-residue interhelical sequence, able to bind Ca2+ with high affinity (with a pentagonal bipyramidal symmetry): calcium binding to proteins acts as a signal in a variety of cellular processes. Sorcin is a homodimer in the absence of calcium [4]; each monomer is formed by two domains, i.e., the flexible glycine-rich N-terminal domain (residues 1–32) and the C-terminal Ca2+ binding domain (SCBD, residues 33–198) containing with five EF-hands. Usually, Ca2+ binding proteins are endowed with an even number of EF-hands, both structurally and functionally coupled. In sorcin, EF-hands are coupled via short two-stranded β-sheets, such that EF1-EF2 and EF3-EF4 pairs are formed; EF5, although uncoupled in sorcin monomers, pairs with another EF5 hand (belonging to the second monomer) in dimeric sorcin, thereby forming part of the dimer interface [29,30,36].
Sorcin activation is calcium-dependent. Upon Ca2+ binding to EF1-3 hands, sorcin undergoes a large conformational change [4,5,80] (Figure 2) that involves a 21° movement of the long D-helix that joins the EF1-EF2 subdomain to EF3, opens EF1, and exposes hydrophobic surfaces in the EF1-EF3 region (Figure 3). This allows sorcin to aggregate in the absence of protein targets or to bind and regulate several proteins in a Ca2+-dependent manner [4,5,6,7,8,29,80]. Peptide phage display experiments identified two consensus sequences by which target proteins bind sorcin upon Ca2+ binding, i.e., a Φ/Gly/Met-Φ/Gly/Met-x-P motif, where Φ is an aromatic residue (Trp, Tyr, or Phe) and x is any amino acid, and an acidic-Φ motif [29]. The Φ/Gly/Met-Φ/Gly/Met-x-P motif is consistent with the sequence of sorcin N-terminal peptide, found in the hydrophobic pocket exposed in the D helix-EF3 region, which comprises residues Trp105 and His108, possibly the most important residues for interaction with targets (Figure 3) [5,6,29,80].
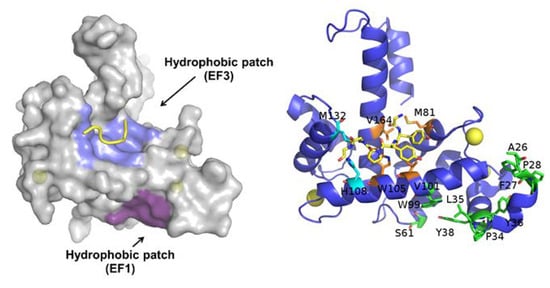
Figure 3.
Ca2+-bound sorcin in complex with a peptide belonging to the N-terminal domain. Left: Upon calcium binding to sorcin, two hydrophobic patches are exposed to the solvent and likely mediate target binding. One patch (violet) arises from the opening of EF1, the other (blue) from EF3. The peptide belonging to the sorcin N-terminal domain is shown in yellow. Right: detail of the residues involved in the exposure of the hydrophobic surfaces upon calcium binding to sorcin, belonging to the A-helix and EF1 hand (green), and to the C-helix, D-helix, EF4 loop, and G-helix (orange and cyan). The peptide belonging to the sorcin N-terminal domain (in yellow) and the residues interacting with it are represented as sticks.
2.2. Sorcin Mechanisms of Action: Role in Calcium Homeostasis, ER Stress, and Apoptosis
Sorcin is highly expressed in many tissues: it is among the top 3% expressed proteins of the human proteome and one of the most expressed Ca2+ binding proteins (source PaxDb, https://pax-db.org/), and has an essential role in calcium homeostasis [22].
Sorcin participates in several processes in the cell and is essential for mitotic progression and cytokinesis: sorcin silencing determines important problems in mitosis and cytokinesis, increases the number of polynucleated rounded cells, and results in blockage of the cell cycle in G2/M, apoptosis and cell death [22].
In 3T3-L1 fibroblasts, sorcin localizes dynamically during cell cycle progression. In interphase, sorcin is in the nucleus (where it is distributed in a speckled fashion and excluded from the nucleoli), in the plasma membrane, in the endoplasmic reticulum (ER) and in ER-derived vesicles localized along the microtubules. These vesicles are positive to Ryanodine Receptors (RyRs), sarcoplasmic/endoplasmic (SR/ER) reticulum Ca2+-ATPase (SERCA), Rab10, and calreticulin. At the beginning of mitosis, i.e., in prophase and upon disruption of the nuclear envelope, sorcin accumulates in the apical zone of the mitotic spindle, while in metaphase, upon chromosome separation, most sorcin accumulates in the central region of the spindle. In the early telophase, sorcin localizes to the cleavage furrow, while in late telophase, most sorcin moves back to the reforming nuclei, but a significant part flanks the central region of the midbody [22].
Sorcin regulates size and Ca2+ content of the ER and ER vesicles, inhibiting RyR, and activating SERCA (Figure 4). Sorcin regulates Ca2+ homeostasis in the cells. In the heart, sorcin participates in the regulation of cardiac excitation-contraction coupling, through its critical role in maintaining calcium homeostasis and regulating Ca2+ fluxes in the cardiomyocyte.
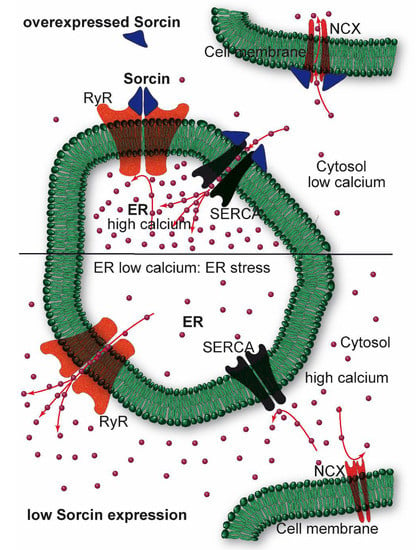
Figure 4.
Sorcin inhibits Ryanodine Receptors (RyRs) and activates sarco/endoplasmic reticulum Ca2+−ATPase (SERCA) and Na+/Ca2+ exchanger (NCX), thereby increasing Ca2+ load of the endoplasmic reticulum (ER) and decreasing ER stress (top). When sorcin expression is low, ER Ca2+ load is decreased, thereby increasing ER stress (bottom).
Cycles of excitation, contraction, and relaxation take place in about 800 ms. The electrical excitation of cardiomyocytes is started by a depolarization wave that opens the voltage-dependent Na+ channels of the T-tubules, resulting in membrane depolarization and calcium entry through voltage-operated Ca2+ channels. Calcium influx locally increases Ca2+ concentration near RyRs, and activates RyR-dependent Ca2+ release from the SR, thereby further increasing cytosolic Ca2+ concentration; the cation binds to troponin C and triggers cardiac contraction. Relaxation follows rapidly: the RyR channels close, and calcium efflux out of the cytosol takes place through SERCA, which pumps the ion back into the SR, and Na+/Ca2+ exchanger (NCX) at the plasma membrane (and mitochondria) [81]: cytosolic calcium concentration decreases rapidly, and calcium dissociates from the myofilaments, switching muscle relaxation.
Upon calcium-dependent activation, sorcin rapidly binds to RyR, inhibiting single-channel activity, thereby attenuating Ca2+-induced Ca2+ release by SR/ER and decreasing Ca2+-triggered membrane depolarization [6,7,9,10]. In addition, sorcin increases SERCA activity, increasing SR/ER calcium load [11] (Figure 4). Further, sorcin increases the activity of the sarcolemmal NCX 14, stimulates voltage-dependent inactivation, and slows Ca2+-dependent inactivation of the L-type voltage-dependent Ca2+ channel (LTCC) [12,13]. Sorcin overexpression enhances cardiac contractility and reverses contractile anomalies of diabetic cardiomyopathy [14,15,16,17], while sorcin KO mice exhibit arrhythmias and sudden death under acute or chronic stress, due to disturbances of Ca2+ fluxes [18]. Sorcin is an important player in other cells where excitation-contraction cycles take place, as outer hair cells, that amplify the acoustic signal in the ear [19]. In general, sorcin regulates calcium homeostasis in all types of cell, decreasing free cytosolic Ca2+ and increasing ER Ca2+ concentration, by binding calcium and by regulating the same channels, pumps, and exchangers, possibly protecting cells from ER stress, dependent on decreased calcium concentration in the organelle (Figure 4).
Besides calcium channels, sorcin interacts in Ca2+-dependent fashion with many protein targets, including Polo-like kinase 1 (PLK1), Aurora A and Aurora B kinases, involved in cell cycle regulation. Sorcin physically interacts with PLK1 and induces PLK1 autophosphorylation, regulating kinase activity [22]. Further, PLK1, Ca2+-calmodulin dependent kinase II (CaMKII), and cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA) phosphorylate sorcin, thus regulating sorcin binding to RyRs and SERCA, and eventually Ca2+ homeostasis [22,30,33]. Additionally, sorcin interacts with the calcium-dependent, phospholipid-binding proteins Annexins A7 and A11 [5,6,22,80,82]; in particular, Annexin A11, like sorcin, is needed for midbody organization and for cytokinesis [83].
Sorcin was identified in many types of vesicles, indicating a particularly significant but still puzzling role in the trafficking of various cell types and tissues. In addition to ER-dependent vesicles, sorcin was identified in nanovesicles released in a Ca2+-dependent fashion from the erythrocytes and containing Annexin A7 [84]; further, sorcin was identified in many types of exosomes, from B-cells, T-cells, mesenchymal stem cells, breast milk, plasma, red blood cells, seminal plasma, human urine and platelets, thymus, dendritic cells, cerebrospinal fluid, and from many types of cancer cells, such as ovarian cancer, prostate cancer, squamous carcinoma, melanoma, lung cancer, chronic lymphocytic leukemia, colorectal cancer, osteosarcoma, astrocytoma, glioblastoma, and neuroblastoma [72,85,86,87,88,89,90,91,92].
In many tumor cells, sorcin is overexpressed (see below, Section 2.4.). Sorcin-overexpressing vincristine- and daunorubicin-resistant Ehrlich ascites cancer cells have lower cytosolic free Ca2+ concentration than the corresponding wild-type cells [23]. In these cells, sorcin silencing increases cytosolic calcium and increases cell death by apoptosis [24], while sorcin overexpression in K562 leukemia cells significantly reduces cytosolic Ca2+ levels, thus protecting cells from etoposide-dependent apoptosis, upregulates Bcl-2 and decreases Bax [25]. Since sorcin increases ER Ca2+ accumulation, thereby limiting ER stress, it is upregulated in ER stress conditions; conversely, its silencing activates caspase 12, caspase 3, and GRP78/BiP, triggering apoptosis with a mechanism possibly involving the mitochondrial chaperone TRAP1 [26,27].
Taxol-resistant, sorcin-overexpressing A549 non-small-cell lung cancer cells show decreased RyR currents, altered ER calcium homeostasis, possibly increased ER Ca2+ reuptake by SERCA and/or increases Ca2+ efflux by NCX, and increased Bcl-2 expression [20].
In myeloma cells, sorcin silencing reduces cell proliferation, cell cycle blockage, and apoptosis, and significantly reduces the expression levels (both mRNA and protein) of the xenobiotic pumps ABCB1 and MRP1, of GST-π, Survivin, Bcl-2, Livin, phospho-Src, Cyclin-D1, p21, C-myc, phospho-Akt, and NF-κB, while significantly increasing the expression of p53 and the activity of caspase 3 and caspase 8 [32].
2.3. Sorcin Under Cellular Stressing Conditions and in Pathologies
Sorcin is important for glucose tolerance and protects vs. lipotoxicity in vivo: sorcin downregulation occurs under lipotoxic stress conditions, such as high-fat diet and exposure to proinflammatory cytokines or palmitate [28,93], while sorcin overexpression protects against ER stress. Sorcin deletion impairs glucose tolerance and glucose-stimulated insulin secretion (GSIS) in transgenic mice, whereas sorcin overexpression in pancreatic β-cells increases glucose tolerance and enhances GSIS during high-fat diet [28]. Sorcin increases intracellular Ca2+ fluxes and ER Ca2+ stores, regulates glucose-6-phosphatase catalytic subunit-2 (G6PC2) via nuclear factor of activated T-cells (NFAT) activation, decreases the levels of C/EBP homologous protein (CHOP) and Grp78/BiP, i.e., of ER stress markers and activates the transcriptional activity of the activating transcription factor 6 ATF6 [28].
In turn, sorcin silencing activates apoptotic caspase-3 and caspase-12, Bcl-2, Bax, Grp78/BiP, c-fos, c-jun, increases mitochondrial Ca2+ concentration and release of cytochrome c [17,22,28]. At low glucose concentrations, sorcin also retains in the cytosol the carbohydrate-responsive element-binding protein ChREBP, i.e., one of the most important mediators of glucotoxicity and regulators of pancreatic β-cell gene expression: sorcin operates as a calcium sensor for glucose-dependent nuclear translocation and for ChREBP-controlled gene activation [35].
High levels of expression in the central nervous system, different in basal and pathological conditions, indicate that sorcin can possibly be a notable player in brain functions and dysfunctions, likely by its capability to modulate calcium homeostasis.
High amounts of sorcin are expressed in the brain (about 5–10 times higher than in the heart): Sorcin is among the most expressed calcium-binding proteins in the amygdala, the hypothalamus, the prefrontal cortex, and in many brain cancers (source GeneAtlas: http://geneatlas.roslin.ed.ac.uk/). The capability to modulate calcium homeostasis makes sorcin a possible player in brain functions and dysfunctions. Moreover, it is highly expressed in brain pathological conditions, e.g., in brains from Alzheimer’s disease (AD) patients vs. controls [37,38], in the frontal cortex of asymptomatic AD patients with respect to symptomatic AD patients [39], in amyloid plaques in sporadic vs. rapidly progressive AD patients, and in AD vs. cerebral amyloid angiopathy patients [40,41], thereby possibly protecting from acceleration progression that takes place in aggressive forms of the disease. Sorcin sequestration by aberrant forms of tau results in impaired calcium homeostasis and resistance to ER stress and may contribute to AD progression [31]. Sorcin is also overexpressed in frontal cortex tissues from frontotemporal dementia, with respect to control patients [42], in substantia nigra of Parkinson’s disease (PD) patients vs. controls [43], and in mitochondrial proteins from substantia nigra pars compacta pathologically verified PD patients vs. controls [44], is upregulated in MPP+-treated cells [36], and in induced pluripotent stem cells (iPSCs) derived from PD patients vs. control cells [45]. Sorcin is overexpressed in seven human and mouse models of Huntington’s disease, under the control of the ERSE-I (ER stress response element) promoter upstream sorcin gene, together with other proteins involved in ER stress and unfolded protein response [46].
The relevant role that sorcin seems to have in neurological processes and diseases, besides calcium homeostasis regulation, could also be due to the direct interaction with some key proteins, such as presenilin 2 (PS2), alpha-synuclein (AS), and the N-methyl-D-aspartate receptor. Sorcin directly interacts in a calcium-dependent fashion (in vitro, in cells and in human brain) with presenilin 2 (PS2) and alpha-synuclein (AS), which are important in AD and PD pathogenesis, respectively [47,48]; sorcin interacts with the C-terminal region of PS2, which is able to form low-conductance calcium channels in lipid bilayers [94], binds to RyR in a calcium-dependent way, and modulates calcium homeostasis [21].
Sorcin also interacts with the ionotropic glutamate receptor NMDAR1 subunit of the non-specific cation channel N-methyl-D-aspartate receptor in the caudate-putamen nucleus [95] and with annexins A7 and A11, that participate in the regulation of calcium homeostasis in astrocytes [96].
Sorcin is important for endometrium development and embryo implantation: it is downregulated in the mid-secretory (receptive) endometrium of women with unexplained infertility with respect to fertile women, and mediates endometrial angiogenesis, endothelial proliferation, migration, and invasion via regulation of the vascular endothelial growth factor (VEGF) pathway involving the vascular endothelial growth factor receptor 2 (VEGFR2), phosphatidylinositol 3-kinase (PI3K), Akt, and nitric oxide synthase (NOS) expression, possibly by regulating calcium homeostasis [76,77].
2.4. Sorcin in Cancer and Multidrug (MD)-resistant Tumors
MDR impairs the efficacy of chemotherapy against tumors, with over 90% treatment failure rate in metastatic cancers. Many mechanisms operate to confer drug resistance (Figure 1) [97]: scarce drug solubility and toxicity to normal tissues limit the doses of chemotherapeutic drugs that can be administered to cancer patients; pharmacokinetic issues, as absorption, distribution, metabolism, and elimination, reduce the amount of chemotherapeutic that effectively reaches cancer cells. Moreover, several mechanisms confer tumor cell drug resistance, e.g., low drug uptake caused by reduced expression or loss of influx transporters, enhanced drug efflux due to overexpression of drug efflux pumps, changes in lipid composition of the cell membrane, increased DNA damage repair, inhibition of apoptosis, alterations of cell cycle or checkpoints, off-target drug compartmentalization, increased drug catabolism, drug target structure modification, epithelial–mesenchymal transition (EMT).
Sorcin contributes to tumorigenesis and to the MDR phenotype via a series of mechanisms (Figure 1, Table 1).
Sorcin has been identified for the first time as a protein overexpressed in vincristine-resistant hamster lung cancer cells and denominated soluble, resistance-related, calcium-binding protein according to its main features [49]. Sorcin is expressed at high levels in many cancers, from many different tissues, usually with MD-resistant phenotype dependent on ABCB1 expression. The SRI gene is located in chromosome 7q21.12, in the same amplicon of ABCB1, the most important ATP-dependent efflux pump, capable of pumping a broad range of drugs and toxins out of cells [49]. Sorcin is “resistance-related” because its gene and ABCB1 are often co-amplified in MD-resistant tumor cells [73]. For a long time, sorcin overexpression in MD-resistant cancer cells was considered as an accidental consequence of such genomic co-amplification [50]; on the contrary, in the last two decades, many studies have demonstrated that sorcin is an oncoprotein, and have revealed its role both as a marker and a cause of MDR.
Sorcin is overexpressed in a number of cancers, such as lymphoma, leukemia (acute lymphoblastic, acute myeloid, chronic myeloid leukemias), myeloma, breast cancer, adenocarcinoma, gastric cancer, colorectal cancer, nasopharyngeal cancer, lung tumor, ovarian cancer, prostate cancer, tobacco-chewing mediated oral cancer, and particularly in MD-resistant tumors [20,24,25,26,34,50,51,52,53,54,55,56,57,58,59,60]; sorcin is overexpressed in glioblastoma, anaplastic astrocytoma, and oligodendroglioma, while is an important marker of poor clinical outcome in embryonal central nervous system tumors and a histological marker for malignant glioma [61,62,63,64,65]. According to the Human Protein Atlas (https://www.proteinatlas.org/ENSG00000075142-SRI), sorcin has moderate to strong cytoplasmic and nuclear positivity in most cancers, with the strongest staining displayed in low-grade gliomas, and is an unfavorable prognostic marker (p < 0.001) in pancreatic cancer (unfavorable), and an unfavorable quasi-marker (0.001 < p < 0.003) for liver cancer, cervical cancer, and endometrial cancer. However, sorcin is a favorable marker in lung cancer.
Sorcin transfection in different cancer cell lines, such as leukemia, lung, gastric, ovarian, and breast tumors, leads to increased drug resistance to chemotherapeutic drugs such as doxorubicin, vincristine, paclitaxel, etoposide, homoharringtonine, and 5-fluorouracil [24,25,34,60,66,74,98,99]. Conversely, sorcin silencing reverses MDR in leukemia, HeLa, breast, and colorectal cancer and nasopharyngeal carcinoma [24,26,52,54,60,66,67,68,69,70]. Human colorectal cancer cells express high amounts of sorcin, whose upregulation induces resistance to oxaliplatin, 5-fluorouracil, and irinotecan, while its downregulation sensitizes cells towards these drugs [26]. Sorcin is upregulated in many cisplatin-resistant cancers and tumor cell lines, such as leukemia, nasopharyngeal carcinoma, and lung cancer, while sorcin silencing increases cisplatin cytotoxicity and glutathione depletion [54,69,71]. Silencing of sorcin in MD-resistant myeloma cell lines increases cellular sensitivity to cisplatin and adriamycin, and decreases cell proliferation, cell cycle blockage, and apoptosis [32]. Moreover, Qu and collaborators showed that sorcin overexpression was associated with gemcitabine resistance and with poor prognosis in non-small cell lung tumor patients [56].
2.5. Sorcin Expression and ABCB1 Expression are Linked
Probably the expression of ABCB1 is the most significant and most characterized mechanism of MDR in which sorcin is involved.
Overexpression of many ATP-dependent efflux pumps, and especially ABCB1 (MDR1 or P-glycoprotein), is an important mechanism of resistance to a wide spectrum of chemotherapeutic drugs, including anthracyclines, taxanes, and Vinca alkaloids, in cancer cell lines and in many cancers, e.g., many solid and hematological tumors [100,101]. Sorcin overexpression increases ABCB1 expression, determining increased drug resistance to several drugs, while sorcin silencing decreases expression of ABCB1, increasing cell death, in gastric cancer cells, lung tumor cells, nasopharyngeal carcinoma, cervical carcinoma cells, and leukemias [24,32,34,52,57,60,66,68,69,74]. Sorcin increases ABCB1 expression by stimulating CREB1 phosphorylation by PKA, and binding of activated CREB1 to the cAMP response element (CRE) in the −716–−709 bp of the promoter of the ABCB1 gene [34]. Sorcin silencing was also shown to inhibit ABCB1 by suppressing ERK and Akt [75].
Sorcin gene is in the same chromosomal region (7q21.12) and in the same amplicon of the ABC transporters ABCB1 and ABCB4, both in human and mouse genomes (the rodent amplicon contains two ABCB1 variants, i.e., ABCB1a and ABCB1b). ABCB1 expression is increased upon treatment with chemotherapeutic drugs, and ABCB1 confers MDR when overexpressed or amplified [102,103,104,105,106,107,108,109,110,111,112]; further, genomic amplification, due to genomic instability or chromosomal rearrangements, is responsible for increased ABCB1 gene copy number and/or transactivation of ABCB1 expression [113,114,115,116,117,118].
Many studies report that a genomic amplification of the ABCB1-containing chromosomal region 7q21.12 occurs in MD-resistant cancers and that overexpression of genes of such region contributes to MDR (Figure 5) [51,73,119,120,121,122,123,124,125,126,127,128,129,130,131]. Amplification of the chromosomal region 7q21 containing ABCB1 and SRI (sorcin gene) was described in multidrug-resistant leukemia, neuroblastoma, and lung tumor cells [122,125,132]. The amplicon includes the genes SRI, ADAM22, DBF4, SLC25A40, RUNDC3B (RPIP9), ABCB1, ABCB4, CROT, the TP53TG1 long non-coding RNA, TMEM243 and DMTF1 (Figure 5), all of which were found to be associated with carcinogenesis and MDR. In particular, sorcin and DBF4 overexpression are drivers of MDR in several types of cancers; the development of inhibitors of sorcin expression and of CDC7-DBF4 activity as potential anti-tumor candidates was recently accomplished. DBF4 is a CDC7 kinase regulatory subunit, important in cell proliferation and DNA replication, overexpressed together with CDC7 in many primary tumors and cancer cell lines, such as diffuse large B-cell lymphoma, colorectal cancer, ovarian cancer, melanoma, breast cancer, and oral squamous cell carcinoma, and are markers of poor prognosis and of advanced tumor grade; many tumors contain extra copies of the DBF4 gene [119,133,134,135,136,137,138]. Overexpression of CDC7-DBF4, reported in several human cancers, is considered a marker of MDR [123,126,133,139].
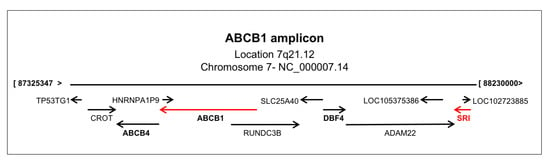
Figure 5.
The ABCB1 amplicon, located in chromosomal region 7q21, containing the sorcin(SOluble Resistance-related Calcium-binding proteIN) (SRI) gene.
2.6. Sorcin, Metastatization, and EMT
Sorcin overexpression in gastric cancer tissue is related closely to the depth of invasion, staging severity of malignant tumors, and lymph node metastasis of gastric cancers [53].
Sorcin induces gastric tumor cell migration and invasion; sorcin silencing downregulates the expression of cathepsin Z, matrix metalloproteinases 2 and 9 (MMP2 and MMP9), and signal transducer and activator of transcription 3 (STAT3), resulting in the suppression of cancer growth and metastasis [78]. Sorcin overexpression facilitates cell migration, invasion, and epithelial–mesenchymal transition (EMT), which generates features associated with high-grade tumors, and leads to metastatic dissemination [67]. In colorectal HCT116 cells, sorcin activates EMT through activation of the PI3K/Akt/mTOR pathway [58]; in breast cancer, sorcin silencing inhibits metastatization and EMT, by increasing the expression of E-cadherin and decreasing that of vascular endothelial growth factor (VEGF) [67].
2.7. Sorcin Directly Binds Chemotherapeutic Drugs
Part of the MD-resistant phenotype can be attributed to the capacity of sorcin to directly bind chemotherapeutic drugs. In fact, sorcin binds with high-affinity doxorubicin, paclitaxel, vincristine, and cisplatin in vitro, as shown by experiments carried out with techniques such as Surface Plasmon Resonance, fluorescence titration, and X-ray diffraction [74]. Recently, a crystal structure of the sorcin–doxorubicin complex has been solved, allowing the identification of one of the two binding sites for doxorubicin, close to the interface of the two sorcin monomers. The doxorubicin molecule is placed between the EF5 loop, the G helix, and the EF4 loop, and is involved in interactions with residues Phe173, Arg174, Asp177, and Tyr188 of the second monomer (Figure 6) [74]. Upon doxorubicin treatment, sorcin cellular localization changes, indicating a possible interaction also inside the cell. These findings show that sorcin can limit the toxic effects of doxorubicin (and possibly also of other drugs) in the cell, acting as a drug scavenger [74].
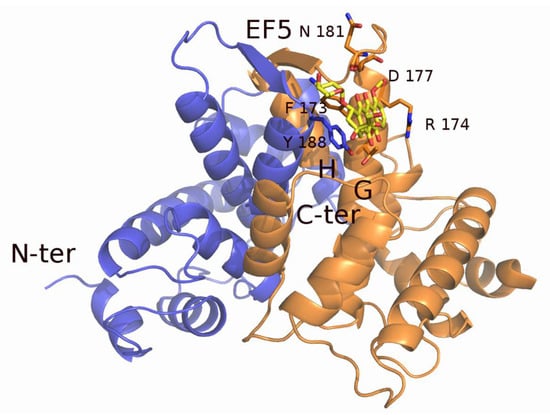
Figure 6.
X-ray crystal structure of sorcin in complex with doxorubicin. The chemotherapeutic drug binds close to residues of the EF5 hand, interacting with residues of sorcin G- and H-helices. The two monomers of the sorcin dimer are colored blue and orange. The doxorubicin molecule (colored in yellow) and the residues interacting with it are represented as sticks.
2.8. Targeting Sorcin
The involvement of sorcin in MDR has been shown for several cancer cell lines and, although it likely occurs through the several mechanisms described, the general inference is that lowering sorcin expression should have the effect to revert MDR. This body of results shows that sorcin can be considered a novel potential anticancer drug target. Studies aimed at targeting sorcin have been recently carried out, using different approaches.
MicroRNAs are negative gene regulators: miR-1, targeting the 3’-UTR region of sorcin gene SRI in its position 29–35, modulates calcium transients in cultured cardiomyocytes, and its expression is downregulated in human heart failure specimen and murine models [140]. In MD-resistant gastric cancer cells, miR-1 is highly downregulated; miR-1 overexpression increases apoptosis and promotes doxorubicin and vincristine accumulation in tumor cells, by acting on sorcin expression [98]. Sorcin overexpression partially reverses the effect of miR-1 in MD-resistant gastric tumor cells [98]. miR-1 may, therefore, be used as a therapeutic molecule vs. sorcin-dependent MDR. Off-target effects of miR-1, however, are possible, since the molecule is known to regulate other targets, such as HSP60, a component of the defense mechanism against diabetic myocardial injury, and the ets1 proto-oncogene, which plays a fundamental role in the extracellular matrix degradation [141,142].
Dihydromyricetin (DMY), a dihydroflavonol compound with anti-oxidant, anti-inflammatory, anti-bacterial, and anti-tumor effects, is able to reverse MDR in adriamycin-dependent MD-resistant breast cancer and leukemia cell lines and in a nude mice model, and to increase adriamycin cytotoxicity, by decreasing sorcin expression (both mRNA and protein), and consequently ABCB1 levels, via ERK/Akt pathways [24,75]. DMY increases intracellular free Ca2+ concentration, reactive oxygen species (ROS) levels, and expression of caspase 12, i.e., markers of ER stress-linked apoptosis, and regulates expression of markers of mitochondrial apoptosis, such as caspase 9, caspase 3, Bcl-2, Bax, and PARP [24]. Ondansetron (OND), an antiemetic drug used during tumor chemotherapy, also has a reversal effect in MDR due to sorcin expression, especially when used in combination with DMY, and restores P53 function by suppressing MDM2/MDMX, thus determining G2/M arrest and apoptosis; both DMY and OND may act by binding sorcin with high affinity [75]. It is possible that other targets may be regulated via the same ERK/Akt pathway, though the effect of DMY seems rather specific and dependent on binding to sorcin.
Haishengsu (HSS), a protein extract from the seashell Tegillarca granosa, promotes apoptosis in adriamycin-resistant leukemia cells inoculated in mice, by reducing expression of sorcin and ABCB1 [143] (other effects on other targets cannot be excluded, considering that this protein extract is poorly characterized from a molecular viewpoint). Administered to patients with acute leukemia in combination with different chemotherapy regimens, HSS increased treatment efficacy (in particular remission rate) and improved quality of life (decreasing nausea and vomiting) [144].
Some small compounds have activity on sorcin expression, among others. Calcitriol, the active form of Vitamin D, binds to the calcitriol receptor, also called vitamin D receptor or VDR. The VDR-calcitriol complex migrates to the nucleus where it acts as a transcription factor activating metabolic pathways with multifaceted effects on calcium homeostasis, leading to differentiation and anti-proliferative action and regulating sorcin expression, mostly increasing the 19-kDa form of the protein [145].
Palmitate, a free fatty acid highly circulating in obese children, induces sorcin downregulation [146], and subsequent increases in glucose-6-phosphatase catalytic subunit-2 levels contribute to lipotoxicity, to ER calcium depletion, and to ER stress in pancreatic β-cells [23]. The addition of metformin during 2-day palmitate exposure normalized oxygen consumption rate and sorcin levels [147].
Triptolide is a diterpenoid epoxide which is produced by the thunder god vine, Tripterygium wilfordii. It has antitumor activity in both non-resistant ovarian cancer SKOV3 and cisplatin-resistant SKOV3/DDP cells, likely through inducing apoptosis and regulating MMP-2, sorcin, and vascular endothelial growth factor expression [148].
Of course, given the high expression of sorcin in normal tissues (especially heart and brain) and its importance in regulating many important cellular events, targeting sorcin in cancer without undesirable off-target toxicity is not easy. This is a problem often encountered with many chemotherapeutic drugs: off-target activity, undesired toxicity vs. normal non-tumor cells, and development of resistance to drugs are the most important problems in anti-cancer treatments. Many approaches have been recently used to widen the therapeutic window of such molecules, and the use of antibodies and antibody-drug conjugates targeting cancer cells, or of genetically modifying techniques is now a reality.
To overcome off-targeting sorcin, we expect that the most useful approaches will be either via specifically targeting sorcin expression or its interaction with other proteins. A deep characterization of sorcin interactome is required to be able to aim at specific interaction and reduce off-target effects. Structural studies performed so far indicate that two hydrophobic regions become exposed upon calcium binding to EF1 and EF3 (Figure 3). Since, at least in principle, the two surfaces could mediate interaction with different partners, targeting different sites on the sorcin surface could allow specific modulation of interaction, resulting in a focused action.
3. Conclusions
In conclusion, sorcin represents an intriguing cancer target, due to its co-amplification with xenobiotic efflux pumps and to its role in cellular calcium signaling and calcium homeostasis. Many efforts have been spent in the dissection of the mechanisms of sorcin action in diverse pathophysiological settings, paving the way towards the development of successful and novel therapeutic strategies. We think that studies on the recently obtained sorcin−/− knock out mouse [18,28] will give important information on the roles of sorcin not only in cancer and in MDR, but also in brain and muscle development, in lipid metabolism, in diabetes, and in neurodegenerative diseases.
Funding
This research was funded by CNR grant Flagship Project Nanomax: “NADINE: Nanotechnology-based Diagnostics In Neurological Diseases and Experimental Oncology”; MIUR grants PRIN 20077PAMRE_003; PRIN 20154JRJPP MIUR; Min. Salute grant Progetto Ricerca Finalizzata RF-2016-02364123 RAREST-JHD to GC and AI; AIRC fellowship "Acqua Vitasnella" id. 22552 to IG.
Conflicts of Interest
The authors display no conflict of interest.
References
- Colotti, G.; Poser, E.; Fiorillo, A.; Genovese, I.; Chiarini, V.; Ilari, A. Sorcin, a calcium binding protein involved in the multidrug resistance mechanisms in cancer cells. Molecules 2014, 19, 13976–13989. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Ilari, A.; Battista, T.; Chiarini, F.; Fiorillo, A.; Colotti, G. Molecular bases of Sorcin-dependent resistance to chemotherapeutic agents. Cancer Drug Resist. 2018, 1, 17. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: an overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Zamparelli, C.; Ilari, A.; Verzili, D.; Giangiacomo, L.; Colotti, G.; Pascarella, S.; Chiancone, E. Structure-function relationships in sorcin, a member of the penta EF-hand family. Interaction of sorcin fragments with the ryanodine receptor and an Escherichia coli model system. Biochemistry 2000, 39, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Colotti, G.; Zamparelli, C.; Verzili, D.; Mella, M.; Loughrey, C.M.; Smith, G.L.; Chiancone, E. The W105G and W99G sorcin mutants demonstrate the role of the D helix in the Ca(2+)-dependent interaction with annexin VII and the cardiac ryanodine receptor. Biochemistry 2006, 45, 12519–12529. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, S.; Ilari, A.; Verzili, D.; Zamparelli, C.; Antaramian, A.; Rueda, A.; Valdivia, H.H.; Chiancone, E.; Colotti, G. Molecular basis for the impaired function of the natural F112L sorcin mutant: X-ray crystal structure, calcium affinity, and interaction with annexin VII and the ryanodine receptor. FASEB J. 2008, 22, 295–306. [Google Scholar] [CrossRef]
- Meyers, M.B.; Pickel, V.M.; Sheu, S.S.; Sharma, V.K.; Scotto, K.W.; Fishman, G.I. Association of sorcin with the cardiac ryanodine receptor. J. Biol. Chem. 1995, 270, 26411–26418. [Google Scholar] [CrossRef]
- Zamparelli, C.; Macquaide, N.; Colotti, G.; Verzili, D.; Seidler, T.; Smith, G.L.; Chiancone, E. Activation of the cardiac Na(+)-Ca(2+) exchanger by sorcin via the interaction of the respective Ca(2+)-binding domains. J. Mol. Cell. Cardiol. 2010, 49, 132–141. [Google Scholar] [CrossRef]
- Farrell, E.F.; Antaramian, A.; Rueda, A.; Gomez, A.M.; Valdivia, H.H. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J. Biol. Chem. 2003, 278, 34660–34666. [Google Scholar] [CrossRef]
- Lokuta, A.J.; Meyers, M.B.; Sander, P.R.; Fishman, G.I.; Valdivia, H.H. Modulation of cardiac ryanodine receptors by sorcin. J. Biol. Chem. 1997, 272, 25333–25338. [Google Scholar] [CrossRef]
- Matsumoto, T.; Hisamatsu, Y.; Ohkusa, T.; Inoue, N.; Sato, T.; Suzuki, S.; Ikeda, Y.; Matsuzaki, M. Sorcin interacts with sarcoplasmic reticulum Ca(2+)-ATPase and modulates excitation-contraction coupling in the heart. Basic Res. Cardiol. 2005, 100, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.R.; Colotti, G.; Chiancone, E.; Higuchi, Y.; Seidler, T.; Smith, G.L. Complex modulation of L-type Ca(2+) current inactivation by sorcin in isolated rabbit cardiomyocytes. Pflug. Arch. 2009, 457, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.R.; Colotti, G.; Chiancone, E.; Smith, G.L.; Fearon, I.M. Sorcin modulates cardiac L-type Ca2+ current by functional interaction with the alpha1C subunit in rabbits. Exp. Physiol. 2008, 93, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.F.; Bolck, B.; Ding, Z.; Krause, D.; Hattebuhr, N.; Malik, A.; Brixius, K.; Hajjar, R.J.; Schrader, J.; Schwinger, R.H. Overexpression of sorcin enhances cardiac contractility in vivo and in vitro. J. Mol. Cell. Cardiol. 2005, 38, 607–615. [Google Scholar] [CrossRef]
- Meyers, M.B.; Fischer, A.; Sun, Y.J.; Lopes, C.M.; Rohacs, T.; Nakamura, T.Y.; Zhou, Y.Y.; Lee, P.C.; Altschuld, R.A.; McCune, S.A.; et al. Sorcin regulates excitation-contraction coupling in the heart. J. Biol. Chem. 2003, 278, 28865–28871. [Google Scholar] [CrossRef]
- Seidler, T.; Miller, S.L.; Loughrey, C.M.; Kania, A.; Burow, A.; Kettlewell, S.; Teucher, N.; Wagner, S.; Kogler, H.; Meyers, M.B.; et al. Effects of adenovirus-mediated sorcin overexpression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Circ. Res. 2003, 93, 132–139. [Google Scholar] [CrossRef]
- Suarez, J.; McDonough, P.M.; Scott, B.T.; Suarez-Ramirez, A.; Wang, H.; Fricovsky, E.S.; Dillmann, W.H. Sorcin modulates mitochondrial Ca(2+) handling and reduces apoptosis in neonatal rat cardiac myocytes. Am. J. Physiol. Cell Physiol. 2013, 304, C248–C256. [Google Scholar] [CrossRef]
- Chen, X.; Weber, C.; Farrell, E.T.; Alvarado, F.J.; Zhao, Y.T.; Gomez, A.M.; Valdivia, H.H. Sorcin ablation plus beta-adrenergic stimulation generate an arrhythmogenic substrate in mouse ventricular myocytes. J. Mol. Cell. Cardiol. 2018, 114, 199–210. [Google Scholar] [CrossRef]
- Ranum, P.T.; Goodwin, A.T.; Yoshimura, H.; Kolbe, D.L.; Walls, W.D.; Koh, J.Y.; He, D.Z.Z.; Smith, R.J.H. Insights into the Biology of Hearing and Deafness Revealed by Single-Cell RNA Sequencing. Cell Rep. 2019, 26, 3160–3171. [Google Scholar] [CrossRef]
- Padar, S.; van Breemen, C.; Thomas, D.W.; Uchizono, J.A.; Livesey, J.C.; Rahimian, R. Differential regulation of calcium homeostasis in adenocarcinoma cell line A549 and its Taxol-resistant subclone. Br. J. Pharmacol. 2004, 142, 305–316. [Google Scholar] [CrossRef]
- Takeda, T.; Asahi, M.; Yamaguchi, O.; Hikoso, S.; Nakayama, H.; Kusakari, Y.; Kawai, M.; Hongo, K.; Higuchi, Y.; Kashiwase, K.; et al. Presenilin 2 regulates the systolic function of heart by modulating Ca2+ signaling. FASEB J. 2005, 19, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, V.S.; Ilari, A.; O’Connell, D.J.; Poser, E.; Sandoval, I.V.; Colotti, G. Sorcin links calcium signaling to vesicle trafficking, regulates Polo-like kinase 1 and is necessary for mitosis. PloS ONE 2014, 9, e85438. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, P.; Friche, E.; Sehested, M.; Jensen, P.B.; Skovsgaard, T. Cytosolic free Ca2+ in daunorubicin and vincristine resistant Ehrlich ascites tumor cells. Drug accumulation is independent of intracellular Ca2+ changes. Biochem. Pharmacol. 1991, 41, 243–253. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Sun, P.; Sun, H.; Ma, X.; Peng, J.; Liu, K. Targeting P-glycoprotein and SORCIN: Dihydromyricetin strengthens anti-proliferative efficiency of adriamycin via MAPK/ERK and Ca(2+) -mediated apoptosis pathways in MCF-7/ADR and K562/ADR. J. Cell. Physiol. 2018, 233, 3066–3079. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, N.; Zhou, Y.; Tan, Y.; Cheng, Y.; Yang, C.; Zhu, Z.; Xiong, D. Overexpression of sorcin in multidrug resistant human leukemia cells and its role in regulating cell apoptosis. Biochem. Biophys. Res. Commun. 2006, 349, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, F.; Laudiero, G.; Piscazzi, A.; Secondo, A.; Scorziello, A.; Lombardi, V.; Matassa, D.S.; Fersini, A.; Neri, V.; Esposito, F.; et al. Sorcin induces a drug-resistant phenotype in human colorectal cancer by modulating Ca(2+) homeostasis. Cancer Res. 2011, 71, 7659–7669. [Google Scholar] [CrossRef]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef]
- Marmugi, A.; Parnis, J.; Chen, X.; Carmichael, L.; Hardy, J.; Mannan, N.; Marchetti, P.; Piemonti, L.; Bosco, D.; Johnson, P.; et al. Sorcin links pancreatic beta cell lipotoxicity to ER Ca2+ stores. Diabetes 2016, 65, 1009–1021. [Google Scholar] [CrossRef]
- Ilari, A.; Fiorillo, A.; Poser, E.; Lalioti, V.S.; Sundell, G.N.; Ivarsson, Y.; Genovese, I.; Colotti, G. Structural basis of Sorcin-mediated calcium-dependent signal transduction. Sci. Rep. 2015, 5, 16828. [Google Scholar] [CrossRef]
- Ilari, A.; Johnson, K.A.; Nastopoulos, V.; Verzili, D.; Zamparelli, C.; Colotti, G.; Tsernoglou, D.; Chiancone, E. The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein. J. Mol. Biol. 2002, 317, 447–458. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, H.J.; Kim, S.S.; Kwon, Y.S.; Chun, W. Sequestration of sorcin by aberrant forms of tau results in the defective calcium homeostasis. Korean J. Physiol. Pharm. 2016, 20, 387–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, P.; Jiang, Y.F.; Wang, J.H. shRNA-mediated silencing of sorcin increases drug chemosensitivity in myeloma KM3/DDP and U266/ADM cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 2300–2310. [Google Scholar] [PubMed]
- Anthony, D.F.; Beattie, J.; Paul, A.; Currie, S. Interaction of calcium/calmodulin-dependent protein kinase IIdeltaC with sorcin indirectly modulates ryanodine receptor function in cardiac myocytes. J. Mol. Cell. Cardiol. 2007, 43, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, N.; Nakao, R.; Kondo, R.; Nishitsuji, M.; Saito, Y.; Kuga, T.; Hatayama, T.; Nakayama, Y. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein. Biochem. Biophys. Res. Commun. 2014, 448, 430–436. [Google Scholar] [CrossRef]
- Noordeen, N.A.; Meur, G.; Rutter, G.A.; Leclerc, I. Glucose-induced nuclear shuttling of ChREBP is mediated by sorcin and Ca(2+) ions in pancreatic beta-cells. Diabetes 2012, 61, 574–585. [Google Scholar] [CrossRef]
- Xie, H.; Chang, M.; Hu, X.; Wang, D.; Tian, M.; Li, G.; Jiang, H.; Wang, Y.; Dong, Z.; Zhang, Y.; et al. Proteomics analysis of MPP+-induced apoptosis in SH-SY5Y cells. Neurol. Sci. 2011, 32, 221–228. [Google Scholar] [CrossRef]
- Andreev, V.P.; Petyuk, V.A.; Brewer, H.M.; Karpievitch, Y.V.; Xie, F.; Clarke, J.; Camp, D.; Smith, R.D.; Lieberman, A.P.; Albin, R.L.; et al. Label-free quantitative LC-MS proteomics of Alzheimer’s disease and normally aged human brains. J. Proteome Res. 2012, 11, 3053–3067. [Google Scholar] [CrossRef]
- Tsuji, T.; Shiozaki, A.; Kohno, R.; Yoshizato, K.; Shimohama, S. Proteomic profiling and neurodegeneration in Alzheimer’s disease. Neurochem Res. 2002, 27, 1245–1253. [Google Scholar] [CrossRef]
- Seyfried, N.T.; Dammer, E.B.; Swarup, V.; Nandakumar, D.; Duong, D.M.; Yin, L.; Deng, Q.; Nguyen, T.; Hales, C.M.; Wingo, T.; et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst 2017, 4, 60–72. [Google Scholar] [CrossRef]
- Drummond, E.; Nayak, S.; Faustin, A.; Pires, G.; Hickman, R.A.; Askenazi, M.; Cohen, M.; Haldiman, T.; Kim, C.; Han, X.; et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol. 2017, 133, 933–954. [Google Scholar] [CrossRef]
- Hondius, D.C.; Eigenhuis, K.N.; Morrema, T.H.J.; van der Schors, R.C.; van Nierop, P.; Bugiani, M.; Li, K.W.; Hoozemans, J.J.M.; Smit, A.B.; Rozemuller, A.J.M. Proteomics analysis identifies new markers associated with capillary cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Umoh, M.E.; Dammer, E.B.; Dai, J.; Duong, D.M.; Lah, J.J.; Levey, A.I.; Gearing, M.; Glass, J.D.; Seyfried, N.T. A proteomic network approach across the ALS-FTD disease spectrum resolves clinical phenotypes and genetic vulnerability in human brain. Embo Mol. Med. 2018, 10, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.J.; Heyny-von Haussen, R.; Mall, G.; Wolf, S. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteome Sci. 2008, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hulette, C.; Wang, Y.; Zhang, T.; Pan, C.; Wadhwa, R.; Zhang, J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol. Cell. Proteom. 2006, 5, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Singh, S.; Lokappa, S.B.; Varkey, J. Gene co-expression network analysis for identifying genetic markers in Parkinson’s disease - a three-way comparative approach. Genomics 2019, 111, 819–830. [Google Scholar] [CrossRef]
- Kalathur, R.K.; Giner-Lamia, J.; Machado, S.; Barata, T.; Ayasolla, K.R.; Futschik, M.E. The unfolded protein response and its potential role in Huntington’s disease elucidated by a systems biology approach. F1000Res 2015, 4, 103. [Google Scholar] [CrossRef]
- Pack-Chung, E.; Meyers, M.B.; Pettingell, W.P.; Moir, R.D.; Brownawell, A.M.; Cheng, I.; Tanzi, R.E.; Kim, T.W. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J. Biol. Chem. 2000, 275, 14440–14445. [Google Scholar] [CrossRef]
- Woods, W.S.; Boettcher, J.M.; Zhou, D.H.; Kloepper, K.D.; Hartman, K.L.; Ladror, D.T.; Qi, Z.; Rienstra, C.M.; George, J.M. Conformation-specific binding of alpha-synuclein to novel protein partners detected by phage display and NMR spectroscopy. J. Biol. Chem. 2007, 282, 34555–34567. [Google Scholar] [CrossRef]
- Meyers, M.B.; Biedler, J.L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem. Biophys. Res. Commun. 1981, 99, 228–235. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Baas, F.; Van der Velde-Koerts, T.; Biedler, J.L.; Meyers, M.B.; Ozols, R.F.; Hamilton, T.C.; Joenje, H.; Borst, P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988, 48, 5927–5932. [Google Scholar]
- Chen, J.; Watanabe, M.; Huang, P.; Sakaguchi, M.; Ochiai, K.; Nasu, Y.; Ouchida, M.; Huh, N.H.; Shimizu, K.; Kashiwakura, Y.; et al. REIC/Dkk-3 stable transfection reduces the malignant phenotype of mouse prostate cancer RM9 cells. Int J. Mol. Med. 2009, 24, 789–794. [Google Scholar] [PubMed]
- Dabaghi, M.; Rahgozar, S.; Moshtaghian, J.; Moafi, A.; Abedi, M.; Pourabutaleb, E. Overexpression of SORCIN is a Prognostic Biomarker for Multidrug-Resistant Pediatric Acute Lymphoblastic Leukemia and Correlates with Upregulated MDR1/P-gp. Genet. Test. Mol. Biomark. 2016, 20, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Su, T.; Leng, A.; Zhang, X.; Xu, M.; Yan, L.; Gu, H.; Zhang, G. Upregulation of soluble resistance-related calcium-binding protein (sorcin) in gastric cancer. Med. Oncol. 2010, 27, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, W.; Liu, X.; Gao, F.; Zhao, X. Reversing effect and mechanism of soluble resistance-related calcium-binding protein on multidrug resistance in human lung cancer A549/DDP cells. Mol. Med. Rep. 2015, 11, 2118–2124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagpal, J.K.; Das, B.R. Identification of differentially expressed genes in tobacco chewing-mediated oral cancer by differential display-polymerase chain reaction. Eur. J. Clin. Investig. 2007, 37, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Yang, Y.; Liu, B.; Xiao, W. Comparative proteomic profiling identified sorcin being associated with gemcitabine resistance in non-small cell lung cancer. Med Oncol. 2010, 27, 1303–1308. [Google Scholar] [CrossRef]
- Tan, Y.; Li, G.; Zhao, C.; Wang, J.; Zhao, H.; Xue, Y.; Han, M.; Yang, C. Expression of sorcin predicts poor outcome in acute myeloid leukemia. Leuk. Res. 2003, 27, 125–131. [Google Scholar] [CrossRef]
- Tong, W.; Sun, D.; Wang, Q.; Suo, J. Sorcin Enhances Metastasis and Promotes Epithelial-to-Mesenchymal Transition of Colorectal Cancer. Cell Biochem. Biophys. 2015, 72, 453–459. [Google Scholar] [CrossRef]
- Yang, Y.X.; Chen, Z.C.; Zhang, G.Y.; Yi, H.; Xiao, Z.Q. A subcelluar proteomic investigation into vincristine-resistant gastric cancer cell line. J. Cell. Biochem. 2008, 104, 1010–1021. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Tan, Y.; Qi, J.; Xiao, Y.; Yang, C.; Zhu, Z.; Xiong, D. Sorcin, an important gene associated with multidrug-resistance in human leukemia cells. Leuk. Res. 2006, 30, 469–476. [Google Scholar] [CrossRef]
- Pomeroy, S.L.; Tamayo, P.; Gaasenbeek, M.; Sturla, L.M.; Angelo, M.; McLaughlin, M.E.; Kim, J.Y.; Goumnerova, L.C.; Black, P.M.; Lau, C.; et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002, 415, 436–442. [Google Scholar] [CrossRef] [PubMed]
- French, P.J.; Swagemakers, S.M.; Nagel, J.H.; Kouwenhoven, M.C.; Brouwer, E.; van der Spek, P.; Luider, T.M.; Kros, J.M.; van den Bent, M.J.; Sillevis Smitt, P.A. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer Res. 2005, 65, 11335–11344. [Google Scholar] [CrossRef] [PubMed]
- Shai, R.; Shi, T.; Kremen, T.J.; Horvath, S.; Liau, L.M.; Cloughesy, T.F.; Mischel, P.S.; Nelson, S.F. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 2003, 22, 4918–4923. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hui, A.M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef]
- Yokota, T.; Kouno, J.; Adachi, K.; Takahashi, H.; Teramoto, A.; Matsumoto, K.; Sugisaki, Y.; Onda, M.; Tsunoda, T. Identification of histological markers for malignant glioma by genome-wide expression analysis: dynein, alpha-PIX and sorcin. Acta Neuropathol. 2006, 111, 29–38. [Google Scholar] [CrossRef]
- He, Q.; Zhang, G.; Hou, D.; Leng, A.; Xu, M.; Peng, J.; Liu, T. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol. Rep. 2011, 25, 237–243. [Google Scholar]
- Hu, Y.; Li, S.; Yang, M.; Yan, C.; Fan, D.; Zhou, Y.; Zhang, Y.; Yague, E.; Xiong, D. Sorcin silencing inhibits epithelial-to-mesenchymal transition and suppresses breast cancer metastasis in vivo. Breast Cancer Res. Treat. 2014, 143, 287–299. [Google Scholar] [CrossRef]
- Kawakami, M.; Nakamura, T.; Okamura, N.; Komoto, C.; Markova, S.; Kobayashi, H.; Hashimoto, N.; Okumura, K.; Sakaeda, T. Knock-down of sorcin induces up-regulation of MDR1 in HeLa cells. Biol. Pharm. Bull. 2007, 30, 1065–1073. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Feng, B.; Liu, G. Reversing effect of sorcin in the drug resistance of human nasopharyngeal carcinoma. Anat. Rec. 2014, 297, 215–221. [Google Scholar] [CrossRef]
- Parekh, H.K.; Deng, H.B.; Choudhary, K.; Houser, S.R.; Simpkins, H. Overexpression of sorcin, a calcium-binding protein, induces a low level of paclitaxel resistance in human ovarian and breast cancer cells. Biochem. Pharmacol. 2002, 63, 1149–1158. [Google Scholar] [CrossRef]
- Demidova, N.S.; Ilyinskaya, G.V.; Shiryaeva, O.A.; Chernova, O.B.; Goncharova, S.A.; Kopnin, B.P. Decreased sensitivity of multidrug-resistant tumor cells to cisplatin is correlated with sorcin gene co-amplification. Neoplasma 1995, 42, 195–201. [Google Scholar] [PubMed]
- Liang, B.; Peng, P.; Chen, S.; Li, L.; Zhang, M.; Cao, D.; Yang, J.; Li, H.; Gui, T.; Li, X.; et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteom. 2013, 80, 171–182. [Google Scholar] [CrossRef]
- Van der Bliek, A M.; Meyers, M.B.; Biedler, J.L.; Hes, E.; Borst, P. A 22-kd protein (sorcin/V19) encoded by an amplified gene in multidrug-resistant cells, is homologous to the calcium-binding light chain of calpain. Embo J. 1986, 5, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Fiorillo, A.; Ilari, A.; Masciarelli, S.; Fazi, F.; Colotti, G. Binding of doxorubicin to Sorcin impairs cell death and increases drug resistance in cancer cells. Cell Death Dis. 2017, 8, e2950. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.; Wang, C.; Meng, Q.; Liu, Z.; Huo, X.; Yang, X.; Sun, P.; Sun, H.; Ma, X.; et al. Combination of dihydromyricetin and ondansetron strengthens antiproliferative efficiency of adriamycin in K562/ADR through downregulation of SORCIN: A new strategy of inhibiting P-glycoprotein. J. Cell. Physiol. 2019, 234, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sirohi, V.K.; Kumari, S.; Shukla, V.; Manohar, M.; Popli, P.; Dwivedi, A. Sorcin is involved during embryo implantation via activating VEGF/PI3K/Akt pathway in mice. J. Mol. Endocrinol. 2018, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Khan, H.; Sirohi, V.K.; Das, V.; Agarwal, A.; Pandey, A.; Siddiqui, W.A.; Dwivedi, A. Alteration in endometrial proteins during early- and mid-secretory phases of the cycle in women with unexplained infertility. PloS ONE 2014, 9, e111687. [Google Scholar] [CrossRef]
- Tuo, H.; Shu, F.; She, S.; Yang, M.; Zou, X.Q.; Huang, J.; Hu, H.D.; Hu, P.; Ren, H.; Peng, S.F.; et al. Sorcin induces gastric cancer cell migration and invasion contributing to STAT3 activation. Oncotarget 2017, 8, 104258–104271. [Google Scholar] [CrossRef]
- Kawasaki, H.; Mizutome, H.; Kretsinger, R.H. Interaction sites of PEF proteins for recognition of their targets. Int. J. Biol. Macromol 2019, 133, 1035–1041. [Google Scholar] [CrossRef]
- Mella, M.; Colotti, G.; Zamparelli, C.; Verzili, D.; Ilari, A.; Chiancone, E. Information transfer in the penta-EF-hand protein sorcin does not operate via the canonical structural/functional pairing. A study with site-specific mutants. J. Biol. Chem. 2003, 278, 24921–24928. [Google Scholar] [CrossRef]
- Bers, D.M.; Despa, S.; Bossuyt, J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann. N. Y. Acad. Sci. 2006, 1080, 165–177. [Google Scholar] [CrossRef]
- Brownawell, A.M.; Creutz, C.E. Calcium-dependent binding of sorcin to the N-terminal domain of synexin (annexin VII). J. Biol. Chem. 1997, 272, 22182–22190. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.; Futter, C.; Moss, S.E. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol. 2004, 165, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Salzer, U.; Hinterdorfer, P.; Hunger, U.; Borken, C.; Prohaska, R. Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 2002, 99, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef]
- Demory Beckler, M.; Higginbotham, J.N.; Franklin, J.L.; Ham, A.J.; Halvey, P.J.; Imasuen, I.E.; Whitwell, C.; Li, M.; Liebler, D.C.; Coffey, R.J. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell. Proteom. 2013, 12, 343–355. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375–15396. [Google Scholar] [CrossRef]
- Kharaziha, P.; Chioureas, D.; Rutishauser, D.; Baltatzis, G.; Lennartsson, L.; Fonseca, P.; Azimi, A.; Hultenby, K.; Zubarev, R.; Ullen, A.; et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget 2015, 6, 21740–21754. [Google Scholar] [CrossRef]
- Pienimaeki-Roemer, A.; Kuhlmann, K.; Bottcher, A.; Konovalova, T.; Black, A.; Orso, E.; Liebisch, G.; Ahrens, M.; Eisenacher, M.; Meyer, H.E.; et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion 2015, 55, 507–521. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Arous, C.; Schvartz, D.; Timper, K.; Sanchez, J.C.; Dermitzakis, E.; Donath, M.Y.; Halban, P.A.; Bouzakri, K. Fractalkine (CX3CL1), a new factor protecting beta-cells against TNFalpha. Mol. Metab. 2014, 3, 731–741. [Google Scholar] [CrossRef]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.F.; Hao, Y.H.; Serneels, L.; De Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 2006, 126, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Gracy, K.N.; Clarke, C.L.; Meyers, M.B.; Pickel, V.M. N-methyl-D-aspartate receptor 1 in the caudate-putamen nucleus: Ultrastructural localization and co-expression with sorcin, a 22,000 mol. wt calcium binding protein. Neuroscience 1999, 90, 107–117. [Google Scholar] [CrossRef]
- Clemen, C.S.; Herr, C.; Hovelmeyer, N.; Noegel, A.A. The lack of annexin A7 affects functions of primary astrocytes. Exp. Cell Res. 2003, 291, 406–414. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Reviews. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Deng, L.M.; Tan, T.; Zhang, T.Y.; Xiao, X.F.; Gu, H. miR1 reverses multidrug resistance in gastric cancer cells via downregulation of sorcin through promoting the accumulation of intracellular drugs and apoptosis of cells. Int. J. Oncol. 2019, 55, 451–461. [Google Scholar]
- Hu, Y.; Cheng, X.; Li, S.; Zhou, Y.; Wang, J.; Cheng, T.; Yang, M.; Xiong, D. Inhibition of sorcin reverses multidrug resistance of K562/A02 cells and MCF-7/A02 cells via regulating apoptosis-related proteins. Cancer Chemother. Pharmacol. 2013, 72, 789–798. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updates 2017, 32, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Abolhoda, A.; Wilson, A.E.; Ross, H.; Danenberg, P.V.; Burt, M.; Scotto, K.W. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin. Cancer Res. 1999, 5, 3352–3356. [Google Scholar]
- Brugger, D.; Brischwein, K.; Liu, C.; Bader, P.; Niethammer, D.; Gekeler, V.; Beck, J.F. Induction of drug resistance and protein kinase C genes in A2780 ovarian cancer cells after incubation with antineoplastic agents at sublethal concentrations. Anticancer Res. 2002, 22, 4229–4232. [Google Scholar] [PubMed]
- Chin, K.V.; Tanaka, S.; Darlington, G.; Pastan, I.; Gottesman, M.M. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J. Biol. Chem. 1990, 265, 221–226. [Google Scholar] [PubMed]
- Fojo, A.T.; Ueda, K.; Slamon, D.J.; Poplack, D.G.; Gottesman, M.M.; Pastan, I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Gekeler, V.; Frese, G.; Diddens, H.; Probst, H. Expression of a P-glycoprotein gene is inducible in a multidrug-resistant human leukemia cell line. Biochem. Biophys. Res. Commun. 1988, 155, 754–760. [Google Scholar] [CrossRef]
- Hu, X.F.; Slater, A.; Wall, D.M.; Kantharidis, P.; Parkin, J.D.; Cowman, A.; Zalcberg, J.R. Rapid up-regulation of mdr1 expression by anthracyclines in a classical multidrug-resistant cell line. Br. J. Cancer 1995, 71, 931–936. [Google Scholar] [CrossRef]
- Liu, Z.L.; Onda, K.; Tanaka, S.; Toma, T.; Hirano, T.; Oka, K. Induction of multidrug resistance in MOLT-4 cells by anticancer agents is closely related to increased expression of functional P-glycoprotein and MDR1 mRNA. Cancer Chemother. Pharmacol. 2002, 49, 391–397. [Google Scholar] [CrossRef]
- Park, J.; Shinohara, N.; Liebert, M.; Noto, L.; Flint, A.; Grossman, H.B. P-glycoprotein expression in bladder cancer. J. Urol. 1994, 151, 43–46. [Google Scholar] [CrossRef]
- Schneider, J.; Efferth, T.; Centeno, M.M.; Mattern, J.; Rodriguez-Escudero, F.J.; Volm, M. High rate of expression of multidrug resistance-associated P-glycoprotein in human endometrial carcinoma and normal endometrial tissue. Eur. J. Cancer 1993, 29A, 554–558. [Google Scholar] [CrossRef]
- Schoenlein, P.V. Molecular cytogenetics of multiple drug resistance. Cytotechnology 1993, 12, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Scotto, K.W.; Biedler, J.L.; Melera, P.W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science 1986, 232, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.K.; Lacayo, N.J.; Duran, G.E.; Wang, Y.; Bangs, C.D.; Rea, S.; Kovacs, M.; Cherry, A.M.; Brown, J.M.; Sikic, B.I. Preferential expression of a mutant allele of the amplified MDR1 (ABCB1) gene in drug-resistant variants of a human sarcoma. Gene Chromosomes Cancer 2002, 34, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, P.; Li, R.; Sachs, R.; Fabarius, A.; Upender, M.B.; Hehlmann, R. Cancer drug resistance: the central role of the karyotype. Drug Resist. Updates 2007, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Shibata, T.; Kokubu, A.; Ojima, H.; Loukopoulos, P.; Kanai, Y.; Kosuge, T.; Fukayama, M.; Kondo, T.; Sakamoto, M.; et al. Genetic profile of hepatocellular carcinoma revealed by array-based comparative genomic hybridization: identification of genetic indicators to predict patient outcome. J. Hepatol. 2005, 43, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.W.; Han, N.; Kim, M.G.; Kim, T.; Oh, J.M. Copy number variability analysis of pharmacogenes in patients with lymphoma, leukemia, hepatocellular, and lung carcinoma using The Cancer Genome Atlas data. Pharm. Genom. 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mickley, L.A.; Spengler, B.A.; Knutsen, T.A.; Biedler, J.L.; Fojo, T. Gene rearrangement: a novel mechanism for MDR-1 gene activation. J. Clin. Investig. 1997, 99, 1947–1957. [Google Scholar] [CrossRef]
- Pang, E.; Hu, Y.; Chan, K.Y.; Lai, P.B.; Squire, J.A.; Macgregor, P.F.; Beheshti, B.; Albert, M.; Leung, T.W.; Wong, N. Karyotypic imbalances and differential gene expressions in the acquired doxorubicin resistance of hepatocellular carcinoma cells. Lab. Investig. 2005, 85, 664–674. [Google Scholar] [CrossRef]
- Bonte, D.; Lindvall, C.; Liu, H.; Dykema, K.; Furge, K.; Weinreich, M. Cdc7-Dbf4 kinase overexpression in multiple cancers and tumor cell lines is correlated with p53 inactivation. Neoplasia 2008, 10, 920–931. [Google Scholar] [CrossRef]
- Chao, C.C.; Ma, C.M.; Lin-Chao, S. Co-amplification and over-expression of two mdr genes in a multidrug-resistant human colon carcinoma cell line. FEBS Lett. 1991, 291, 214–218. [Google Scholar] [CrossRef]
- Finalet Ferreiro, J.; Rouhigharabaei, L.; Urbankova, H.; van der Krogt, J.A.; Michaux, L.; Shetty, S.; Krenacs, L.; Tousseyn, T.; De Paepe, P.; Uyttebroeck, A.; et al. Integrative genomic and transcriptomic analysis identified candidate genes implicated in the pathogenesis of hepatosplenic T-cell lymphoma. PloS ONE 2014, 9, e102977. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, M.; Muhlethaler-Mottet, A.; Martinet, D.; Fattet, S.; Bourloud, K.B.; Auderset, K.; Meier, R.; Schmutz, N.B.; Delattre, O.; Joseph, J.M.; et al. Molecular cytogenetic characterization of doxorubicin-resistant neuroblastoma cell lines: evidence that acquired multidrug resistance results from a unique large amplification of the 7q21 region. Gene Chromosomes Cancer 2006, 45, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.N.; Ehlers, N.S.; Zhu, S.; Thomsen, M.B.; Nielsen, R.L.; Liu, D.; Wang, G.; Hou, Y.; Zhang, X.; Xu, X.; et al. The stepwise evolution of the exome during acquisition of docetaxel resistance in breast cancer cells. Bmc Genom. 2016, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Sterzynska, K.; Zawierucha, P.; Rucinski, M.; Swierczewska, M.; Partyka, M.; Bednarek-Rajewska, K.; Brazert, M.; Nowicki, M.; Zabel, M.; et al. Microarray-based detection and expression analysis of new genes associated with drug resistance in ovarian cancer cell lines. Oncotarget 2017, 8, 49944–49958. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Yamasaki, T. The MDR1/ABCB1 regional amplification in large inverted repeats with asymmetric sequences and microhomologies at the junction sites. Cancer Genet. Cytogenet. 2007, 178, 120–127. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.; Ho, J.N.; Jin, H.; Byun, S.S.; Lee, E. Analysis of resistance-associated gene expression in docetaxel-resistant prostate cancer cells. Oncol. Lett. 2017, 14, 3011–3018. [Google Scholar] [CrossRef]
- Litviakov, N.V.; Cherdyntseva, N.V.; Tsyganov, M.M.; Slonimskaya, E.M.; Ibragimova, M.K.; Kazantseva, P.V.; Kzhyshkowska, J.; Choinzonov, E.L. Deletions of multidrug resistance gene loci in breast cancer leads to the down-regulation of its expression and predict tumor response to neoadjuvant chemotherapy. Oncotarget 2016, 7, 7829–7841. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Torigoe, K.; Sato, S.; Kusaba, H.; Kohno, K.; Kuwano, M.; Okumura, K.; Green, E.D.; Tsui, L.C.; Scherer, S.W.; Schlessinger, D.; et al. A YAC-based contig of 1.5 Mb spanning the human multidrug resistance gene region and delineating the amplification unit in three human multidrug-resistant cell lines. Genome Res. 1995, 5, 233–244. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Van der Velde-Koerts, T.; Ling, V.; Borst, P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol. Cell. Biol. 1986, 6, 1671–1678. [Google Scholar] [CrossRef][Green Version]
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9. [Google Scholar] [CrossRef]
- Kadioglu, O.; Efferth, T. Peptide aptamer identified by molecular docking targeting translationally controlled tumor protein in leukemia cells. Investig. New Drugs 2016, 34, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.N.; Jiang, S.S.; Fan, C.C.; Lo, Y.K.; Kuo, C.Y.; Chen, C.H.; Liu, Y.L.; Lee, C.C.; Chen, W.S.; Huang, T.S.; et al. Increased Cdc7 expression is a marker of oral squamous cell carcinoma and overexpression of Cdc7 contributes to the resistance to DNA-damaging agents. Cancer Lett. 2013, 337, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Choschzick, M.; Lebeau, A.; Marx, A.H.; Tharun, L.; Terracciano, L.; Heilenkotter, U.; Jaenicke, F.; Bokemeyer, C.; Simon, R.; Sauter, G.; et al. Overexpression of cell division cycle 7 homolog is associated with gene amplification frequency in breast cancer. Hum. Pathol. 2010, 41, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Fountaine, T.J.; Hennessy, J.; Bruggeman, R.D.; Clarke, J.T.; Mauger, D.T.; Helm, K.F. Cdc7 expression in melanomas, Spitz tumors and melanocytic nevi. J. Cutan. Pathol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, H.Q.; Ba, Y. High expression of cell division cycle 7 protein correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med Oncol. 2012, 29, 3498–3503. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Kingsbury, S.R.; Tudzarova, S.; Hong, H.K.; Loddo, M.; Rashid, M.; Rodriguez-Acebes, S.; Prevost, A.T.; Ledermann, J.A.; Stoeber, K.; et al. Cdc7 kinase is a predictor of survival and a novel therapeutic target in epithelial ovarian carcinoma. Clin. Cancer Res. 2009, 15, 2417–2425. [Google Scholar] [CrossRef]
- Nambiar, S.; Mirmohammadsadegh, A.; Hassan, M.; Mota, R.; Marini, A.; Alaoui, A.; Tannapfel, A.; Hegemann, J.H.; Hengge, U.R. Identification and functional characterization of ASK/Dbf4, a novel cell survival gene in cutaneous melanoma with prognostic relevance. Carcinogenesis 2007, 28, 2501–2510. [Google Scholar] [CrossRef]
- Sasi, N.K.; Bhutkar, A.; Lanning, N.J.; MacKeigan, J.P.; Weinreich, M. DDK Promotes Tumor Chemoresistance and Survival via Multiple Pathways. Neoplasia 2017, 19, 439–450. [Google Scholar] [CrossRef]
- Ali, R.; Huang, Y.; Maher, S.E.; Kim, R.W.; Giordano, F.J.; Tellides, G.; Geirsson, A. miR-1 mediated suppression of Sorcin regulates myocardial contractility through modulation of Ca2+ signaling. J. Mol. Cell. Cardiol. 2012, 52, 1027–1037. [Google Scholar] [CrossRef]
- Shan, Z.X.; Lin, Q.X.; Deng, C.Y.; Zhu, J.N.; Mai, L.P.; Liu, J.L.; Fu, Y.H.; Liu, X.Y.; Li, Y.X.; Zhang, Y.Y.; et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. Febs Lett. 2010, 584, 3592–3600. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, Z.; Fu, H.; Zhang, H.; Wu, Y.; Zheng, X. MicroRNA-1 and microRNA-499 downregulate the expression of the ets1 proto-oncogene in HepG2 cells. Oncol. Rep. 2012, 28, 701–706. [Google Scholar] [CrossRef]
- Li, G.Y.; Liu, J.Z.; Zhang, B.; Yang, M.; Chen, S.G.; Hou, M.; Wang, L.X. Tegillarca granosa extract Haishengsu (HSS) suppresses expression of mdr1, BCR/ABL and sorcin in drug-resistant K562/ADM tumors in mice. Adv. Med Sci. 2013, 58, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Zhang, L.; Liu, J.Z.; Chen, S.G.; Xiao, T.W.; Liu, G.Z.; Wang, J.X.; Wang, L.X.; Hou, M. Marine drug Haishengsu increases chemosensitivity to conventional chemotherapy and improves quality of life in patients with acute leukemia. Biomed. Pharmacother. 2016, 81, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.J.; Tchack, L.; Angelo, G.; Pratt, R.E.; Sonna, L.A. DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol. Genom. 2004, 17, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Groebe, K.; Cen, J.; Schvartz, D.; Sargsyan, E.; Chowdhury, A.; Roomp, K.; Schneider, R.; Alderborn, A.; Sanchez, J.C.; Bergsten, P. Palmitate-Induced Insulin Hypersecretion and Later Secretory Decline Associated with Changes in Protein Expression Patterns in Human Pancreatic Islets. J. Proteome Res. 2018, 17, 3824–3836. [Google Scholar] [CrossRef]
- Cen, J.; Sargsyan, E.; Forslund, A.; Bergsten, P. Mechanisms of beneficial effects of metformin on fatty acid-treated human islets. J. Mol. Endocrinol.s 2018, 61, 91–99. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, S.; Tong, Y.; Huang, G.; Tan, B.; Yang, L. Antitumor activity of triptolide in SKOV3 cells and SKOV3/DDP in vivo and in vitro. Anticancer Drugs 2020. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).