Abstract
Radiation therapy (RT) is an important component of cancer therapy, with >50% of cancer patients receiving RT. As the number of cancer survivors increases, the short- and long-term side effects of cancer therapy are of growing concern. Side effects of RT for thoracic tumors, notably cardiac and pulmonary toxicities, can cause morbidity and mortality in long-term cancer survivors. An understanding of the biological pathways and mechanisms involved in normal tissue toxicity from RT will improve future cancer treatments by reducing the risk of long-term side effects. Many of these mechanistic studies are performed in animal models of radiation exposure. In this area of research, the use of small animal image-guided RT with treatment planning systems that allow more accurate dose determination has the potential to revolutionize knowledge of clinically relevant tumor and normal tissue radiobiology. However, there are still a number of challenges to overcome to optimize such radiation delivery, including dose verification and calibration, determination of doses received by adjacent normal tissues that can affect outcomes, and motion management and identifying variation in doses due to animal heterogeneity. In addition, recent studies have begun to determine how animal strain and sex affect normal tissue radiation injuries. This review article discusses the known and potential benefits and caveats of newer technologies and methods used for small animal radiation delivery, as well as how the choice of animal models, including variables such as species, strain, and age, can alter the severity of cardiac radiation toxicities and impact their clinical relevance.
1. Introduction
Over 17 million cases of cancer were diagnosed worldwide in 2018, and roughly 9.5 million cancer deaths were reported [1]. Since the early 1900s, ionizing radiation has been used to treat cancers [2], and today radiation remains a major modality in cancer treatment, with over half of all cancer patients receiving radiation therapy (RT). Because of overall growth and aging of the population, it is estimated that by 2040 the global incidence of cancer will rise to over 27 million new cases, and more than 16 million cancer deaths will occur [1]. As a consequence, the global cancer burden will give rise to a growing population of survivors that may develop short- and long-term side effects of cancer therapy.
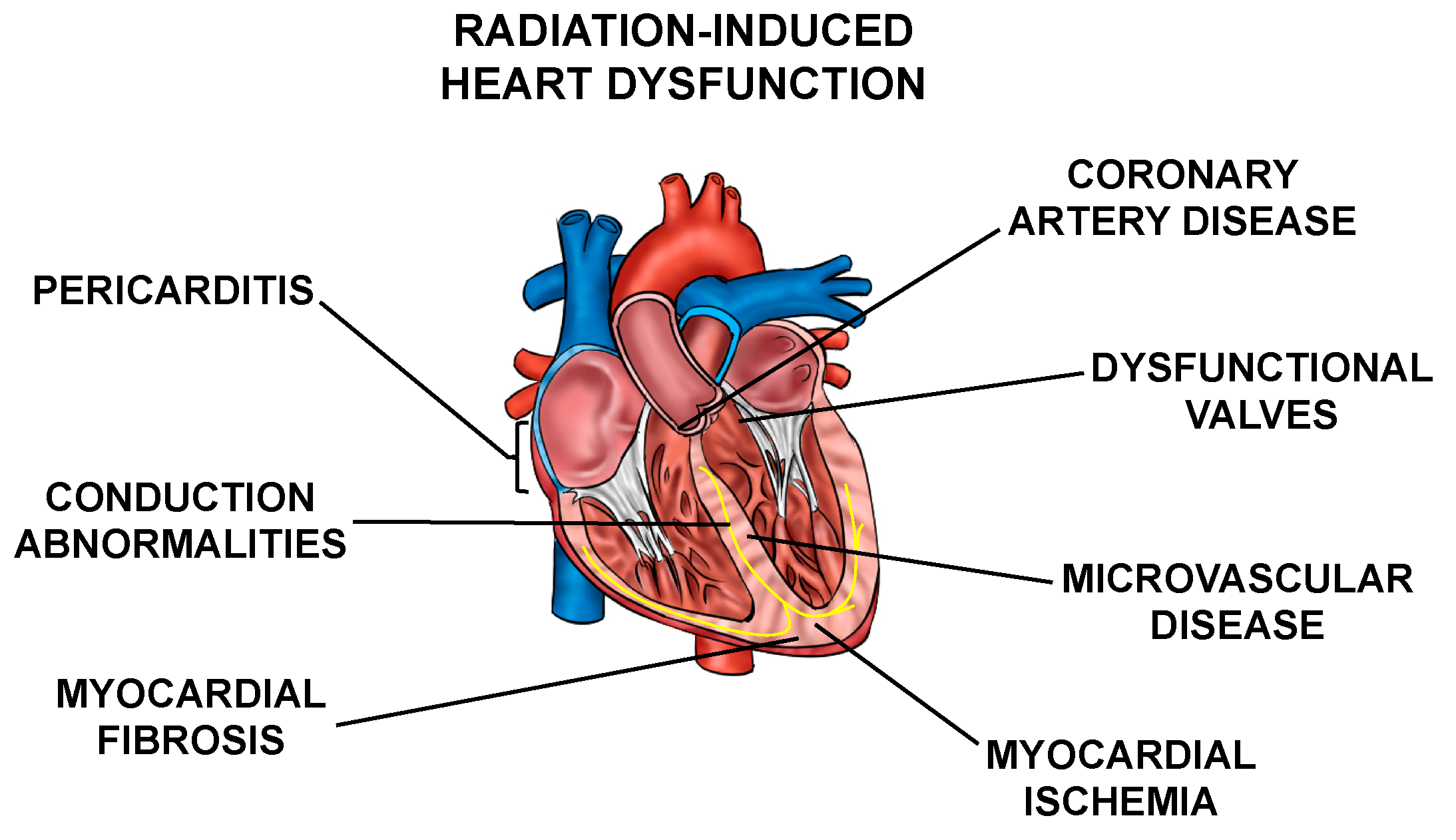
Normal tissue toxicities, mainly in the heart and lungs, can occur after RT in patients with thoracic tumors. The most common toxicities include acute pneumonitis and chronic fibrosis due to radiation exposure of the lung [3,4], and cardiac dysfunction, including pericarditis, ischemic heart disease, conduction abnormalities, myocardial fibrosis, and valvular abnormalities collectively called radiation-induced heart dysfunction (RIHD) (Figure 1) from incidental radiation to the heart and surrounding vasculature [5,6,7,8,9,10]. These side effects may present clinically months to years after RT, affecting patient quality of life and at times even leading to increased mortality [6,9,10,11,12,13,14,15,16]. For example, patients who received tangential RT for left-sided breast cancer in the 1970s and 1980s had an increased risk for cardiovascular mortality at 15 years post-treatment [17]. In patients that received mediastinal radiation for Hodgkin’s disease in the 1960s–1990s, there was a higher prevalence of cardiac abnormalities when compared to the Framingham population [18]. In addition, non-small cell lung cancer (NSCLC) patients may experience RIHD within two years of radiation exposure [14,19,20,21,22]. Numerous other groups have highlighted similar increases in cardiac toxicity-related morbidity and mortality among patients that have received thoracic radiation [23,24,25,26,27].
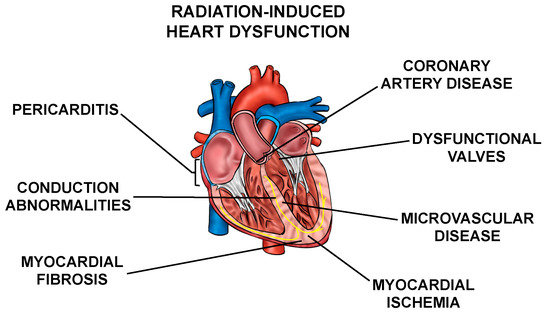
Figure 1.
Cardiac radiation exposure causes a number of abnormalities. Exposure of the heart and surrounding vasculature to radiation may lead to several adverse structural and functional changes in the heart, in this article collectively referred to as radiation-induced heart dysfunction.
A number of advances in radiation oncology have made radiation delivery more precise and allow more effectively delivery of doses to the target volume while reducing the radiation doses to surrounding normal tissues [28,29,30,31,32,33]. However, numerous studies have shown that modern RT technology has not fully eliminated the risk of RIHD [34,35,36]. In breast cancer patients, it has been estimated that there is an approximately 4–16% relative increase in heart disease and/or major coronary events for each 1 Gy in mean heart dose received [6,9,10]. A recent national multicenter NSCLC trial and other single institution reviews have shown a correlation between early death and radiation dose to the heart [12,14,19,20,21,22]. However, efforts towards severely limiting incidental heart dose could potentially compromise RT’s effectiveness in treating tumors in patients with mediastinal lymphomas, thymomas, and breast, lung, or esophageal cancers [7].
Thus, there is a need for understanding the mechanisms by which radiation causes cardiovascular disease, and potentially providing targeted interventions that prevent or reverse RIHD while maintaining optimal radiation doses to the target(s) for maximum tumor control. There are several preclinical studies that aim to elucidate the molecular and cellular mechanisms of RIHD (reviewed in [37,38]). However, translating the biological mechanisms involved in the normal tissue radiation response into therapeutic targets in patients remains a critical challenge given the current limitations of preclinical models in accurately characterizing all facets of human disease. Developing preclinical models of RT with improved representation of human physiology, as well as appropriate modeling of current radiation delivery in the clinic will contribute to overcoming this challenge.
2. Small Animal Radiotherapy Delivery
2.1. Target Volume and Methods of Radiation Delivery in Preclinical Studies of RIHD
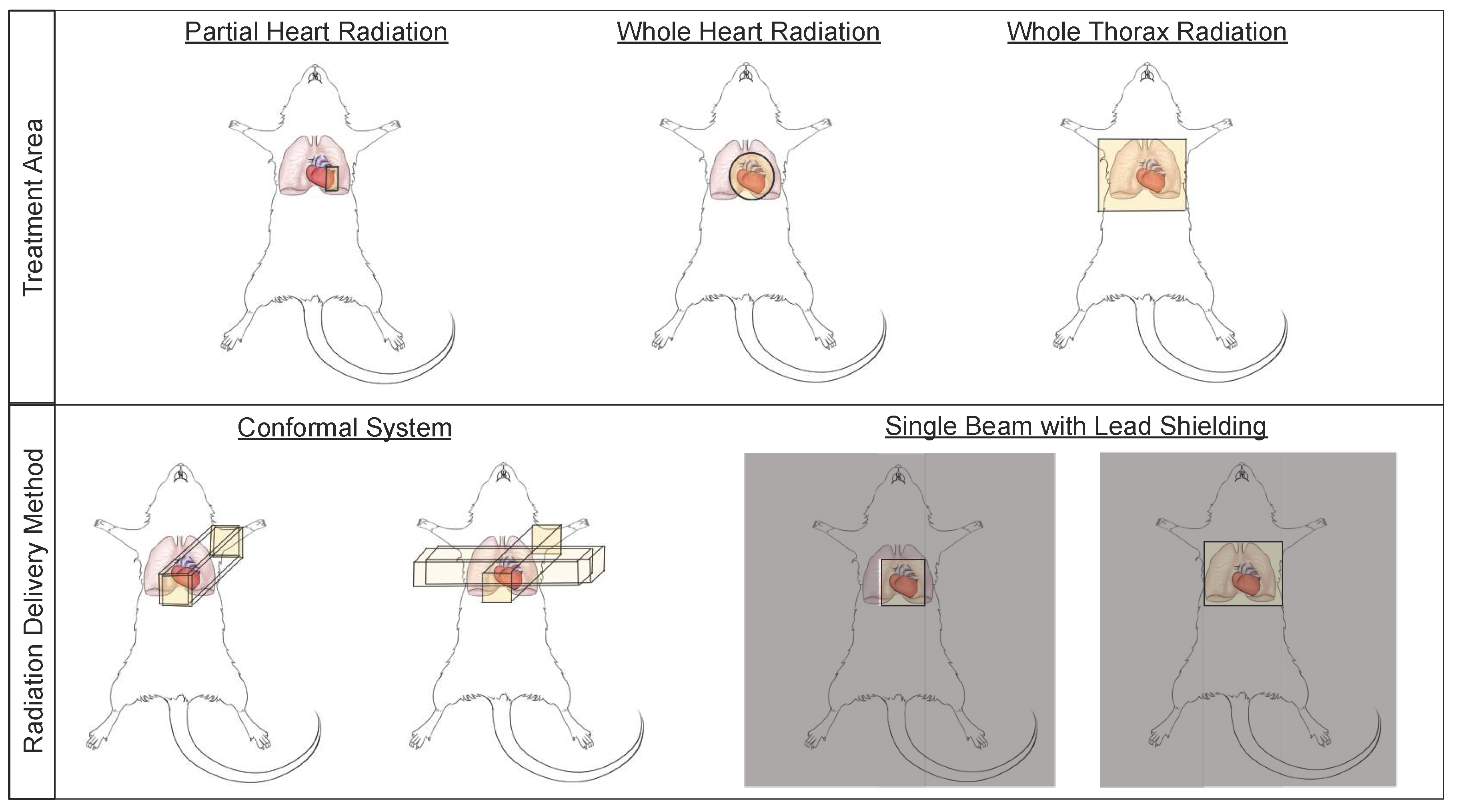
Small animal models have been used to study cardiac radiation toxicity for many decades [39,40,41] based on the physiological similarities of these models to humans [42]. In these models, many current treatment paradigms exist to study RIHD, including whole thorax, whole heart, and partial heart irradiation (Figure 2). Whole thorax irradiation is a method used extensively in the past [43,44,45,46], as it does not require precise image guidance during radiation delivery. Unfortunately, when irradiating the whole thorax, damage occurs not only to the heart, but also the lungs, a dose limiting organ, thereby increasing morbidity and mortality and making it difficult to distinguish whether the resulting damage is from heart or lung irradiation alone, or due to irradiation of both the heart and lungs [45,47] (see also Section 2.3). Modern thoracic RT techniques aim to shield the heart and other organs at risk. However, more advanced techniques and modalities such as intensity-modulated radiation therapy (IMRT) and proton therapy have not been extensively explored in detail in pre-clinical normal tissue toxicity models, although modern small animal irradiators can allow for arc therapy and this technique is beginning to be utilized in pre-clinical models [48].
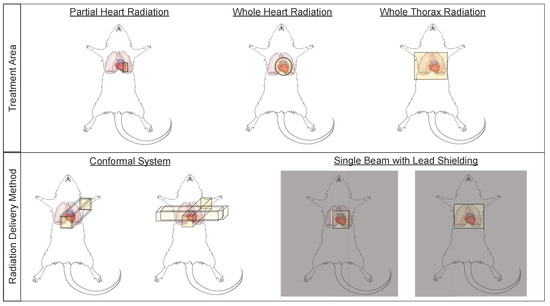
Figure 2.
Small animal irradiation techniques allow a wide variety of cardiac and pulmonary exposures and a number of delivery methods. Schematic illustrations of radiation field options that are commonly used to deliver cardiac radiation (top panel), and methods of radiation delivery (bottom panel) in small animal models of radiation-induced heart dysfunction (RIHD).
Whole heart irradiation has been a relatively new technique to deliver radiation in preclinical models. This technique requires imaging, such as fluoroscopy, x-ray or cone beam computed tomography (CBCT), to accurately deliver radiation to the heart using beam sizes that are mainly focused on the heart, thus limiting lung doses [49,50,51,52]. The use of image-guidance aims to irradiate smaller target volumes with improved accuracy. Currently, two systems to perform CBCT-guided image-guided local irradiation of small experimental animals are commercially available, which can provide accuracy that is similar to clinical RT: the Small Animal Radiotherapy Research Platform (SARRP, Xstrahl) and the X-Rad SmART research platform (Precision X-ray) [53]. In addition, several other non-commercial systems have been developed to deliver localized, image-guided radiotherapy [51,54,55,56,57,58,59]. These image guided systems are conformal systems (i.e., have multiple beam angles), which allow the systems to deliver high doses to small, targeted regions [54,60] and aim to mimic clinical RT devices (Figure 2).
Research groups that do not have access to image-guided radiation delivery platforms rely on lead shielding to target the heart while aiming to reduce the radiation dose to surrounding normal tissues (Figure 2) [61,62]. In addition to decreased accuracy of targeting the heart and non-uniform radiation delivery from inability to account for differences in heart/thorax sizes between animals, this technique may also be less clinically relevant by delivering the radiation in only one or two fields [54]. Bazolava et al. have shown that the conformal systems, when compared to single-field irradiation without image-guidance, result in lower mean dose to tissues nearby the target region [54]. In a simulation of treatment plans for a mouse model bearing a lung tumor, they also showed that the mean dose to heart and the contralateral lung were lower when using a conformal, imagine-guided system to deliver radiation to the tumor, compared to single-field, non-image guided irradiation [54,62]. Other groups have also shown similar benefits of using image-guided, conformal radiation systems to target small areas like the heart [51,53,60,63].
Clinically, patients receiving thoracic RT often receive radiation to only part of the heart, instead of a fairly uniform radiation dose to the whole heart. While preclinical studies using whole heart irradiation have advanced our knowledge of RIHD and contributed to the mechanistic understanding of normal tissue radiation injury, whole heart radiation might not completely represent the clinical pathophysiology spectrum of RIHD [64,65,66,67]. Lee et al. showed that partial heart irradiation of ⅓ of the mouse left ventricle causes left ventricular dilation and increased fibrosis in the myocardium and pericardium, whereas the same phenotype is not observed with whole heart irradiation [66]. Lee et al. showed that partial heart irradiation of ⅓ of the mouse left ventricle causes left ventricular dilation and increased fibrosis in the myocardium and pericardium [67], whereas the same phenotype is not observed with whole heart irradiation [68]. The minimal fibrosis observed with the whole heart RT may be attributed to the rapid progression of myocardial necrosis and heart failure post-RT. The histological features of myocardial fibrosis and pericarditis seen in the partial-heart irradiation model seemed to mimic the changes observed in humans [67,69]. Moreover, numerous studies have shown relationships between the severity or specific pathophysiology of RIHD and the substructures of the heart receiving high doses of radiation [17,25,64,70,71,72]. While in previous clinical studies, mean heart dose was related to the likelihood of RIHD [12], and the risk of major coronary events in breast cancer patients increased linearly by approximately 4–16% for each 1 Gy in mean heart dose received [6,9,10], other studies have shown that dose to the coronary arteries may also be an indicator of risk of developing coronary artery stenosis, an important aspect of RIHD [73]. Thus, there is a need for irradiation of small segments of the heart in pre-clinical models, which may include key sections of coronary arteries, to accurately predict risk factors and successfully develop interventions in RIHD [64,74,75,76,77,78]. Partial heart irradiation in small animals may also be used to elucidate ways in which high-dose radiation treatment of segments of the heart can decrease ventricular tachycardia events in patients [79].
Partial heart irradiation in small animal models presents a myriad of potential technical obstacles that must be considered. For example, even with the advanced and widely available imaging modalities in clinics, researchers have found it difficult to delineate subregions of the heart based upon CT, especially non-contrast CT, which is the most widely used imaging modality for treatment planning [64]. The small size of experimental animal hearts makes precise identification of cardiac substructures in CT scans even more difficult than in the clinic. Moreover, the heart’s motion during respiratory and cardiac cycles as well as the complex anatomy and variability of the coronary vessels, adds to the challenges of standardizing subregion borders [11,64]. Newer imaging techniques like magnetic resonance imaging (MRI) might help overcome some of these challenges [80], but its use in RT treatment planning is limited clinically, and nearly absent in preclinical studies [64]. Furthermore, deep breath holding techniques or respiratory gating have been used clinically to decrease radiation dose to the heart [29,81], and similar techniques could potentially be useful for motion management in animals during precise heart and lung irradiation. However, since small animals will not hold their breath voluntarily and free-breathing gated treatment requires complex oversight during treatment administration, there are numerous technical challenges in using breath hold and other gating technique in preclinical studies. Overall, the cost, availability of advanced imaging modalities and software, and technical challenges in animal positioning, are some major limitations in 3D dose-volume mapping in small animals. After RT delivery, there are many methods available to obtain anatomic and physiologic information regarding cardiac function in preclinical studies. These include non-invasive methods such as echocardiogram, MRI, micro-CT, single-photon emission computerized tomography (SPECT), and positron emission tomography (PET) [67]. While imaging post-RT provides meaningful data in understanding how RT affects heart function, improved techniques to image while administering RT are greatly needed to improve the field of precise cardiac RT delivery.
2.2. Inconsistencies in Radiation Dose Delivery
Small animal radiation delivery to the whole heart or regions of the heart and/or lung, requires careful planning of dose distribution [82,83]. However, no standards have been followed for the performance and reporting of radiation dosimetry in small animal studies, which has made it difficult to reproduce previously published results [84]. In 2011, members of the Centers for Medical Countermeasures against Radiation (CMCR) outlined specific issues related to small animal irradiator calibration and dosimetry, in order to highlight the importance of “accurate and reproducible dosimetry” in preclinical radiobiology studies [85]. Gafchromic film dosimetry can be a valuable tool for characterization of small kV radiation beams [86]. However, even if the incident radiation field on a given animal is accurately assessed and uniform, the true delivered dose to the target volume will vary based on the incident radiation energy, the atomic number of the radiation source, and density of the tissue (e.g., lung, bone, soft-tissue) [85]. This effect can be difficult to measure and could potentially be overlooked depending on the geometry of the radiation field and the study goals. However, given the close interaction between the heart and lungs in the pathophysiology of radiation-induced toxicities (see also Section 2.3), and major differences in their tissue densities, these factors might be worth accounting for in preclinical studies that are aimed at isolating the effects within individual organs. For experiments where the exact absorbed dose is important to assess, researchers have developed microdosimetry techniques that allow calculation of non-homogeneous dose-distribution [87,88,89]. Additionally, in an effort to account for tissue-density based factors, groups have used Monte Carlo and image-based models to calculate absorbed dose [90,91,92].
Respiratory motion also adds complexity to the accurate delivery of thoracic RT. Studies have reported in mice a degree of motion in the order of 5 mm, and likely to be greater in rats [45,93]. The Verhaegen group performed a quantitative analysis of the impact of respiratory motion on a mouse lung tumor irradiation using a four-dimensional digital mouse whole body phantom. They reported respiratory motion resulted in an overestimation of a mean tumor dose of up to 11%, depending upon the placement of the tumor [94]. Similar errors in dose delivery may be expected in studies aiming to irradiate precise areas of normal tissues. Considering the heart in the context of respiration motion, radiation dose to the portions of the heart could either be overestimated or underestimated, as the heart moves in and out of the radiation field (see also Section 2.1). Therefore, respiratory motion is an important factor in considering treatment plans and improving clinical models. Efforts are underway to implement motion management or respiration gating in small animal RT [95,96], and temporary abdominal or chest compression may help to limit breathing motion in some small animals [97].
2.3. Cardiopulmonary Tissue Toxicity from RT
In addition to the heart, the lungs are also at risk of developing short- and long-term injuries after exposure to ionizing radiation [98,99,100]. In the pathogenesis of radiation-induced lung injury, several phases are recognized [100]. An early inflammatory phase is followed by the gradual development of radiation fibrosis and loss of lung function. Interestingly, studies in small animal models to determine mechanisms of radiation-induced lung injury face challenges similar to the ones described here for the heart [45]. The group of Coppes et al. have studied the interaction between lung and heart radiation injury by using proton beams to precisely irradiate the rat heart, alone or in combination with various volumes of the lung. The tolerance dose for loss of lung function was dependent upon the concomitant irradiation of the heart [101,102,103]. Conversely, manifestations of RIHD were more severe when both heart and lungs were exposed [66], and echocardiography after whole thorax or leg-out partial body irradiation revealed changes, including evidence of right-sided heart dysfunction, during periods when radiation pneumonitis was occurring [104,105]. Human studies have also suggested that both heart and lung doses can influence the development of cardiac and/or lung toxicities [14,106,107,108]. Thus, given the growing evidence that suggests that RIHD is linked to radiation-induced lung disease and/or lung doses received, it is important for researchers interested in understanding radiation injury in either organ alone to be aware of and control the dose volume to both the heart and lungs [65].
3. Models to Study Cardiac Radiation Toxicities: Animal Species, Strain, and Genotype
3.1. Animal Species Used in Preclinical Studies of RIHD
Historically, a number of pre-characterized mouse and rat models of cardiovascular disease have become available and used on a wide scale. The low maintenance and housing costs, gestational time and lifespan, and suitability for genetic selection and transgenic strain production, make rodents excellent models for proof-of-concept studies (Table 1). While rodent models are practical and provide us with critical insight into mechanisms by which radiation may injure the cardiovascular system, they do have some disadvantages. They are phylogenetically distant from humans, may have different physiologies and pathophysiologies, and can respond differently to pharmaceutical therapies [109,110,111,112]. However, many features of RIHD can be modeled in rodents, and much has been learned regarding the proteins and pathways involved in radiation-induced cardiotoxicity from these models [5,38]. Some of the limitations in rodents can be overcome by using rabbit models. Rabbits are more physiologically similar to humans from a cardiovascular standpoint (e.g., ion channel and Ca2+ transporter function), and are medium-sized animals, serving as a practical alternative to larger-sized animals [113]. Additionally, there are a myriad of transgenic rabbit models of cardiovascular disease, as well as several commercially available rabbit-specific antibodies, thereby making rabbit studies relatively feasible [113]. New Zealand White rabbits were first used by Fajardo and Stewart in 1968 to study RIHD [40]. The researchers concluded that the cardiac lesions developed in rabbit hearts after single dose x-ray exposure resembled the lesions seen in humans [40]. Rabbits have since only been used by a few researchers to study RIHD [41,114,115,116,117,118], although this species is commonly used to study cardiovascular disease from other causes [119,120,121,122,123,124,125]. There might be unique benefits to studying electrophysiological changes caused by radiation in rabbit models over rat models, as the rabbits’ Ca2+ transport, action potential duration, and main ionic currents underlying repolarization are similar to humans [113,126,127].

Table 1.
Comparison of the main characteristics, advantages, and disadvantages of different animal species used in the study of RIHD. Items are ranked from highly optimal (++++) to less optimal (+).
Despite the similarities in cardiovascular physiology between the rabbit and human, a major disadvantage in using both rabbit and rodent models include difference in the physical dimensions of the hearts, heart rates, and body weights when compared to humans. This might affect studies looking at arrhythmias [113], an effect of cardiac radiation exposure that has received renewed interest due to the use of radiation to treat ventricular tachycardia [79]. Additionally, certain molecular mechanisms of drug responses in large animals such as dogs and non-human primates are more similar to humans when compared to rodents, and thus may be better-suited for drug screening and toxicities [128,129]. Even among the larger animals, different species offer differing benefits. For example, models need to be selected carefully to address radiation-induced coronary alterations, an important component of human RIHD that needs additional study [6,122,123,124,125,126,127]. The coronary circulation of pigs is similar to young human hearts (e.g., no anastomoses between branches of the vasculature), while the coronary circulation in dogs is more similar to older human hearts with ischemic heart disease (highly collateralized circulation) [129,130,131]. These differences between species are important to keep in mind [6,132,133,134,135,136,137]. Few groups have used canine models for understanding the physiology of RIHD and for non-invasive imaging following local heart irradiation [138,139,140,141,142]. However, due to cultural values, unfavorable public opinion, more complex approval processes, and tight regulations in some countries, the use of canine models have decreased over time [143,144,145]. Other large animals such as non-human primates and pigs have been used by a few groups to study RIHD [146,147,148], but these are uncommon given the high cost and low throughput nature of large animal models.
3.2. Influence of Strain on Manifestations of RIHD
In an effort to understand heritable genetic traits that could modify cardiac radiation sensitivity, our group utilized Salt-Sensitive (SS) and Brown Norway (BN) strain consomic rats. The inbred SS rat strain is more sensitive to RIHD than the inbred BN rat strain [49], as well as consomic SS.BN3 rats (genetically identical to SS rats except for chromosome 3, which is substituted from the BN rats) [49]. These results demonstrated that the BN rat chromosome 3 contains one or more genetic variations that play a protective role in the development of RIHD. These studies not only highlight the importance of heritable factors in determining the sensitivity radiation-induced cardiotoxicity, but also highlight the importance of selecting the proper rat strain for a particular research question [49]. Since studies in this field of research have made use of different mouse and rat strains to study the biological effects of cardiac irradiation [39,149,150], care must be taken when comparing outcomes from individual studies using difference strains.
3.3. Use of Genetically Modified Animals to Study Biological Mechanisms of RIHD
Genetically engineered mouse models allow researchers to mechanistically examine the effects of genetic changes on radiation damage in malignant and healthy tissue [151]. For example, researchers have used ApoE-deficient mice that are prone to atherosclerosis to study changes in inflammation and thrombosis following radiation delivery to the heart, aortic arch, and carotid arteries [152,153,154,155]. Lee et al. used mice with an endothelial cell-specific knock-out of p53 and p21 to understand the role of endothelial cells and the p53 pathway in radiation-induced heart damage [68]. The researchers used Cre-loxP technology to not only develop the p53-deficient mouse model, but also other mouse models that are more prone to radiation-induced normal tissue injury (i.e., faster onset, more prominent phenotype, etc.) [43,151] and models that are useful for studying cardiac damage following partial heart irradiation [67]. These genetically engineered mouse models are crucial for elucidating the role of specific signaling pathways and changes in the tissue microenvironment on RIHD, and the use of such models is expected to increase dramatically over time [151].
4. Preclinical Models to Study Cardiac Radiation Toxicities: Animal Age, Size, Dose/Fractionation, and Sex
As discussed above, attention must be paid to the choice of animal species, strain, and genotype when studying radiation-induced cardiotoxicity. However, studies show that additional variations in parameters such as animal age, size, and sex may influence outcome. These variables need to be accurately reported and considered in statistical analyses of results, as they may cause inconsistencies in findings between experimental groups within a research project and between separate studies. Radiation exposure at a young age has long been known to lead to more severe long-term normal tissue radiation injury compared to radiation exposure in adults [156]. Mulrooney et al. reported health outcomes in a cohort of just over 14,000 survivors of childhood and adolescent cancer and found that this population was at substantial risk for cardiovascular disease, with a two- to six-fold increased relative risk for coronary events in patients who had received >15 Gy to the heart compared to non-irradiated survivors [157]. Moreover, the relative risk of cardiovascular events was reported up to 60-fold higher in childhood cancer survivors that received a higher cardiac mean dose of >30 Gy compared to patients who received either no RT or a dose to the heart of 0.1 Gy or less [158]. While some studies report exposure at an older age may also be a risk factor for more severe normal tissue toxicity [159,160], a rodent study reported increased mortality rates and incidence of RT pneumonitis compared to geriatric rats after 13 Gy partial body irradiation [161]. Therefore, in preclinical research it is important to treat animals at an age that is most appropriate for the clinical scenario that is under investigation, which may also be dependent upon the sex and strain of the animal. However, many preclinical studies use young or young adult animals, due in large part to reduce the costs of the research.
A small number of clinical studies has addressed potential differences in the sensitivity to develop normal tissue radiation injury between male and female patients, including in the cardiopulmonary system [162,163,164,165]. Interestingly, as also described for preclinical animal models, an increased pulmonary radiation toxicity in women compared to men may be at least in part related to a smaller total lung volume in women and therefore a relatively larger percent volume of the female lung exposed to radiation [166,167]. The vast majority of preclinical studies of RIHD have used either male or female animals, and only few studies have included both sexes (examples include [49,168]). Because of the observations of an influence of sex on normal tissue radiation injury in human subjects, and with the increased call for studying both sexes in preclinical animal models, future studies should make direct comparisons between male and female animals in radiation-induced cardiopulmonary injury.
A number of different dose and fractionation regimens have been utilized in preclinical studies of RIHD. These range from single, large fractions of cardiac, to more clinically relevant fractionated regimens. However, due to the time and cost required for image-guided radiation therapy and potential anesthesia requirements of daily fractionated treatments, it may be difficult to model multi-week treatments using small fractions similar to commonly used clinical regimens (i.e., 1.8–2.7 Gy daily). Thus, a number of studies utilize larger daily fractions for more limited time periods, such as cardiac exposures to 9 Gy for five daily treatments [49,169].
5. Studying Cardiac Toxicity from Combined Cancer Therapies
Many cancer patients who receive RT as part of their treatment plan often also undergo surgery, chemotherapy, hormonal therapy, and/or immunotherapy. Numerous chemotherapy agents commonly used to treat thoracic cancers (e.g., doxorubicin) have been shown to cause cardiac toxicities on their own [170]. Whole heart x-ray exposure and Adriamycin (doxorubicin) have synergistic effects on heart toxicity in a New Zealand White rabbit model [171]. Therefore, additional cardioprotective measures might need to be taken prior to treatment or after therapy with radiation and anthracyclines. The cardiac effects of the combination of radiation with other anti-cancer agents are less commonly studied. For instance, while whole heart x-rays in combination with a tyrosine kinase inhibitor in rats may have more severe effects on cardiac mitochondrial structure than each of the treatments alone, long-term effects on cardiac function with these therapies are not yet known [169]. As more and more patients receive multiple and more diverse personalized therapies, further studies need to be conducted to study how multiple therapies interact to cause short- and long-term cardiovascular effects. In studying such combined therapies in animal models, one has to be careful in determining clinically relevant treatment doses and optimizing the order of the treatments and the time between the therapies.
5.1. Influence of Anesthesia on the Study of Combined Cancer Therapies
Experimental animals are often anesthetized prior to targeted radiation of the heart. However, the myocardial depressant effects of many anesthetics could potentially increase the severity of cardiotoxicity from chemotherapy, and possibly even radiation [172]. Additionally, anesthesia has been shown to reduce proliferation rates of natural killer cells and T-cells in rats and humans, which could potentially alter the effects of combining radiation and immunotherapy [173,174]. Thus, appropriate controls are necessary for understanding the effects of experimental conditions such as anesthesia.
5.2. Radiation Therapy and the Immune Response
The immune system has crucial housekeeping functions in the heart [175]. In some circumstances, such as after an infection or myocardial infarction, the immune system mediates healing and removal of dead tissue [175]. However, immune responses can also cause adverse tissue remodeling and irreversible damage [175,176]. For example, inflammation leads to induction of programmed death ligand 1 (PD-L1) in cardiac endothelial cells, which seems to protect the myocardium from cytotoxic T-lymphocyte-mediated cardiac injury [177]. Programmed death protein 1 (PD-1)-deficient mice develop autoimmune-mediated cardiomyopathy, but the development of cardiac disease is dependent upon the genetic background of the mouse model [177,178]. Thus, careful selection of animal strain and genetic background is crucial for accurate modeling of immune responses. These known roles of the PD-L1 pathway in adverse cardiac remodeling are also of significance in immune checkpoint inhibitor therapy. Ionizing radiation activates the anticancer immune response by exposing tumor-specific antigens and increasing tumor immunogenicity [179]. Moreover, it leads to the release of damage-associated molecular signals (including ATP, reactive oxygen species, heat shock proteins, and short DNA/RNA) following radiation-induced cell damage. These triggers mediate inflammation and innate immune responses [177,180]. Therefore, the combination of radiation with immune checkpoint inhibitors has increased anti-cancer response rates in preclinical and clinical studies. However, this combined treatment could also potentially have an adverse impact on normal tissues [181,182,183,184,185].
Du et al. studied RIHD in conjunction with inhibition of PD-1. C57BL/6 mice were given image-guided whole heart irradiation concurrently with PD-1 blockade. Increased mortality and cardiac dysfunction (illustrated by reduced ejection fraction) were observed in mice receiving cardiac RT with PD-1 blockade versus mice that only received RT [185]. Myers and Lu treated C57BL/6 mice with whole thorax irradiation in combination with an anti-PD-1 antibody and also observed reduced survival and increased numbers of T-cells in lung and heart compared to radiation alone [186]. These studies have shown that radiation-induced cardiac toxicity can be altered by PD-1 through cytotoxic T lymphocytes and suggest that PD-1 blockade should be administered with purposeful cardiac RT planning to ensure both positive treatment outcome and patient safety.
Although inbred mouse strains such as C57BL/6 mice have been widely used in anti-tumor and immunology studies, they have highly variable immune responses, and do not accurately mimic the human immune system [187]. Sanmamed et al. reviewed the benefits and shortcomings of various murine models for studying immune checkpoint inhibitors. The murine models that currently are closest to mimicking the human immune system are humanized mouse models, which have the most promise in testing the antitumor effects in immunotherapy strategies [188]. Humanized mouse models are developed using genetic, tissue, or environmental engineering methods, and have many immunologic factors that resemble humans [189]. However, the utility of a given model for studying RIHD is dependent on the method and type of immune engraftment. For example, CB-17-scid, NOD-scid, and NOG mice are highly suitable for oncological studies but have a low tolerance to radiation [190]. In studying normal tissue radiation toxicities in these mouse models, radiation doses may have to be reduced to obtain the same normal tissue pathologies as in common wild-type mice. Additionally, human microbiota have been shown to play a crucial role in the efficacy of cancer therapies and development of the host immune system [191,192,193,194]. While designing preclinical studies for combination therapies, particularly ones that require human immune responses, consideration of the immune population within humanized mice, differences in responses between inbred mice and humans, microbiota differences in animal models, and normal tissue radiation sensitivity should be considered.
5.3. Influence of Environmental Factors on Radiation Therapy
In addition to chemotherapy influencing RT responses and possible potentiation of side effects, a number lifestyle factors and environmental exposures may also alter RT side effects, yet preclinical studies to investigate these effects are thus far limited. For example, the effects of cigarette smoking during chemotherapy and/or RT revealed increased symptom burden compared to nonsmokers, including weight loss, skin problems, and nausea, in patients being treated for a number of different types of cancer [195]. In addition, studies have suggested that head and neck cancer patients who smoke during RT have lower rates of response and survival compared to patients that do not smoke [196]. The effects of smoking on cardiac RT sensitivity has not been well-studied, with little to no pre-clinical data reported.
Another environmental factor that may impact RT sensitivity is exercise, and exercise-oncology is an emerging area of interest as cancer therapy becomes more personalized. Again, clinical and preclinical studies to study this interaction are severely limited. Clinically, cardiorespiratory fitness was assessed following thoracic RT in breast or lung cancer patients that received significant heart exposure (>10% heart volume receiving 5 Gy). Patients displayed a dose-dependent relation between cardiac dose received and impairment in peak oxygen consumption, a marker of impaired cardiovascular reserve [197]. Exercise has also been reported to change redox signaling in cancer, as well as drive changes in immune system response, metabolism, and inflammation [198]. Because of the high level of redox signaling in the mitochondria and heart, a direct study to determine whether exercise influences cardiac sensitivity to RT would be valuable in the field regarding during cancer treatment as well as patient quality of life post-RT. Finally, diet has been implicated in affecting many diseases including heart disease and cancer. A preclinical study revealed fasting reduced intestinal radiotoxicity using C57BL/6J mice, assessed by fasting improved intestinal stem cell regeneration and improved survival to lethal doses of abdominal RT [199]. Musa and Shabeeb reported natural products may have a potential for protection against RIHD, reviewing products such as hesperidin, curcumin, melatonin, zingerone, and Shen-Mai San (SMS), where SMS is examined in a clinical trial for cancer patients receiving chemotherapy or RT (NCT01580358) [200]. Overall, there are currently limited published preclinical as well as clinical studies that investigate whether lifestyle factors affect incidence of RIHD.
6. Conclusions
Advances in the delivery of thoracic radiation therapy in preclinical models have translated into improved outcomes for cancer patients receiving RT. In return, advances in the clinic including image-guided irradiation have been implemented in research laboratories to further drive discoveries in the fields of cancer radiation therapy and normal tissue radiation toxicity. With current advancements in imaging, radiation delivery, and radiation dosimetry in preclinical research, we now have an opportunity to build our understanding of the normal tissue radiation response by improving the setup and dose distribution to better mimic clinical therapy. However, in each pre-clinical study this needs to be done with careful radiation dosimetry and reporting of the experimental setup to promote reproducibility. Lastly, understanding the differences and limitations of thoracic RT in preclinical models across research labs will aid in the proper interpretation of results and elucidation of pathways and mechanisms of radiation-induced cardiopulmonary damage.
Author Contributions
R.A.S., G.S., M.B., and C.B. conceptualized, wrote, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Institute of General Medical Sciences, grant number P20 GM109005 (MB), and Department of Defense, grant number W81XWH1910737 (MB). Additional support was provided by NIH 1R01HL147884 (CB), the Mary Kay Foundation Award Grant No. 017-29 (CB), Susan G. Komen® CCR17483233 (CB), the Nancy Laning Sobczak, PhD, Breast Cancer Research Award (CB), the Medical College of Wisconsin Cancer Center (CB), the Michael H. Keelan, Jr., MD, Research Foundation Grant (CB), and the Cardiovascular Center at the Medical College of Wisconsin (CB).
Conflicts of Interest
The authors declare no conflict of interest. Bergom receives research funding from Innovation Pathways, Palo Alto, CA.
References
- Street, W. Cancer Facts & Figures 2019. Am. Cancer Soc. 2018, 76. [Google Scholar]
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Berman, A.T. Radiation Pneumonitis: Old Problem, New Tricks. Cancers 2018, 10, e222. [Google Scholar] [CrossRef] [PubMed]
- Libshitz, H.I. Radiation Changes in the Lung. Semin. Roentgenol. 1993, 28, 303–320. [Google Scholar] [CrossRef]
- Boerma, M. Experimental Radiation-Induced Heart Disease: Past, Present, and Future. Radiat. Res. 2012, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Venkatesulu, B.P.; Mahadevan, L.S.; Krishnan, S. Radiation-Induced Cardiovascular Disease: A Clinical Perspective. Front. Cardiovasc. Med. 2017, 4, e66. [Google Scholar] [CrossRef]
- Donnellan, E.; Phelan, D.; McCarthy, C.P.; Collier, P.; Desai, M.; Griffin, B. Radiation-Induced Heart Disease: A Practical Guide to Diagnosis and Management. Cleve. Clin. J. Med. 2016, 83, 914–922. [Google Scholar] [CrossRef]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Van den Bogaard, V.A.B.; Ta, B.D.P.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.H.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.J.; Marteijn, L.A.W.; de Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef]
- Gagliardi, G.; Lax, I.; Rutqvist, L.E. Partial Irradiation of the Heart. Semin. Radiat. Oncol. 2001, 11, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Dess, R.T.; Sun, Y.; Matuszak, M.M.; Sun, G.; Soni, P.D.; Bazzi, L.; Murthy, V.L.; Hearn, J.W.D.; Kong, F.-M.; Kalemkerian, G.P.; et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [PubMed]
- Speirs, C.K.; DeWees, T.A.; Rehman, S.; Molotievschi, A.; Velez, M.A.; Mullen, D.; Fergus, S.; Trovo, M.; Bradley, J.D.; Robinson, C.G. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Bouillon, K.; Haddy, N.; Delaloge, S.; Garbay, J.-R.; Garsi, J.-P.; Brindel, P.; Mousannif, A.; Lê, M.G.; Labbe, M.; Arriagada, R.; et al. Long-Term Cardiovascular Mortality after Radiotherapy for Breast Cancer. J. Am. Coll. Cardiol. 2011, 57, 445–452. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef]
- Roychoudhuri, R.; Robinson, D.; Putcha, V.; Cuzick, J.; Darby, S.; Møller, H. Increased Cardiovascular Mortality More than Fifteen Years after Radiotherapy for Breast Cancer: A Population-Based Study. BMC Cancer 2007, 7, e9. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic Cardiac Disease Following Mediastinal Irradiation. J. Am. Coll. Cardiol. 2003, 42, 743–749. [Google Scholar] [CrossRef]
- Haque, W.; Verma, V.; Fakhreddine, M.; Butler, E.B.; Teh, B.S.; Simone, C.B. Trends in Cardiac Mortality in Patients with Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 470–477. [Google Scholar] [CrossRef]
- Wang, K.; Pearlstein, K.A.; Patchett, N.D.; Deal, A.M.; Mavroidis, P.; Jensen, B.C.; Lipner, M.B.; Zagar, T.M.; Wang, Y.; Lee, C.B.; et al. Heart Dosimetric Analysis of Three Types of Cardiac Toxicity in Patients Treated on Dose-Escalation Trials for Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. 2017, 125, 293–300. [Google Scholar] [CrossRef]
- Yegya-Raman, N.; Wang, K.; Kim, S.; Reyhan, M.; Deek, M.P.; Sayan, M.; Li, D.; Patel, M.; Malhotra, J.; Aisner, J.; et al. Dosimetric Predictors of Symptomatic Cardiac Events After Conventional-Dose Chemoradiation Therapy for Inoperable NSCLC. J. Thorac. Oncol. 2018, 13, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-Dose versus High-Dose Conformal Radiotherapy with Concurrent and Consolidation Carboplatin plus Paclitaxel with or without Cetuximab for Patients with Stage IIIA or IIIB Non-Small-Cell Lung Cancer (RTOG 0617): A Randomised, Two-by-Two Factorial Phase 3 Study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [PubMed]
- Heidenreich, P.A.; Hancock, S.L.; Vagelos, R.H.; Lee, B.K.; Schnittger, I. Diastolic Dysfunction after Mediastinal Irradiation. Am. Heart J. 2005, 150, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Schnittger, I.; Strauss, H.W.; Vagelos, R.H.; Lee, B.K.; Mariscal, C.S.; Tate, D.J.; Horning, S.J.; Hoppe, R.T.; Hancock, S.L. Screening for Coronary Artery Disease after Mediastinal Irradiation for Hodgkin’s Disease. J. Clin. Oncol. 2007, 25, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lipsitz, S.R.; Colan, S.D.; Tarbell, N.J.; Treves, S.T.; Diller, L.; Greenbaum, N.; Mauch, P.; Lipshultz, S.E. Cardiovascular Status in Long-Term Survivors of Hodgkin’s Disease Treated with Chest Radiotherapy. J. Clin. Oncol. 2004, 22, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Tsagalou, E.P.; Kanakakis, J.; Anastasiou-Nana, M.I. Complete Heart Block after Mediastinal Irradiation in a Patient with the Wolff-Parkinson-White Syndrome. Int. J. Cardiol. 2005, 104, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Aeppli, D.; Nierengarten, M.E. The Need for Long-Term Surveillance for Patients Treated with Curative Radiotherapy for Hodgkin’s Disease: University of Minnesota Experience. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 169–179. [Google Scholar] [CrossRef]
- Bortfeld, T.; Jeraj, R. The Physical Basis and Future of Radiation Therapy. Br. J. Radiol. 2011, 84, 485–498. [Google Scholar] [CrossRef]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front. Oncol. 2018, 8, e87. [Google Scholar] [CrossRef]
- Bergom, C.; Kelly, T.; Morrow, N.; Wilson, J.F.; Walker, A.; Xiang, Q.; Ahn, K.W.; White, J. Prone Whole-Breast Irradiation Using Three-Dimensional Conformal Radiotherapy in Women Undergoing Breast Conservation for Early Disease Yields High Rates of Excellent to Good Cosmetic Outcomes in Patients with Large and/or Pendulous Breasts. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 821–828. [Google Scholar] [CrossRef]
- Bradley, J.A.; Mendenhall, N.P. Novel Radiotherapy Techniques for Breast Cancer. Annu. Rev. Med. 2018, 69, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Modiri, A.; Sabouri, P.; Gu, X.; Timmerman, R.; Sawant, A. Inversed-Planned Respiratory Phase Gating in Lung Conformal Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, S.; Perrin, R.; Peroni, M.; Bernatowicz, K.; Parkel, T.; Pytko, I.; Klöck, S.; Guckenberger, M.; Tanadini-Lang, S.; Weber, D.C.; et al. Respiratory Motion-Management in Stereotactic Body Radiation Therapy for Lung Cancer—A Dosimetric Comparison in an Anthropomorphic Lung Phantom (LuCa). Radiother. Oncol. 2016, 121, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Saiki, H.; Petersen, I.A.; Scott, C.G.; Bailey, K.R.; Dunlay, S.M.; Finley, R.R.; Ruddy, K.J.; Yan, E.; Redfield, M.M. Risk of Heart Failure with Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017, 135, 1388–1396. [Google Scholar] [CrossRef]
- Boero, I.J.; Paravati, A.J.; Triplett, D.P.; Hwang, L.; Matsuno, R.K.; Gillespie, E.F.; Yashar, C.M.; Moiseenko, V.; Einck, J.P.; Mell, L.K.; et al. Modern Radiation Therapy and Cardiac Outcomes in Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 700–708. [Google Scholar] [CrossRef]
- Boekel, N.B.; Schaapveld, M.; Gietema, J.A.; Russell, N.S.; Poortmans, P.; Theuws, J.C.M.; Schinagl, D.A.X.; Rietveld, D.H.F.; Versteegh, M.I.M.; Visser, O.; et al. Cardiovascular Disease Risk in a Large, Population-Based Cohort of Breast Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1061–1072. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-Induced Heart Disease: A Review of Classification, Mechanism and Prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef]
- Boerma, M.; Hauer-Jensen, M. Preclinical Research into Basic Mechanisms of Radiation-Induced Heart Disease. Cardiol. Res. Pract. 2010, 2011. [Google Scholar] [CrossRef]
- Lauk, S.; Kiszel, Z.; Buschmann, J.; Trott, K.-R. Radiation-Induced Heart Disease in Rats. Int. J. Radiat. Oncol. 1985, 11, 801–808. [Google Scholar] [CrossRef]
- Stewart, J.R.; Fajardo, L.F.; Cohn, K.E.; Page, V. Experimental Radiation-Induced Heart Disease in Rabbits. Radiology 1968, 91, 814–817. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Stewart, J.R. Experimental Radiation-Induced Heart Disease. I. Light Microscopic Studies. Am. J. Pathol. 1970, 59, 299–316. [Google Scholar] [PubMed]
- Perlman, R.L. Mouse Models of Human Disease. Evol. Med. Public Health 2016, 2016, 170–176. [Google Scholar] [PubMed]
- Mezzaroma, E.; Di, X.; Graves, P.; Toldo, S.; Van Tassell, B.W.; Kannan, H.; Baumgarten, C.; Voelkel, N.; Gewirtz, D.A.; Abbate, A. A Mouse Model of Radiation-Induced Cardiomyopathy. Int. J. Cardiol. 2012, 156, 231–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dabjan, M.B.; Buck, C.M.; Jackson, I.L.; Vujaskovic, Z.; Marples, B.; Down, J.D. A Survey of Changing Trends in Modelling Radiation Lung Injury in Mice: Bringing out the Good, the Bad, and the Uncertain. Lab. Invest. 2016, 96, 936–949. [Google Scholar] [CrossRef]
- Ghita, M.; Dunne, V.; Hanna, G.G.; Prise, K.M.; Williams, J.P.; Butterworth, K.T. Preclinical Models of Radiation-Induced Lung Damage: Challenges and Opportunities for Small Animal Radiotherapy. Br. J. Radiol. 2019, 92, e20180473. [Google Scholar] [CrossRef]
- Terry, N.H.; Tucker, S.L.; Travis, E.L. Residual Radiation Damage in Murine Lung Assessed by Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 929–938. [Google Scholar] [CrossRef]
- Carver, J.R.; Shapiro, C.L.; Ng, A.; Jacobs, L.; Schwartz, C.; Virgo, K.S.; Hagerty, K.L.; Somerfield, M.R.; Vaughn, D.J.; ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology Clinical Evidence Review on the Ongoing Care of Adult Cancer Survivors: Cardiac and Pulmonary Late Effects. J. Clin. Oncol. 2007, 25, 3991–4008. [Google Scholar] [CrossRef]
- Gomarteli, K.; Fleckenstein, J.; Kirschner, S.; Bobu, V.; Brockmann, M.A.; Henzler, T.; Meyer, M.; Riffel, P.; Schönberg, S.O.; Veldwijk, M.R.; et al. Radiation-Induced Malignancies after Intensity-Modulated versus Conventional Mediastinal Radiotherapy in a Small Animal Model. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Schlaak, R.A.; Frei, A.; Schottstaedt, A.M.; Tsaih, S.-W.; Fish, B.L.; Harmann, L.; Liu, Q.; Gasperetti, T.; Medhora, M.; North, P.E.; et al. Mapping Genetic Modifiers of Radiation-Induced Cardiotoxicity to Rat Chromosome 3. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1267–H1280. [Google Scholar] [CrossRef]
- Verhaegen, F.; Granton, P.; Tryggestad, E. Small Animal Radiotherapy Research Platforms. Phys. Med. Biol. 2011, 56, R55–R83. [Google Scholar] [CrossRef]
- Sharma, S.; Moros, E.G.; Boerma, M.; Sridharan, V.; Han, E.Y.; Clarkson, R.; Hauer-Jensen, M.; Corry, P.M. A Novel Technique for Image-Guided Local Heart Irradiation in the Rat. Technol. Cancer Res. Treat. 2014, 13, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Udayakumar, T.S.; Johnson, P.B.; Dogan, N.; Pollack, A.; Yang, Y. An Image Guided Small Animal Stereotactic Radiotherapy System. Oncotarget 2016, 7, 18825–18836. [Google Scholar] [CrossRef] [PubMed]
- Ghita, M.; Brown, K.H.; Kelada, O.J.; Graves, E.E.; Butterworth, K.T. Integrating Small Animal Irradiators with Functional Imaging for Advanced Preclinical Radiotherapy Research. Cancers 2019, 11, e170. [Google Scholar] [CrossRef] [PubMed]
- Bazalova, M.; Nelson, G.; Noll, J.M.; Graves, E.E. Modality Comparison for Small Animal Radiotherapy: A Simulation Study. Med. Phys. 2014, 41, e011710. [Google Scholar] [CrossRef]
- Jensen, M.D.; Hrinivich, W.T.; Jung, J.A.; Holdsworth, D.W.; Drangova, M.; Chen, J.; Wong, E. Implementation and Commissioning of an Integrated Micro-CT/RT System with Computerized Independent Jaw Collimation. Med. Phys. 2013, 40, e081706. [Google Scholar] [CrossRef]
- Felix, M.C.; Fleckenstein, J.; Kirschner, S.; Hartmann, L.; Wenz, F.; Brockmann, M.A.; Glatting, G.; Giordano, F.A. Image-Guided Radiotherapy Using a Modified Industrial Micro-CT for Preclinical Applications. PLoS ONE 2015, 10, e0126246. [Google Scholar] [CrossRef]
- Zhou, H.; Rodriguez, M.; van den Haak, F.; Nelson, G.; Jogani, R.; Xu, J.; Zhu, X.; Xian, Y.; Tran, P.T.; Felsher, D.W.; et al. Development of a Micro-Computed Tomography-Based Image-Guided Conformal Radiotherapy System for Small Animals. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 297–305. [Google Scholar] [CrossRef]
- Sharma, S.; Narayanasamy, G.; Przybyla, B.; Webber, J.; Boerma, M.; Clarkson, R.; Moros, E.G.; Corry, P.M.; Griffin, R.J. Advanced Small Animal Conformal Radiation Therapy Device. Technol. Cancer Res. Treat. 2017, 16, 45–56. [Google Scholar] [CrossRef]
- Tillner, F.; Thute, P.; Löck, S.; Dietrich, A.; Fursov, A.; Haase, R.; Lukas, M.; Rimarzig, B.; Sobiella, M.; Krause, M.; et al. Precise Image-Guided Irradiation of Small Animals: A Flexible Non-Profit Platform. Phys. Med. Biol. 2016, 61, 3084–3108. [Google Scholar] [CrossRef]
- Grover, A.R.; Kimler, B.F.; Duncan, F.E. Use of a Small Animal Radiation Research Platform (SARRP) Facilitates Analysis of Systemic versus Targeted Radiation Effects in the Mouse Ovary. J. Ovarian Res. 2018, 11, e72. [Google Scholar] [CrossRef]
- Sag, C.M.; Wolff, H.A.; Neumann, K.; Opiela, M.-K.; Zhang, J.; Steuer, F.; Sowa, T.; Gupta, S.; Schirmer, M.; Hünlich, M.; et al. Ionizing Radiation Regulates Cardiac Ca Handling via Increased ROS and Activated CaMKII. Basic Res. Cardiol. 2013, 108, e385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boerma, M.; Wang, J.; Wondergem, J.; Joseph, J.; Qiu, X.; Kennedy, R.H.; Hauer-Jensen, M. Influence of Mast Cells on Structural and Functional Manifestations of Radiation-Induced Heart Disease. Cancer Res. 2005, 65, 3100–3107. [Google Scholar] [CrossRef] [PubMed]
- Brodin, N.P.; Velcich, A.; Guha, C.; Tomé, W.A. A Model for Precise and Uniform Pelvic- and Limb-Sparing Abdominal Irradiation to Study the Radiation-Induced Gastrointestinal Syndrome in Mice Using Small Animal Irradiation Systems. Dose-Response Publ. Int. Hormesis Soc. 2017, 15, e1559325816685798. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G.; Constine, L.S.; Moiseenko, V.; Correa, C.; Pierce, L.J.; Allen, A.M.; Marks, L.B. Radiation Dose-Volume Effects in the Heart. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 77–85. [Google Scholar] [CrossRef]
- Sievert, W.; Stangl, S.; Steiger, K.; Multhoff, G. Improved Overall Survival of Mice by Reducing Lung Side Effects After High-Precision Heart Irradiation Using a Small Animal Radiation Research Platform. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 671–679. [Google Scholar] [CrossRef]
- Ghobadi, G.; van der Veen, S.; Bartelds, B.; de Boer, R.A.; Dickinson, M.G.; de Jong, J.R.; Faber, H.; Niemantsverdriet, M.; Brandenburg, S.; Berger, R.M.F.; et al. Physiological Interaction of Heart and Lung in Thoracic Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 639–646. [Google Scholar] [CrossRef]
- Lee, C.-L.; Min, H.; Befera, N.; Clark, D.; Qi, Y.; Das, S.; Johnson, G.A.; Badea, C.T.; Kirsch, D.G. Assessing Cardiac Injury in Mice with Dual Energy-MicroCT, 4D-MicroCT, and MicroSPECT Imaging after Partial Heart Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 686–693. [Google Scholar] [CrossRef]
- Lee, C.-L.; Moding, E.J.; Cuneo, K.C.; Li, Y.; Sullivan, J.M.; Mao, L.; Washington, I.; Jeffords, L.B.; Rodrigues, R.C.; Ma, Y.; et al. P53 Functions in Endothelial Cells to Prevent Radiation-Induced Myocardial Injury in Mice. Sci. Signal. 2012, 5, ra52. [Google Scholar] [CrossRef]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-Related Heart Disease: Current Knowledge and Future Prospects. Int. J. Radiat. Oncol. 2010, 76, 656–665. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; van Leeuwen, F.E. Long-Term Risk of Cardiovascular Disease in 10-Year Survivors of Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef]
- Moignier, A.; Broggio, D.; Derreumaux, S.; El Baf, F.; Mandin, A.-M.; Girinsky, T.; Paul, J.-F.; Chea, M.; Jenny, C.; Franck, D.; et al. Dependence of Coronary 3-Dimensional Dose Maps on Coronary Topologies and Beam Set in Breast Radiation Therapy: A Study Based on CT Angiographies. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.F.; Hutchison, G.B.; Lubin, J.H.; Mauch, P. Coronary Artery Disease Mortality in Patients Treated for Hodgkin’s Disease. Cancer 1992, 69, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Moignier, A.; Broggio, D.; Derreumaux, S.; Beaudré, A.; Girinsky, T.; Paul, J.-F.; Drubay, D.; Lefkopoulos, D.; Franck, D.; Aubert, B.; et al. Coronary Stenosis Risk Analysis Following Hodgkin Lymphoma Radiotherapy: A Study Based on Patient Specific Artery Segments Dose Calculation. Radiother. Oncol. 2015, 117, 467–472. [Google Scholar] [CrossRef]
- Jacob, S.; Pathak, A.; Franck, D.; Latorzeff, I.; Jimenez, G.; Fondard, O.; Lapeyre, M.; Colombier, D.; Bruguiere, E.; Lairez, O.; et al. Early Detection and Prediction of Cardiotoxicity after Radiation Therapy for Breast Cancer: The BACCARAT Prospective Cohort Study. Radiat. Oncol. 2016, 11, e54. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, S.A.; Varghese, B.; Gladish, G.W.; Choi, A.D.; Dong, W.; Patel, Z.S.; Chung, C.C.; Rao, A.; Pinnix, C.C.; Gunther, J.R.; et al. Coronary Artery Dose-Volume Parameters Predict Risk of Calcification After Radiation Therapy. J. Cardiovasc. Imaging 2019, 27, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Bates, J.E.; Mendenhall, N.P.; Morris, C.G.; Louis, D.; Ho, M.W.; Hoppe, R.; Shaikh, M.; Li, Z.; Flampouri, S. The Meaningless Meaning of Mean Heart Dose in Mediastinal Lymphoma in the Modern Radiotherapy Era. Pract. Radiat. Oncol. 2019. pii: S1879-8500(19)30279-6. [Google Scholar] [CrossRef]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.-O.; Laurier, D.; et al. Is Mean Heart Dose a Relevant Surrogate Parameter of Left Ventricle and Coronary Arteries Exposure during Breast Cancer Radiotherapy: A Dosimetric Evaluation Based on Individually-Determined Radiation Dose (BACCARAT Study). Radiat. Oncol. 2019, 14, e29. [Google Scholar] [CrossRef]
- Cutter, D.J.; Schaapveld, M.; Darby, S.C.; Hauptmann, M.; van Nimwegen, F.A.; Krol, A.D.G.; Janus, C.P.M.; van Leeuwen, F.E.; Aleman, B.M.P. Risk of Valvular Heart Disease after Treatment for Hodgkin Lymphoma. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Robinson, C.G.; Samson, P.P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation 2019, 139, 313–321. [Google Scholar] [CrossRef]
- Morris, E.D.; Ghanem, A.I.; Pantelic, M.V.; Walker, E.M.; Han, X.; Glide-Hurst, C.K. Cardiac Substructure Segmentation and Dosimetry Using a Novel Hybrid Magnetic Resonance and Computed Tomography Cardiac Atlas. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 985–993. [Google Scholar] [CrossRef]
- Desai, N.; Currey, A.; Kelly, T.; Bergom, C. Nationwide Trends in Heart-Sparing Techniques Utilized in Radiation Therapy for Breast Cancer. Adv. Radiat. Oncol. 2019, 4, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Motomura, A.R.; Bazalova, M.; Zhou, H.; Keall, P.J.; Graves, E.E. Investigation of the Effects of Treatment Planning Variables in Small Animal Radiotherapy Dose Distributions. Med. Phys. 2010, 37, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Biglin, E.R.; Price, G.J.; Chadwick, A.L.; Aitkenhead, A.H.; Williams, K.J.; Kirkby, K.J. Preclinical Dosimetry: Exploring the Use of Small Animal Phantoms. Radiat. Oncol. 2019, 14, e134. [Google Scholar] [CrossRef] [PubMed]
- Draeger, E.; Sawant, A.; Johnstone, C.; Koger, B.; Becker, S.; Vujaskovic, Z.; Jackson, I.-L.; Poirier, Y. A Dose of Reality: How 20 Years of Incomplete Physics and Dosimetry Reporting in Radiobiology Studies May Have Contributed to the Reproducibility Crisis. Int. J. Radiat. Oncol. Biol. Phys. 2019, 106, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Brady, S.L.; Robbins, M.E.; Bourland, J.D. Specific Issues in Small Animal Dosimetry and Irradiator Calibration. Int. J. Radiat. Biol. 2011, 87, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Wack, L.; Ngwa, W.; Tryggestad, E.; Tsiamas, P.; Berbeco, R.; Ng, S.K.; Hesser, J.; Zygmanski, P. High Throughput Film Dosimetry in Homogeneous and Heterogeneous Media for a Small Animal Irradiator. Phys. Medica 2014, 30, 36–46. [Google Scholar] [CrossRef]
- Kellerer, A.M. Radiobiological Challenges Posed by Microdosimetry. Health Phys. 1996, 70, 832–836. [Google Scholar] [CrossRef]
- Waker, A.J. Techniques for Radiation Measurements: Microdosimetry and Dosimetry. Radiat. Prot. Dosimetry 2006, 122, 369–373. [Google Scholar] [CrossRef]
- Goodhead, D.T. Energy Deposition Stochastics and Track Structure: What about the Target? Radiat. Prot. Dosimetry 2006, 122, 3–15. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Luo, Q.; Liu, Q. An Image-Based Rat Model for Monte Carlo Organ Dose Calculations. Med. Phys. 2008, 35, 3759–3764. [Google Scholar] [CrossRef]
- Marcelin, B.; Kjäll, P.; Johansson, J.; Lundin, A.; Nordström, H.; Eriksson, M.; Bernard, C.; Régis, J. Using Monte-Carlo-Simulated Radiation Transport to Calculate Dose Distribution in Rats before Irradiation with Leksell Gamma Knife 4C: Technical Note. Stereotact. Funct. Neurosurg. 2010, 88, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Tsiamas, P.; Velarde, E.; Tryggestad, E.; Jacques, R.; Berbeco, R.; McNutt, T.; Kazanzides, P.; Wong, J. Validation of GPU-Accelerated Superposition-Convolution Dose Computations for the Small Animal Radiation Research Platform. Med. Phys. 2018, 45, 2252–2265. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Vojnovic, B. Implications of Respiratory Motion for Small Animal Image-Guided Radiotherapy. Br. J. Radiol. 2017, 90, e20160482. [Google Scholar] [CrossRef] [PubMed]
- van der Heyden, B.; van Hoof, S.J.; Schyns, L.E.J.R.; Verhaegen, F. The Influence of Respiratory Motion on Dose Delivery in a Mouse Lung Tumour Irradiation Using the 4D MOBY Phantom. Br. J. Radiol. 2017, 90, e20160419. [Google Scholar] [CrossRef] [PubMed]
- Frelin, A.-M.; Beaudouin, V.; Le Deroff, C.; Roger, T. Implementation and Evaluation of Respiratory Gating in Small Animal Radiotherapy. Phys. Med. Biol. 2018, 63, e215024. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Thompson, J.M.; Kavanagh, A.; Tullis, I.D.C.; Newman, R.G.; Prentice, J.; Beech, J.; Gilchrist, S.; Smart, S.; Fokas, E.; et al. The Development of Technology for Effective Respiratory-Gated Irradiation Using an Image-Guided Small Animal Irradiator. Radiat. Res. 2017, 188, 247–263. [Google Scholar] [CrossRef]
- Iiyori, N.; Ide, T.; Isono, S.; Tagaito, Y.; Nishino, T. Ventilatory Load Compensation Response to Long-Term Chest Compression in Rat Model. Respir. Physiol. Neurobiol. 2003, 136, 55–63. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, e877. [Google Scholar] [CrossRef]
- Lu, L.; Sun, C.; Su, Q.; Wang, Y.; Li, J.; Guo, Z.; Chen, L.; Zhang, H. Radiation-Induced Lung Injury: Latest Molecular Developments, Therapeutic Approaches, and Clinical Guidance. Clin. Exp. Med. 2019, 19, 417–426. [Google Scholar] [CrossRef]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Van Luijk, P.; Novakova-Jiresova, A.; Faber, H.; Schippers, J.M.; Kampinga, H.H.; Meertens, H.; Coppes, R.P. Radiation Damage to the Heart Enhances Early Radiation-Induced Lung Function Loss. Cancer Res. 2005, 65, 6509–6511. [Google Scholar] [CrossRef]
- van Luijk, P.; Novakova-Jiresova, A.; Faber, H.; Steneker, M.N.J.; Kampinga, H.H.; Meertens, H.; Coppes, R.P. Relation between Radiation-Induced Whole Lung Functional Loss and Regional Structural Changes in Partial Irradiated Rat Lung. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1495–1502. [Google Scholar] [CrossRef]
- van Luijk, P.; Faber, H.; Meertens, H.; Schippers, J.M.; Langendijk, J.A.; Brandenburg, S.; Kampinga, H.H.; Coppes, R.P. The Impact of Heart Irradiation on Dose-Volume Effects in the Rat Lung. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Medhora, M.; Gao, F.; Glisch, C.; Narayanan, J.; Sharma, A.; Harmann, L.M.; Lawlor, M.W.; Snyder, L.A.; Fish, B.L.; Down, J.D.; et al. Whole-Thorax Irradiation Induces Hypoxic Respiratory Failure, Pleural Effusions and Cardiac Remodeling. J. Radiat. Res. 2015, 56, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.R.; Narayanan, J.; Fish, B.L.; Gao, F.; Harmann, L.M.; Bergom, C.; Gasperetti, T.; Strande, J.L.; Medhora, M. Cardiac Remodeling and Reversible Pulmonary Hypertension During Pneumonitis in Rats after 13-Gy Partial-Body Irradiation with Minimal Bone Marrow Sparing: Effect of Lisinopril. Health Phys. 2019, 116, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.X.; Hope, A.J.; Lindsay, P.E.; Trovo, M.; El Naqa, I.; Deasy, J.O.; Bradley, J.D. Heart Irradiation as a Risk Factor for Radiation Pneumonitis. Acta Oncol. 2011, 50, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yorke, E.D.; Jackson, A.; Kuo, L.C.; Ojo, A.; Panchoo, K.; Adusumilli, P.; Zauderer, M.G.; Rusch, V.W.; Shepherd, A.; Rimner, A. Heart Dosimetry Is Correlated with Risk of Radiation Pneumonitis After Lung-Sparing Hemithoracic Pleural Intensity Modulated Radiation Therapy for Malignant Pleural Mesothelioma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.; Oh, J.H.; Deasy, J.O.; Palma, G.; Liuzzi, R.; D’avino, V.; Conson, M.; Picardi, M.; Salvatore, M.; Pacelli, R. Predicting Radiation-Induced Valvular Heart Damage. Acta Oncol. 2015, 54, 1796–1804. [Google Scholar] [CrossRef]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Small Mammalian Animal Models of Heart Disease. Am. J. Cardiovasc. Dis. 2016, 6, 70–80. [Google Scholar]
- Mayosi, B.M.; Kardos, A.; Davies, C.H.; Gumedze, F.; Hovnanian, A.; Burge, S.; Watkins, H. Heterozygous Disruption of SERCA2a Is Not Associated with Impairment of Cardiac Performance in Humans: Implications for SERCA2a as a Therapeutic Target in Heart Failure. Heart Br. Card. Soc. 2006, 92, 105–109. [Google Scholar] [CrossRef]
- Vegter, E.L.; Ovchinnikova, E.S.; Silljé, H.H.W.; Meems, L.M.G.; van der Pol, A.; van der Velde, A.R.; Berezikov, E.; Voors, A.A.; de Boer, R.A.; van der Meer, P. Rodent Heart Failure Models Do Not Reflect the Human Circulating MicroRNA Signature in Heart Failure. PLoS ONE 2017, 12, e0177242. [Google Scholar] [CrossRef] [PubMed]
- Swynghedauw, B. Developmental and Functional Adaptation of Contractile Proteins in Cardiac and Skeletal Muscles. Physiol. Rev. 1986, 66, 710–771. [Google Scholar] [CrossRef] [PubMed]
- Pogwizd, S.M.; Bers, D.M. Rabbit Models of Heart Disease. Drug Discov. Today Dis. Models 2008, 5, 185–193. [Google Scholar] [CrossRef]
- Reeves, W.C.; Stryker, J.A.; Abt, A.B.; Chung, C.K.; Whitesell, L.; Zelis, R. Early Corticosteroid Administration in Experimental Radiation-Induced Heart Disease. Radiology 1980, 134, 533–535. [Google Scholar] [CrossRef]
- Reeves, W.C.; Cunningham, D.; Schwiter, E.J.; Abt, A.; Skarlatos, S.; Wood, M.A.; Whitesell, L. Myocardial Hydroxyproline Reduced by Early Administration of Methylprednisolone or Ibuprofen to Rabbits with Radiation-Induced Heart Disease. Circulation 1982, 65, 924–927. [Google Scholar] [CrossRef]
- Maeda, S. Pathology of Experimental Radiation Pancarditis. I. Observation on Radiation-Induced Heart Injuries Following a Single Dose of X-ray Irradiation to Rabbit Heart with Special Reference to Its Pathogenesis. Acta Pathol. Jpn. 1980, 30, 59–78. [Google Scholar]
- Stewart, J.R.; Fajardo, L.F. Dose Response in Human and Experimental Radiation-Induced Heart Disease. Application of the Nominal Standard Dose (NSD) Concept. Radiology 1971, 99, 403–408. [Google Scholar] [CrossRef]
- Mikhaleva, N.P.; Mochalova, I.N.; Ivanov, I.I. [Biochemical indices of blood serum and myocardium in local irradiation in the region of the heart in the rabbit with hard deceleration in radiation]. Biull. Eksp. Biol. Med. 1969, 68, 55–57. [Google Scholar] [CrossRef]
- Fontes-Sousa, A.P.; Moura, C.; Carneiro, C.S.; Teixeira-Pinto, A.; Areias, J.C.; Leite-Moreira, A.F. Echocardiographic Evaluation Including Tissue Doppler Imaging in New Zealand White Rabbits Sedated with Ketamine and Midazolam. Vet. J. 2009, 181, 326–331. [Google Scholar] [CrossRef][Green Version]
- Almotrefi, A.A.; Bukhari, I.A.; Alhumayyd, M.S. Investigation of the Antifibrillatory Drug Interactions between Amiodarone and Ibutilide in Isolated, Perfused Rabbit Hearts. Fundam. Clin. Pharmacol. 2015, 29, 553–557. [Google Scholar] [CrossRef]
- Nezasa, Y.; Kodama, I.; Toyama, J. Effects of OPC-88117, a New Antiarrhythmic Agent, on the Electrophysiological Properties of Rabbit Isolated Hearts. Br. J. Pharmacol. 1989, 98, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Ponnaluri, A.V.S.; Perotti, L.E.; Liu, M.; Qu, Z.; Weiss, J.N.; Ennis, D.B.; Klug, W.S.; Garfinkel, A. Electrophysiology of Heart Failure Using a Rabbit Model: From the Failing Myocyte to Ventricular Fibrillation. PLoS Comput. Biol. 2016, 12, e1004968. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Kohl, P. Rabbit Models of Cardiac Mechano-Electric and Mechano-Mechanical Coupling. Prog. Biophys. Mol. Biol. 2016, 121, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.E.; Kohl, P. Follow the White Rabbit: Experimental and Computational Models of the Rabbit Heart Provide Insights into Cardiac (Patho-) Physiology. Prog. Biophys. Mol. Biol. 2016, 121, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Chamberlain, J.; Francis, S.E.; Gunn, J. Role of Animal Models in Coronary Stenting. Ann. Biomed. Eng. 2016, 44, 453–465. [Google Scholar] [CrossRef]
- Bers, D.M. Altered Cardiac Myocyte Ca Regulation in Heart Failure. Physiol. 2006, 21, 380–387. [Google Scholar] [CrossRef]
- Pogwizd, S.M.; Bers, D.M. Cellular Basis of Triggered Arrhythmias in Heart Failure. Trends Cardiovasc. Med. 2004, 14, 61–66. [Google Scholar] [CrossRef]
- Graves, J.A.M. Background and Overview of Comparative Genomics. ILAR J. 1998, 39, 48–65. [Google Scholar] [CrossRef]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Large Mammalian Animal Models of Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, e30. [Google Scholar] [CrossRef]
- Recchia, F.A.; Lionetti, V. Animal Models of Dilated Cardiomyopathy for Translational Research. Vet. Res. Commun. 2007, 31, 35–41. [Google Scholar] [CrossRef]
- Hearse, D.J.; Sutherland, F.J. Experimental Models for the Study of Cardiovascular Function and Disease. Pharmacol. Res. 2000, 41, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.P.; Vliegenthart, R.; Schoepf, U.J.; Pilz, L.R.; Schoenberg, S.O.; Morris, P.B.; Henzler, T.; Apfaltrer, P. Coronary Artery Calcium in Breast Cancer Survivors after Radiation Therapy. Int. J. Cardiovasc. Imaging 2017, 33, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Wethal, T.; Günther, A.; Fosså, A.; Edvardsen, T.; Fosså, S.D.; Kjekshus, J. Relation of Coronary Artery Calcium Score to Premature Coronary Artery Disease in Survivors >15 Years of Hodgkin’s Lymphoma. Am. J. Cardiol. 2010, 105, 149–152. [Google Scholar] [CrossRef] [PubMed]
- van Nimwegen, F.A.; Schaapveld, M.; Cutter, D.J.; Janus, C.P.M.; Krol, A.D.G.; Hauptmann, M.; Kooijman, K.; Roesink, J.; van der Maazen, R.; Darby, S.C.; et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016, 34, 235–243. [Google Scholar] [CrossRef]
- van Leeuwen-Segarceanu, E.M.; Bos, W.-J.W.; Dorresteijn, L.D.A.; Rensing, B.J.W.M.; van der Heyden, J.A.S.; Vogels, O.J.M.; Biesma, D.H. Screening Hodgkin Lymphoma Survivors for Radiotherapy Induced Cardiovascular Disease. Cancer Treat. Rev. 2011, 37, 391–403. [Google Scholar] [CrossRef]
- Dunsmore, L.D.; LoPonte, M.A.; Dunsmore, R.A. Radiation-Induced Coronary Artery Disease. J. Am. Coll. Cardiol. 1986, 8, 239–244. [Google Scholar] [CrossRef][Green Version]
- DeZorzi, C. Radiation-Induced Coronary Artery Disease and Its Treatment: A Quick Review of Current Evidence. Cardiol. Res. Pract. 2018, 2018, e8367268. [Google Scholar] [CrossRef]
- El-Sherif, O.; Xhaferllari, I.; Sykes, J.; Butler, J.; deKemp, R.A.; Renaud, J.; Yin, H.; Wilk, B.; Sullivan, R.; Pickering, J.G.; et al. 18F-FDG Cardiac PET Imaging in a Canine Model of Radiation-Induced Cardiovascular Disease Associated with Breast Cancer Radiotherapy. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H586–H595. [Google Scholar] [CrossRef]
- Song, J.; Yan, R.; Wu, Z.; Li, J.; Yan, M.; Hao, X.; Liu, J.; Li, S. 13N-Ammonia PET/CT Detection of Myocardial Perfusion Abnormalities in Beagle Dogs After Local Heart Irradiation. J. Nucl. Med. 2017, 58, 605–610. [Google Scholar] [CrossRef]
- McChesney, S.L.; Gillette, E.L.; Powers, B.E. Radiation-Induced Cardiomyopathy in the Dog. Radiat. Res. 1988, 113, 120–132. [Google Scholar] [CrossRef]
- Barnes, M.; Pass, H.; DeLuca, A.; Tochner, Z.; Potter, D.; Terrill, R.; Sindelar, W.F.; Kinsella, T.J. Response of the Mediastinal and Thoracic Viscera of the Dog to Intraoperative Radiation Therapy (IORT). Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 371–378. [Google Scholar] [CrossRef]
- Yan, R.; Song, J.; Wu, Z.; Guo, M.; Liu, J.; Li, J.; Hao, X.; Li, S. Detection of Myocardial Metabolic Abnormalities by 18F-FDG PET/CT and Corresponding Pathological Changes in Beagles with Local Heart Irradiation. Korean J. Radiol. 2015, 16, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Hasiwa, N.; Bailey, J.; Clausing, P.; Daneshian, M.; Eileraas, M.; Farkas, S.; Gyertyán, I.; Hubrecht, R.; Kobel, W.; Krummenacher, G.; et al. Critical Evaluation of the Use of Dogs in Biomedical Research and Testing in Europe. ALTEX 2011, 28, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Spannbauer, A.; Traxler, D.; Zlabinger, K.; Gugerell, A.; Winkler, J.; Mester-Tonczar, J.; Lukovic, D.; Müller, C.; Riesenhuber, M.; Pavo, N.; et al. Large Animal Models of Heart Failure with Reduced Ejection Fraction (HFrEF). Front. Cardiovasc. Med. 2019, 6, e117. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Janssen, P.M.L. Small and Large Animal Models in Cardiac Contraction Research: Advantages and Disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef]
- Virmani, R.; Farb, A.; Carter, A.J.; Jones, R.M. Pathology of Radiation-Induced Coronary Artery Disease in Human and Pig. Cardiovasc. Radiat. Med. 1999, 1, 98–101. [Google Scholar] [CrossRef]
- DeBo, R.J.; Lees, C.J.; Dugan, G.O.; Caudell, D.L.; Michalson, K.T.; Hanbury, D.B.; Kavanagh, K.; Cline, J.M.; Register, T.C. Late Effects of Total-Body Gamma Irradiation on Cardiac Structure and Function in Male Rhesus Macaques. Radiat. Res. 2016, 186, 55–64. [Google Scholar] [CrossRef]
- Virmani, R.; Farb, A.; Carter, A.J.; Jones, R.M. Comparative Pathology: Radiation-Induced Coronary Artery Disease in Man and Animals. Semin. Interv. Cardiol. SIIC 1998, 3, 163–172. [Google Scholar]
- Sridharan, V.; Aykin-Burns, N.; Tripathi, P.; Krager, K.J.; Sharma, S.K.; Moros, E.G.; Corry, P.M.; Nowak, G.; Hauer-Jensen, M.; Boerma, M. Radiation-Induced Alterations in Mitochondria of the Rat Heart. Radiat. Res. 2014, 181, 324–334. [Google Scholar] [CrossRef]
- Sridharan, V.; Tripathi, P.; Aykin-Burns, N.; Krager, K.J.; Sharma, S.K.; Moros, E.G.; Melnyk, S.B.; Pavliv, O.; Hauer-Jensen, M.; Boerma, M. A Tocotrienol-Enriched Formulation Protects against Radiation-Induced Changes in Cardiac Mitochondria without Modifying Late Cardiac Function or Structure. Radiat. Res. 2015, 183, 357–366. [Google Scholar] [CrossRef]
- Castle, K.D.; Chen, M.; Wisdom, A.J.; Kirsch, D.G. Genetically Engineered Mouse Models for Studying Radiation Biology. Transl. Cancer Res. 2017, 6, S900–S913. [Google Scholar] [CrossRef] [PubMed]
- Patties, I.; Haagen, J.; Dörr, W.; Hildebrandt, G.; Glasow, A. Late Inflammatory and Thrombotic Changes in Irradiated Hearts of C57BL/6 Wild-Type and Atherosclerosis-Prone ApoE-Deficient Mice. Strahlenther. Onkol. 2015, 191, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, K.; Hoving, S.; Gijbels, M.J.; Pol, J.F.; te Poele, J.A.; Biessen, E.A.; Daemen, M.J.; Stewart, F.A.; Heeneman, S. Irradiation of Existing Atherosclerotic Lesions Increased Inflammation by Favoring Pro-Inflammatory Macrophages. Radiother. Oncol. 2014, 110, 455–460. [Google Scholar] [CrossRef]
- Gabriels, K.; Hoving, S.; Seemann, I.; Visser, N.L.; Gijbels, M.J.; Pol, J.F.; Daemen, M.J.; Stewart, F.A.; Heeneman, S. Local Heart Irradiation of ApoE(-/-) Mice Induces Microvascular and Endocardial Damage and Accelerates Coronary Atherosclerosis. Radiother. Oncol. 2012, 105, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Hoving, S.; Heeneman, S.; Gijbels, M.J.J.; Te Poele, J.A.M.; Visser, N.; Cleutjens, J.; Russell, N.S.; Daemen, M.J.A.P.; Stewart, F.A. Irradiation Induces Different Inflammatory and Thrombotic Responses in Carotid Arteries of Wildtype C57BL/6J and Atherosclerosis-Prone ApoE(-/-) Mice. Radiother. Oncol. 2012, 105, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Turaka, A. Predictors and Management of Chest Wall Toxicity after Lung Stereotactic Body Radiotherapy. Cancer Treat. Rev. 2014, 40, 1215–1220. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac Outcomes in a Cohort of Adult Survivors of Childhood and Adolescent Cancer: Retrospective Analysis of the Childhood Cancer Survivor Study Cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef]
- Haddy, N.; Diallo, S.; El-Fayech, C.; Schwartz, B.; Pein, F.; Hawkins, M.; Veres, C.; Oberlin, O.; Guibout, C.; Pacquement, H.; et al. Cardiac Diseases Following Childhood Cancer Treatment: Cohort Study. Circulation 2016, 133, 31–38. [Google Scholar] [CrossRef]
- Zhao, J.; Yorke, E.D.; Li, L.; Kavanagh, B.D.; Li, X.A.; Das, S.; Miften, M.; Rimner, A.; Campbell, J.; Xue, J.; et al. Simple Factors Associated with Radiation-Induced Lung Toxicity after Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1357–1366. [Google Scholar] [CrossRef]
- Hernández, L.; Terradas, M.; Camps, J.; Martín, M.; Tusell, L.; Genescà, A. Aging and Radiation: Bad Companions. Aging Cell 2015, 14, 153–161. [Google Scholar] [CrossRef]
- Medhora, M.; Gao, F.; Gasperetti, T.; Narayanan, J.; Khan, A.H.; Jacobs, E.R.; Fish, B.L. Delayed Effects of Acute Radiation Exposure (Deare) in Juvenile and Old Rats: Mitigation by Lisinopril. Health Phys. 2019, 116, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Sklar, C.A.; Hudson, M.M.; Robison, L.L. Long-Term Health Status among Survivors of Childhood Cancer: Does Sex Matter? J. Clin. Oncol. 2007, 25, 4477–4489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, W.; Wang, T.J. Gender Difference in Radiotherapy-Induced Carotid Stenosis. J. Biol. Regul. Homeost. Agents 2017, 31, 631–637. [Google Scholar] [PubMed]
- Christiansen, J.R.; Massey, R.; Dalen, H.; Kanellopoulos, A.; Hamre, H.; Ruud, E.; Kiserud, C.E.; Fosså, S.D.; Aakhus, S. Right Ventricular Function in Long-Term Adult Survivors of Childhood Lymphoma and Acute Lymphoblastic Leukaemia. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 735–741. [Google Scholar] [CrossRef]
- Bates, J.E.; Howell, R.M.; Liu, Q.; Yasui, Y.; Mulrooney, D.A.; Dhakal, S.; Smith, S.A.; Leisenring, W.M.; Indelicato, D.J.; Gibson, T.M.; et al. Therapy-Related Cardiac Risk in Childhood Cancer Survivors: An Analysis of the Childhood Cancer Survivor Study. J. Clin. Oncol. 2019, 37, 1090–1101. [Google Scholar] [CrossRef]
- Briere, T.M.; Krafft, S.; Liao, Z.; Martel, M.K. Lung Size and the Risk of Radiation Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 377–384. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Vaporciyan, A.A.; Tucker, S.L.; Liu, H.; Wei, X.; Swisher, S.; Ajani, J.A.; Cox, J.D.; Komaki, R. Investigation of Clinical and Dosimetric Factors Associated with Postoperative Pulmonary Complications in Esophageal Cancer Patients Treated with Concurrent Chemoradiotherapy Followed by Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 692–699. [Google Scholar] [CrossRef]
- Unthank, J.L.; Miller, S.J.; Quickery, A.K.; Ferguson, E.L.; Wang, M.; Sampson, C.H.; Chua, H.L.; DiStasi, M.R.; Feng, H.; Fisher, A.; et al. Delayed Effects of Acute Radiation Exposure in a Murine Model of the H-ARS: Multiple-Organ Injury Consequent to <10 Gy Total Body Irradiation. Health Phys. 2015, 109, 511–521. [Google Scholar] [CrossRef]
- Sridharan, V.; Thomas, C.J.; Cao, M.; Melnyk, S.B.; Pavliv, O.; Joseph, J.; Singh, S.P.; Sharma, S.; Moros, E.G.; Boerma, M. Effects of Local Irradiation Combined with Sunitinib on Early Remodeling, Mitochondria, and Oxidative Stress in the Rat Heart. Radiother. Oncol. 2016, 119, 259–264. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F.; et al. Cardiovascular Toxicity Induced by Chemotherapy, Targeted Agents and Radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23, 155–166. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Eltringham, J.R.; Steward, J.R. Combined Cardiotoxicity of Adriamycin and X-Radiation. Lab. Investig. 1976, 34, 86–96. [Google Scholar] [PubMed]
- Allan, N.; Siller, C.; Breen, A. Anaesthetic Implications of Chemotherapy. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 52–56. [Google Scholar] [CrossRef]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.-H. Combining Surgery and Immunotherapy: Turning an Immunosuppressive Effect into a Therapeutic Opportunity. J. Immunother. Cancer 2018, 6, e86. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, B.; Sacerdote, P.; Bianchi, M.; Locatelli, L.; Veljic-Radulovic, J.; Panerai, A.E. Evidence for an Opioid Inhibitory Effect on T Cell Proliferation. J. Neuroimmunol. 1993, 44, 43–48. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The Immune System in Cardiac Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune Mechanisms in Heart Failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef]
- Grabie, N.; Gotsman, I.; DaCosta, R.; Pang, H.; Stavrakis, G.; Butte, M.J.; Keir, M.E.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Endothelial Programmed Death-1 Ligand 1 (PD-L1) Regulates CD8+ T-Cell Mediated Injury in the Heart. Circulation 2007, 116, 2062–2071. [Google Scholar] [CrossRef]
- Okazaki, T.; Iwai, Y.; Honjo, T. New Regulatory Co-Receptors: Inducible Co-Stimulator and PD-1. Curr. Opin. Immunol. 2002, 14, 779–782. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.-P.; Elemento, O.; Formenti, S.C.; Demaria, S. Radiation Therapy and Anti-Tumor Immunity: Exposing Immunogenic Mutations to the Immune System. Genome Med. 2019, 11, e40. [Google Scholar] [CrossRef]
- Brickey, W.J.; Neuringer, I.P.; Walton, W.; Hua, X.; Wang, E.Y.; Jha, S.; Sempowski, G.D.; Yang, X.; Kirby, S.L.; Tilley, S.L.; et al. MyD88 Provides a Protective Role in Long-Term Radiation-Induced Lung Injury. Int. J. Radiat. Biol. 2012, 88, 335–347. [Google Scholar] [CrossRef][Green Version]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus Placebo after Radiotherapy in Patients with Metastatic Castration-Resistant Prostate Cancer That Had Progressed after Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab Alone or in Combination with Radiotherapy in Metastatic Castration-Resistant Prostate Cancer: Results from an Open-Label, Multicenter Phase I/II Study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of Non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Du, S.; Zhou, L.; Alexander, G.S.; Park, K.; Yang, L.; Wang, N.; Zaorsky, N.G.; Ma, X.; Wang, Y.; Dicker, A.P.; et al. PD-1 Modulates Radiation-Induced Cardiac Toxicity through Cytotoxic T Lymphocytes. J. Thorac. Oncol. 2018, 13, 510–520. [Google Scholar] [CrossRef]
- Myers, C.J.; Lu, B. Decreased Survival After Combining Thoracic Irradiation and an Anti-PD-1 Antibody Correlated with Increased T-Cell Infiltration into Cardiac and Lung Tissues. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1129–1136. [Google Scholar] [CrossRef]
- Sellers, R.S.; Clifford, C.B.; Treuting, P.M.; Brayton, C. Immunological Variation between Inbred Laboratory Mouse Strains: Points to Consider in Phenotyping Genetically Immunomodified Mice. Vet. Pathol. 2012, 49, 32–43. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chester, C.; Melero, I.; Kohrt, H. Defining the Optimal Murine Models to Investigate Immune Checkpoint Blockers and Their Combination with Other Immunotherapies. Ann. Oncol. 2016, 27, 1190–1198. [Google Scholar] [CrossRef]
- Wagar, L.E.; DiFazio, R.M.; Davis, M.M. Advanced Model Systems and Tools for Basic and Translational Human Immunology. Genome Med. 2018, 10, e73. [Google Scholar] [CrossRef]
- Yong, K.S.M.; Her, Z.; Chen, Q. Humanized Mice as Unique Tools for Human-Specific Studies. Arch. Immunol. Ther. Exp. 2018, 66, 245–266. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; McCoy, K.D.; Macpherson, A.J. Use of Axenic Animals in Studying the Adaptation of Mammals to Their Commensal Intestinal Microbiota. Semin. Immunol. 2007, 19, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Dzutsev, A.; Goldszmid, R.S.; Viaud, S.; Zitvogel, L.; Trinchieri, G. The Role of the Microbiota in Inflammation, Carcinogenesis, and Cancer Therapy. Eur. J. Immunol. 2015, 45, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.; Kim, K.; Kim, S.-H.; Chung, Y.-J.; Lee, C. Studying Cancer Immunotherapy Using Patient-Derived Xenografts (PDXs) in Humanized Mice. Exp. Mol. Med. 2018, 50, e99. [Google Scholar] [CrossRef]
- Peppone, L.J.; Mustian, K.M.; Morrow, G.R.; Dozier, A.M.; Ossip, D.J.; Janelsins, M.C.; Sprod, L.K.; McIntosh, S. The Effect of Cigarette Smoking on Cancer Treatment–Related Side Effects. Oncologist 2011, 16, 1784–1792. [Google Scholar] [CrossRef]
- Browman, G.P.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; McAlpine, L.; Skingley, P.; Levine, M.N. Influence of Cigarette Smoking on the Efficacy of Radiation Therapy in Head and Neck Cancer. N. Engl. J. Med. 1993, 328, 159–163. [Google Scholar] [CrossRef]
- Canada, J.M.; Trankle, C.R.; Carbone, S.; Buckley, L.F.; de Chazal, M.; Billingsley, H.; Evans, R.K.; Garten, R.; Van Tassell, B.W.; Kadariya, D.; et al. Determinants of Cardiorespiratory Fitness Following Thoracic Radiotherapy in Lung or Breast Cancer Survivors. Am. J. Cardiol. 2019. [Google Scholar] [CrossRef]
- Assi, M.; Dufresne, S.; Rébillard, A. Exercise Shapes Redox Signaling in Cancer. Redox Biol. 2020, 101439. [Google Scholar] [CrossRef]
- de la Cruz Bonilla, M.; Stemler, K.M.; Jeter-Jones, S.; Fujimoto, T.N.; Molkentine, J.; Asencio Torres, G.M.; Zhang, X.; Broaddus, R.R.; Taniguchi, C.M.; Piwnica-Worms, H. Fasting Reduces Intestinal Radiotoxicity, Enabling Dose-Escalated Radiation Therapy for Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 537–547. [Google Scholar] [CrossRef]
- Musa, A.E.; Shabeeb, D. Radiation-Induced Heart Diseases: Protective Effects of Natural Products. Medicina 2019, 55. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).