Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma
Abstract
1. Introduction
2. Results
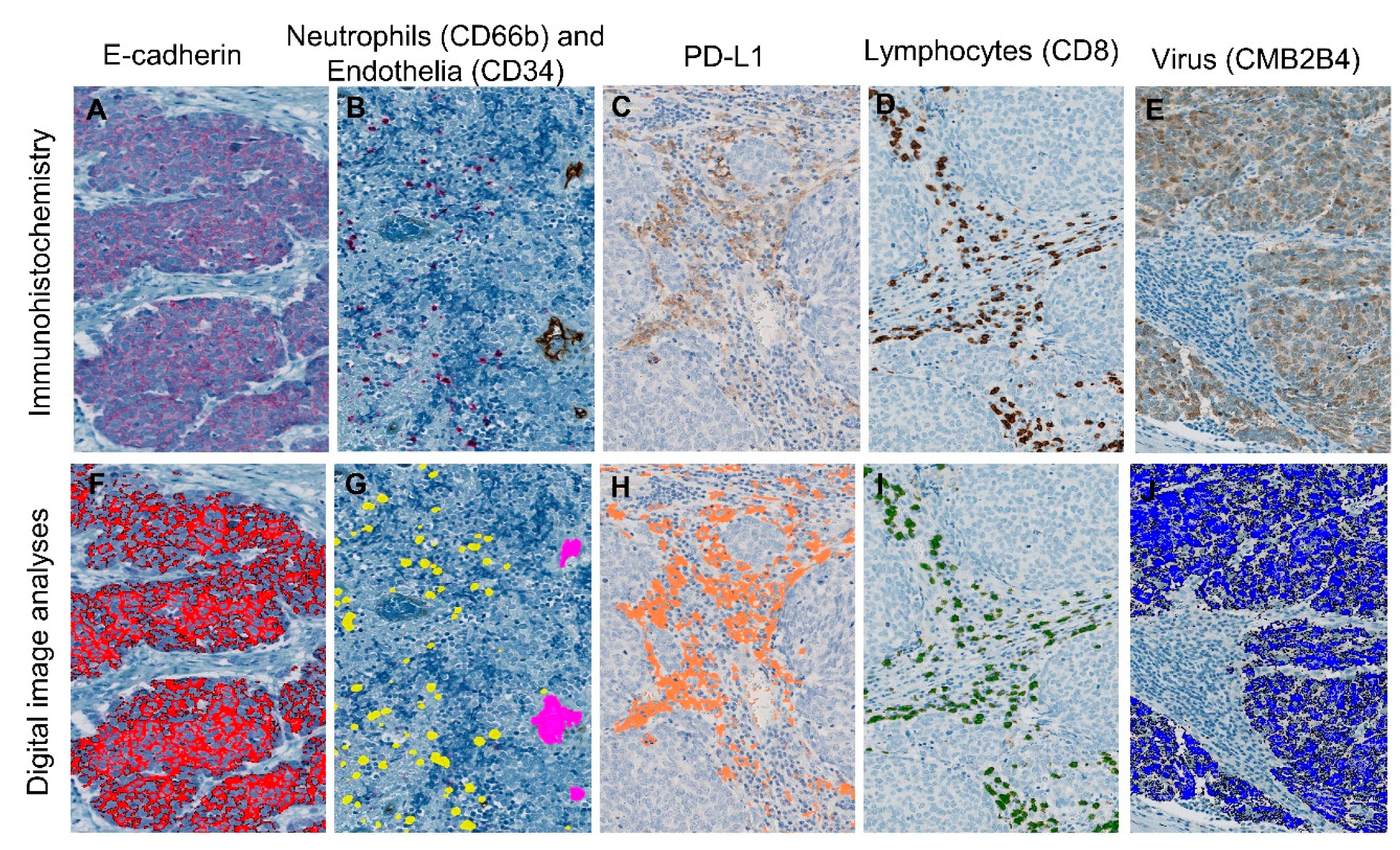
2.1. Ulceration in MCC Is Associated with Increased Infiltration of Neutrophils and Decreased Infiltration of CD8 Lymphocytes
2.2. Ulceration Is Associated with Virus-Negative MCC
2.3. Virus-Positive MCC Presents Higher Densities of PD-L1, Lower Neutrophil-to-CD8 Lymphocyte Ratio and Lower Density of E-Cadherin
2.4. Density of CD8 Lymphocytes and PD-L1 Are Associated
2.5. Density of CD8 Lymphocytes, Neutrophil-to-CD8 Lymphocyte Ratio, Virus-Positive Status, Ulceration and Nodal Involvement Have Independent Impact on MCC Specific Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Tumor Specimens
4.3. DNA Extraction and Quantification
4.4. Real-time Taqman Polymerase Chain Reaction
4.5. Immunohistochemical Staining
4.6. Digital Pathology
4.7. Ulceration Status
4.8. Viral Status
4.9. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Toker, C. Trabecular carcinoma of the skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.L.; Dennis, S.; Kachare, S.D.; Vohra, N.A.; Wong, J.H.; Zervos, E.E. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the USA. Am. Surg. 2015, 81, 802–806. [Google Scholar] [PubMed]
- Lyhne, D.; Lock-Andersen, J.; Dahlstrom, K.; Drzewiecki, K.T.; Balslev, E.; Muhic, A.; Krarup-Hansen, A. Rising incidence of Merkel cell carcinoma. J. Plast. Surg. Hand Surg. 2011, 45, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Soyer, H.P.; Youl, P.H.; Fritschi, L.; Baade, P.D. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. Jama Dermatol. 2014, 150, 864–872. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Wong, S.Q.; Waldeck, K.; Vergara, I.A.; Schroder, J.; Madore, J.; Wilmott, J.S.; Colebatch, A.J.; De Paoli-Iseppi, R.; Li, J.; Lupat, R.; et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015, 75, 5228–5234. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Vincent, J.G.; Loyo, M.; Kagohara, L.T.; Luber, B.S.; Wang, H.; Xu, H.; Nayar, S.K.; Wang, T.S.; Sidransky, D.; et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol. Res. 2013, 1, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Bohling, T.; Kavola, H.; Koljonen, V.; Salmi, M.; Jalkanen, S.; Joensuu, H. Tumor infiltrating immune cells and outcome of Merkel cell carcinoma: A population-based study. Clin. Cancer Res. 2012, 18, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Church, C.D.; Dong, L.; Crispin, D.; Fitzgibbon, M.P.; Lachance, K.; Jing, L.; Shinohara, M.; Gavvovidis, I.; Willimsky, G.; et al. Tumor-Infiltrating Merkel Cell Polyomavirus-Specific T Cells Are Diverse and Associated with Improved Patient Survival. Cancer Immunol. Res. 2017, 5, 137–147. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Simonson, W.T.; Blom, A.; Thibodeau, R.M.; Schmidt, M.; Pietromonaco, S.; Sokil, M.; Warton, E.M.; Asgari, M.M.; et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: A population-based study. Am. J. Clin. Pathol. 2014, 142, 452–458. [Google Scholar] [CrossRef]
- Antonio, N.; Bonnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Bonnelykke-Behrndtz, M.L.; Steiniche, T.; Norgaard, P.; Danielsen, A.V.; Damsgaard, T.E.; Christensen, I.J.; Bastholt, L.; Moller, H.J.; Schmidt, H. Loss of E-cadherin as Part of a Migratory Phenotype in Melanoma Is Associated With Ulceration. Am. J. Dermatopathol. 2017, 39, 672–678. [Google Scholar] [CrossRef]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Homig-Holzel, C.; et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.S.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Milella, M.; Brownell, I.; et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after >/=1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer 2018, 6, 7. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F. Update on the melanoma staging system: The importance of sentinel node staging and primary tumor mitotic rate. J. Surg. Oncol. 2011, 104, 379–385. [Google Scholar] [CrossRef]
- Grabowski, J.; Saltzstein, S.L.; Sadler, G.R.; Tahir, Z.; Blair, S. A comparison of merkel cell carcinoma and melanoma: Results from the california cancer registry. Clin. Med. Oncol. 2008, 2, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Frohm, M.L.; Griffith, K.A.; Harms, K.L.; Hayman, J.A.; Fullen, D.R.; Nelson, C.C.; Wong, S.L.; Schwartz, J.L.; Bichakjian, C.K. Recurrence and Survival in Patients With Merkel Cell Carcinoma Undergoing Surgery Without Adjuvant Radiation Therapy to the Primary Site. Jama Dermatol. 2016, 152, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Andea, A.A.; Coit, D.G.; Amin, B.; Busam, K.J. Merkel cell carcinoma: Histologic features and prognosis. Cancer 2008, 113, 2549–2558. [Google Scholar] [CrossRef]
- Mott, R.T.; Smoller, B.R.; Morgan, M.B. Merkel cell carcinoma: A clinicopathologic study with prognostic implications. J. Cutan. Pathol. 2004, 31, 217–223. [Google Scholar] [CrossRef]
- Skelton, H.G.; Smith, K.J.; Hitchcock, C.L.; McCarthy, W.F.; Lupton, G.P.; Graham, J.H. Merkel cell carcinoma: Analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J. Am. Acad. Dermatol. 1997, 37, 734–739. [Google Scholar] [CrossRef]
- Bob, A.; Nielen, F.; Krediet, J.; Schmitter, J.; Freundt, D.; Terhorst, D.; Rowert-Huber, J.; Kanitakis, J.; Stockfleth, E.; Ulrich, C.; et al. Tumor vascularization and clinicopathologic parameters as prognostic factors in merkel cell carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 1999–2010. [Google Scholar] [CrossRef]
- Llombart, B.; Monteagudo, C.; Lopez-Guerrero, J.A.; Carda, C.; Jorda, E.; Sanmartin, O.; Almenar, S.; Molina, I.; Martin, J.M.; Llombart-Bosch, A. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology 2005, 46, 622–634. [Google Scholar] [CrossRef]
- Nagase, K.; Kimura-Kaku, H.; Inoue, T.; Shinogi, T.; Narisawa, Y. Usefulness of ulceration and hyperkeratosis as clinical predictors of Merkel cell polyomavirus-negative and combined Merkel cell carcinoma: A retrospective study. J. Dermatol. 2019, 46, 103–109. [Google Scholar] [CrossRef]
- Michaeli, J.; Shaul, M.E.; Mishalian, I.; Hovav, A.H.; Levy, L.; Zolotriov, L.; Granot, Z.; Fridlender, Z.G. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFalpha and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology 2017, 6, e1356965. [Google Scholar] [CrossRef]
- Rana, S.; Byrne, S.N.; MacDonald, L.J.; Chan, C.Y.; Halliday, G.M. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am. J. Pathol. 2008, 172, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Coursaget, P.; Samimi, M.; Nicol, J.T.; Gardair, C.; Touze, A. Human Merkel cell polyomavirus: Virological background and clinical implications. Apmis 2013, 121, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Garneski, K.M.; Warcola, A.H.; Feng, Q.; Kiviat, N.B.; Leonard, J.H.; Nghiem, P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J. Investig. Dermatol. 2009, 129, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Cheng, J.; Wardzala, J.; DoRosario, A.; Scanlon, J.J.; Laga, A.C.; Martinez-Fernandez, A.; Barletta, J.A.; Bellizzi, A.M.; Sadasivam, S.; et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J. Clin. Investig. 2012, 122, 4645–4653. [Google Scholar] [CrossRef]
- Touzé, A.; Gaitan, J.; Maruani, A.; Le Bidre, E.; Doussinaud, A.; Clavel, C.; Durlach, A.; Aubin, F.; Guyétant, S.; Lorette, G.; et al. Merkel Cell Polyomavirus Strains in Patients with Merkel Cell Carcinoma. Emerg. Infect. Dis 2009, 15, 960–962. [Google Scholar]
- Harms, P.W.; Vats, P.; Verhaegen, M.E.; Robinson, D.R.; Wu, Y.M.; Dhanasekaran, S.M.; Palanisamy, N.; Siddiqui, J.; Cao, X.; Su, F.; et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015, 75, 3720–3727. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 2018, 37, 1409–1416. [Google Scholar] [CrossRef]
- Krishna, S.M.; Kattoor, J.; Balaram, P. Down regulation of adhesion protein E-cadherin in Epstein-Barr virus infected nasopharyngeal carcinomas. Cancer Biomark 2005, 1, 271–277. [Google Scholar] [CrossRef]
- Arora, P.; Kim, E.O.; Jung, J.K.; Jang, K.L. Hepatitis C virus core protein downregulates E-cadherin expression via activation of DNA methyltransferase 1 and 3b. Cancer Lett. 2008, 261, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Iyer, J.G.; Tegeder, A.R.; Thibodeau, R.; Schelter, J.; Koba, S.; Schrama, D.; Simonson, W.T.; Lemos, B.D.; Byrd, D.R.; et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J. Clin. Oncol. 2011, 29, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Hudgens, C.W.; Ray-Lyons, G.; Nagarajan, P.; Aung, P.P.; Curry, J.L.; Torres-Cabala, C.A.; Mino, B.; Rodriguez-Canales, J.; Reuben, A.; et al. Density, Distribution, and Composition of Immune Infiltrates Correlate with Survival in Merkel Cell Carcinoma. Clin. Cancer Res. 2016, 22, 5553–5563. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra137. [Google Scholar] [CrossRef]
- Zaragoza, J.; Kervarrec, T.; Touze, A.; Avenel-Audran, M.; Beneton, N.; Esteve, E.; Wierzbicka Hainaut, E.; Aubin, F.; Machet, L.; Samimi, M. A high neutrophil-to-lymphocyte ratio as a potential marker of mortality in patients with Merkel cell carcinoma: A retrospective study. J. Am. Acad. Dermatol. 2016, 75, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Carus, A.; Donskov, F.; Nielsen, P.; Hager, H.; Nedergaard, B.; Steiniche, T.; Ladekarl, M. Strong Prognostic Value of Tumor-infiltrating Neutrophils and Lymphocytes Assessed by Automated Digital Image Analysis in Early Stage Cervical Cancer: A Comparator Study with Observer-assisted Stereological Assessments. J. OncoPathol. 2014, 2. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Schowalter, R.M.; Pastrana, D.V.; Pumphrey, K.A.; Moyer, A.L.; Buck, C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 2010, 7, 509–515. [Google Scholar] [CrossRef]
| Mean Area Marker (%) | Virus-Positive MCC Mean Area Fraction of Marker (95% CI) | Virus-Negative MCC Mean Area Fraction of Marker (95% CI) | p-Value |
|---|---|---|---|
| Lymphocytes (CD8, intratumoral) | 0.23 (0.06–0.89) | 0.06 (0.02–0.19) | p = 0.11 |
| PD-L1 (intratumoral) | 59.28 × 10−3 (9.46 × 10−3–371.29 × 10−3) | 4.36 × 10−3 (0.84 × 10−3–22.68 × 10−3) | p = 0.03 |
| Neutrophils (CD66b, intratumoral) | 0.19 × 10−3 (0.07 × 10−3–0.52 × 10−3) | 0.89 × 10−3 (0.19 × 10−3–4.07 × 10−3) | p = 0.09 |
| Neutrophil-to-lymphocyte ratio, (CD66b/CD8, intratumoral) * | 0.81 × 10−3 (0.16 × 10−3–4.12 × 10−3) | 15.93 × 10−3 (2.20 × 10−3–115.16 × 10−3) | p = 0.02 |
| E-cadherin (intratumoral) | 0.27 × 10−3 (0.04 × 10−3–2.04 × 10−3) | 56.57 × 10−3 (6.44 × 10−3–497.02 × 10−3) | p = 0.0005 |
| Endothelia (CD34, intratumoral) | 3.74 (0.65–21.39) | 4.40 (1.23–15.78) | p = 0.87 |
| Characteristics | Number of Patients (n) | Univariate Analysis HR (95% CI) | p-Value |
|---|---|---|---|
| Presence of virus | 90 | 0.47 (0.22–1.00) | p = 0.05 |
| Presence of ulceration | 78 | 2.49 (1.18–5.25) | p = 0.02 |
| Lymphocytes (CD8, intratumoral) | 90 | 0.70 (0.57– 0.87) | p = 0.001 |
| Neutrophils (CD66b, intratumoral) | 89 | 1.10 (0.91–1.34) | p = 0.32 |
| Neutrophil-to-lymphocyte ratio (CD66b/CD8, intratumoral) | 89 | 1.21 (1.06–1.37) | p = 0.004 |
| Endothelia (CD34, intratumoral) | 89 | 0.97 (0.82–1.15) | p = 0.74 |
| E-cadherin (intratumoral) | 89 | 0.98 (0.88–1.10) | p = 0.73 |
| PD-L1 (intratumoral) | 38 | 0.81 (0.59–1.12) | p = 0.21 |
| Characteristics | Number of Patients (n) | Multivariate Analysis HR (95% CI) | p-Value |
|---|---|---|---|
| Presence of virus | 82 | 0.32 (0.13–0.78) | p = 0.01 |
| Presence of ulceration | 70 | 2.22 (0.99–4.98) | p = 0.05 |
| Lymphocytes (CD8, intratumoral) | 82 | 0.68 (0.54–0.85) | p = 0.001 |
| Neutrophils (CD66b, intratumoral) | 81 | 1.02 (0.82–1.26) | p = 0.87 |
| Neutrophil-to-lymphocyte ratio (CD66b/CD8, intratumoral) | 89 | 1.14 (1.00–1.31) | p = 0.04 |
| Endothelia (CD34, intratumoral) | 81 | 1.03 (0.86–1.23) | p = 0.77 |
| E-cadherin (intratumoral) | 81 | 0.98 (0.86–1.11) | p = 0.73 |
| PD-L1 (intratumoral) | 31 | 0.80 (0.53–1.20) | p = 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri, S.; Steiniche, T.; Bæhr Georgsen, J.; Thomsen, R.; Ladekarl, M.; Heje, M.; Engberg Damsgaard, T.; Bønnelykke-Behrndtz, M.L. Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma. Cancers 2020, 12, 888. https://doi.org/10.3390/cancers12040888
Naseri S, Steiniche T, Bæhr Georgsen J, Thomsen R, Ladekarl M, Heje M, Engberg Damsgaard T, Bønnelykke-Behrndtz ML. Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma. Cancers. 2020; 12(4):888. https://doi.org/10.3390/cancers12040888
Chicago/Turabian StyleNaseri, Simon, Torben Steiniche, Jeanette Bæhr Georgsen, Rune Thomsen, Morten Ladekarl, Martin Heje, Tine Engberg Damsgaard, and Marie Louise Bønnelykke-Behrndtz. 2020. "Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma" Cancers 12, no. 4: 888. https://doi.org/10.3390/cancers12040888
APA StyleNaseri, S., Steiniche, T., Bæhr Georgsen, J., Thomsen, R., Ladekarl, M., Heje, M., Engberg Damsgaard, T., & Bønnelykke-Behrndtz, M. L. (2020). Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma. Cancers, 12(4), 888. https://doi.org/10.3390/cancers12040888







