Riboflavin-Targeted Drug Delivery
Abstract
:1. Introduction
2. Riboflavin and Its Transport
2.1. Riboflavin Carrier Protein
2.2. Riboflavin Transporters
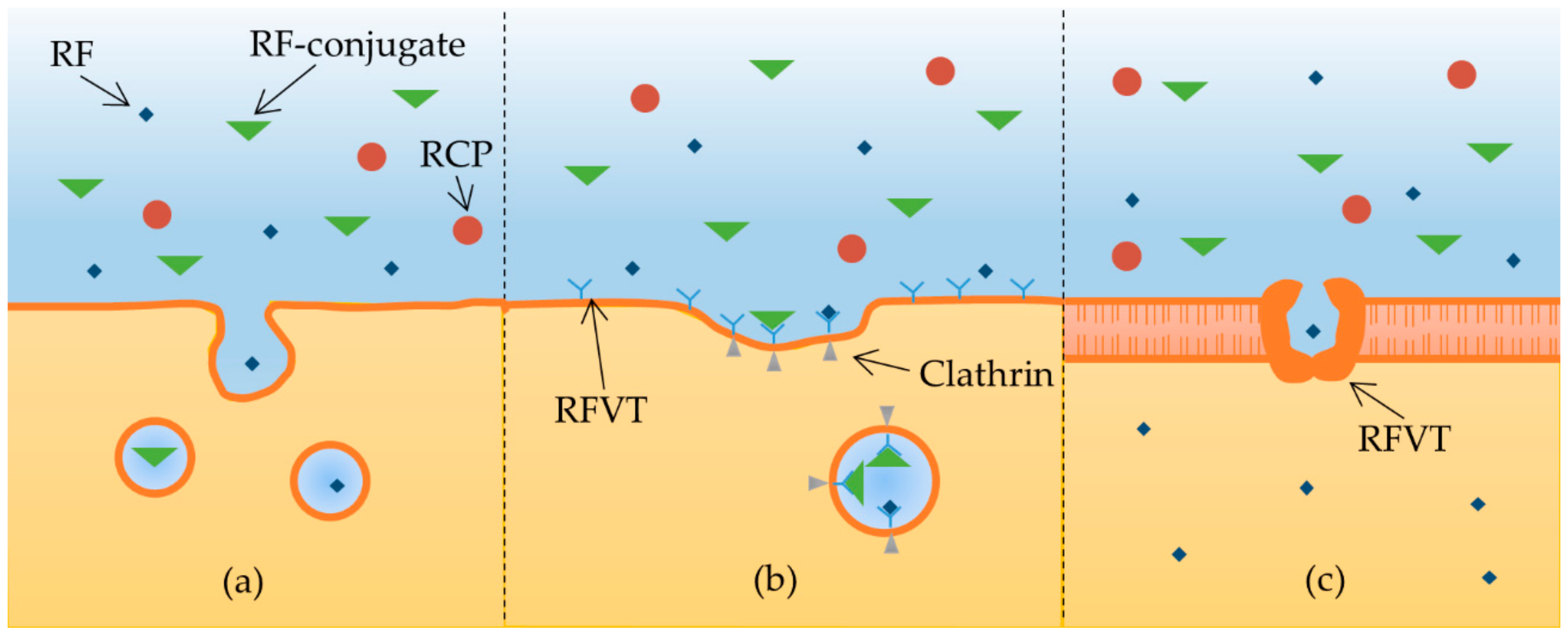
2.3. Riboflavin Internalization
3. Riboflavin Targeting
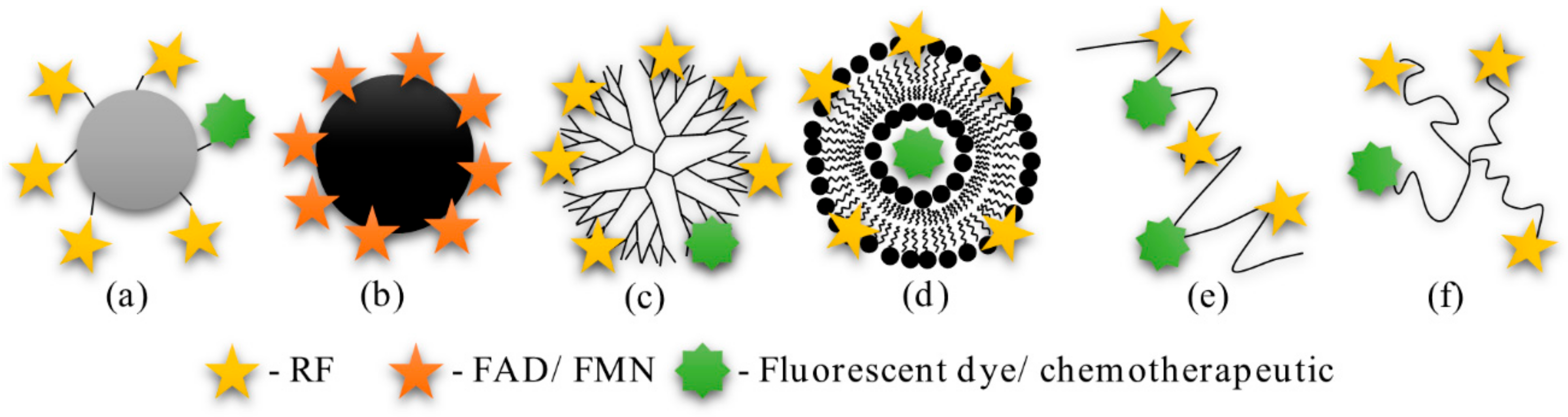
3.1. Bioconjugates
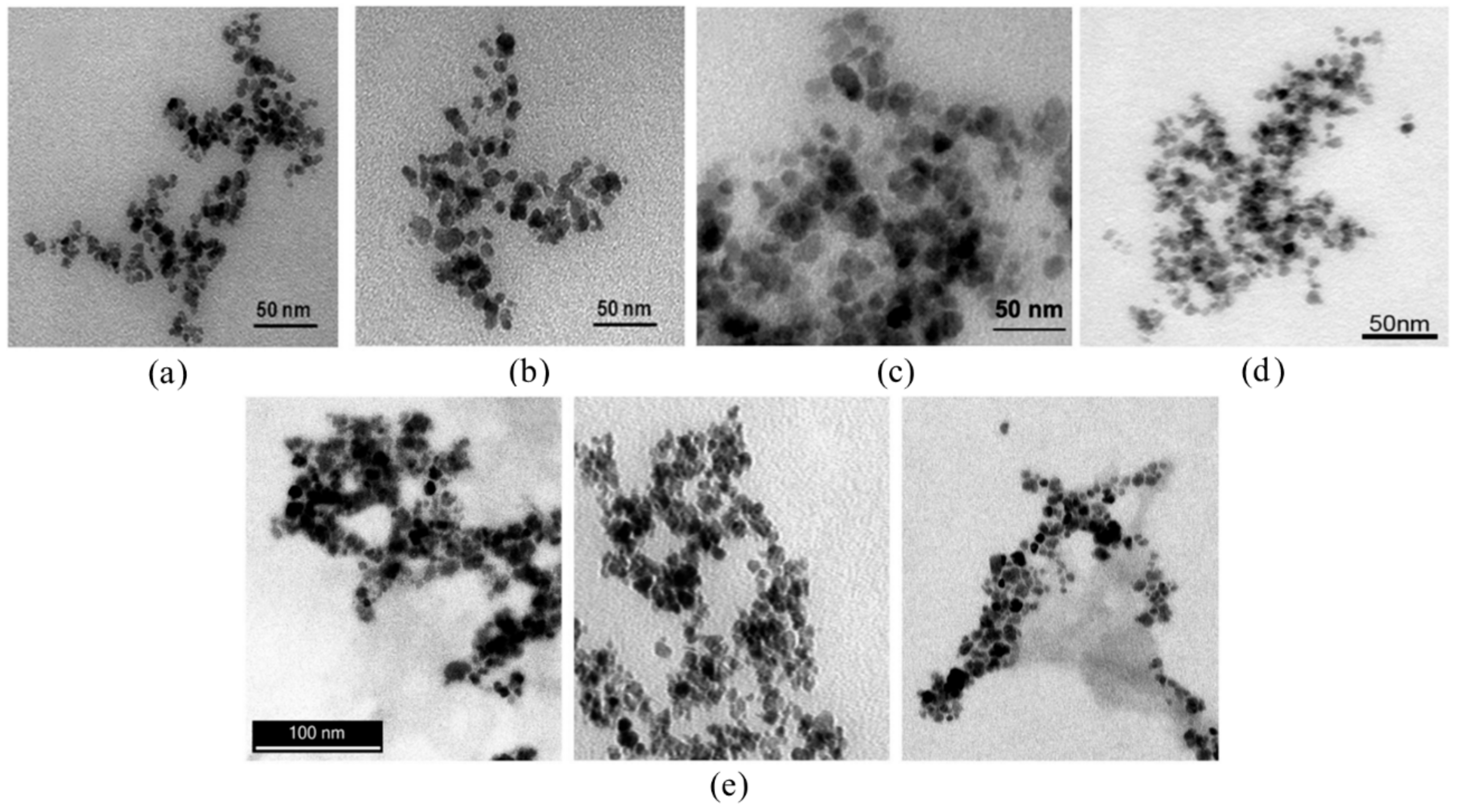
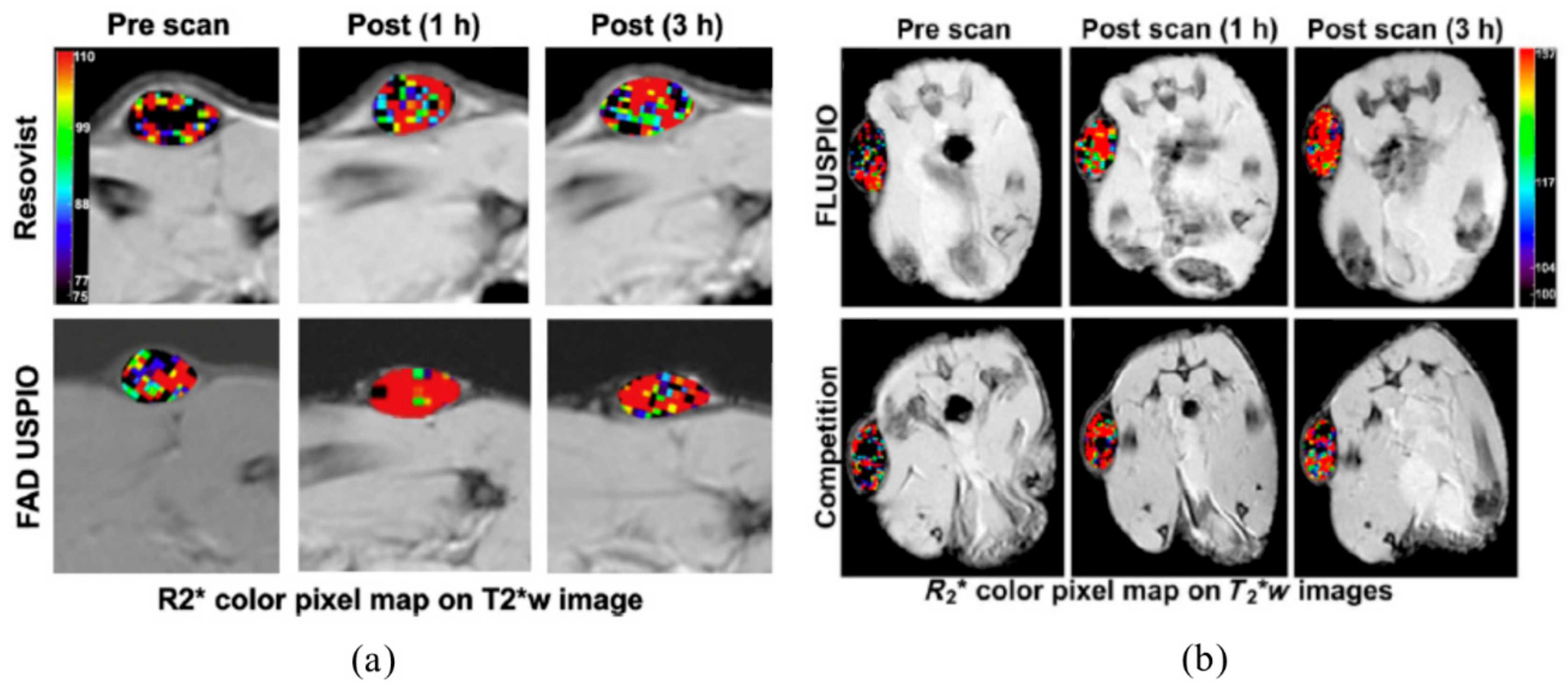
3.2. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles
3.3. Dendrimers
3.4. Liposomes
3.5. Polymers
4. Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A431 | Human epidermoid carcinoma |
| A549 | Human lung adenocarcinoma cells |
| ADP | Adenosine diphosphate |
| AFM | Atomic force microscope |
| AMP | Adenosine monophosphate |
| ARPE-19 | Human retinal pigment epithelial cells |
| ATP | Adenosine triphosphate |
| AuNP | Gold nanoparticles |
| BeWo | Human trophoblast cells |
| BRE4 | Rat brain capillary endothelial cells |
| BSA | Bovine serum albumin |
| Caco-2 | Human intestinal epithelial cells |
| cRCP | Chicken riboflavin carrier protein |
| CT/FMT | Computed tomography/fluorescence molecular tomography |
| DNP | 2,4-dinitrophenol |
| DSC | Differential scanning calorimetry |
| DU-145 | Human prostate adenocarcinoma cells |
| EPR | Enhanced permeability and retention |
| FACS | Fluorescence-activated cell sorting |
| FAD | Flavin adenine dinucleotide |
| FLUSPIO | FMN/FAD coated USPIO |
| FMN | Flavin mononucleotide |
| GMP | Guanosine monophosphate |
| HEL293 | Human embryonic kidney cells |
| HeLa | Human cervix adenocarcinoma cells |
| Hep G2 | Human liver cells |
| HK-2 | Human renal proximal tubule epithelial cells |
| hRCP | Human riboflavin carrier protein |
| HUVEC | Human umbilical vein epithelial cells |
| ITC | Isothermal titration calorimetry |
| KB | Human nasopharyngeal carcinoma cells |
| LCL | Long circulating liposomes |
| LnCaP | Human prostate adenocarcinoma cells |
| MCF-7 | Human breast adenocarcinoma cells |
| MLS | Human ovarian serous cystadenocarcinoma |
| MMC | Mitomycin C |
| MR | Magnetic resonance |
| MRI | Magnetic resonance imaging |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MTX | Methotrexate |
| NCM460 | Human colonic epithelial cells |
| PAMAM | Polyamidoamine |
| PBMC | Human peripheral blood mononuclear cells |
| PC3 | Human prostate adenocarcinoma cells |
| p-CMPS | p-chloromercuriphenyl sulfonate |
| PDI | Polydispersity index |
| PEG | Polyethylene glycol |
| PHPMA | Poly(N-(2-Hydroxypropyl) methacrylamide) |
| pI | Isoelectric point |
| RCP | Riboflavin carrier protein |
| RF | Riboflavin |
| RFVT | Riboflavin transporter |
| rPCEC | Rabbit corneal epithelial cells |
| SCC | Squamous cell carcinoma |
| SKBR-3 | Human adenocarcinoma cells |
| SK-LU-1 | Human lung adenocarcinoma cells |
| SK-OV | Human ovary adenocarcinoma cells |
| SLC | Solute carriers |
| SPR | Surface plasmon resonance |
| T84 | Human colorectal carcinoma cells |
| TUNEL | Transferase-mediated deoxyuridine triphosphate nick end tunneling |
| USPIO | Ultrasmall superparamagnetic iron oxide nanoparticles |
| XTT | 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
| Y-79 | Human retinoblastoma cells |
| β-TC-6 | Human pancreatic cells |
References
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleck, L.M. The Costs of Caring: Who Pays? Who Profits? Who Panders? Hastings Cent. Rep. 2006, 36, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Conover, C.; Shi, C.; Whitlow, M.; Filpula, D. Prolonged Circulating Lives of Single-Chain Fv Proteins Conjugated with Polyethylene Glycol: A Comparison of Conjugation Chemistries and Compounds. Bioconjug. Chem. 1999, 10, 973–981. [Google Scholar] [CrossRef]
- Suzuki, T.; Ikeda, K.; Tomono, T. Physicochemical and biological properties of poly(ethylene glycol)-coupled immunoglobulin G. Part II. Effect of molecular weight of poly(ethylene glycol). J. Biomater. Sci. Polym. Ed. 1989, 1, 71–84. [Google Scholar] [CrossRef]
- Kitamura, K.; Takahashi, T.; Yamaguchi, T.; Noguchi, A.; Noguchi, A.; Takashina, K.; Tsurumi, H.; Inagake, M.; Toyokuni, T.; Hakomori, S. Chemical engineering of the monoclonal antibody A7 by polyethylene glycol for targeting cancer chemotherapy. Cancer Res. 1991, 51, 4310–4315. [Google Scholar]
- Campbell, I.G.; Jones, T.A.; Foulkes, W.D.; Trowsdale, J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991, 51, 5329–5338. [Google Scholar]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.H.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Toffoli, G.; Cernigoi, C.; Russo, A.; Gallo, A.; Bagnoli, M.; Boiocchi, M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. [Google Scholar] [CrossRef]
- Lutz, R.J. Targeting the folate receptor for the treatment of ovarian cancer. Transl. Cancer Res. 2015, 4, 118–126. [Google Scholar]
- Klein, B.P. Handbook of vitamins: Nutritional, biochemical, and clinical aspects. Edited by Lawrence J. Machlin. Marcel Dekker, Inc., New York, NY. 1984. 632 pp. ISBN 0–8247-7051-X. $;9.50. J. Pharm. Sci. 1985, 74, 1024–1025. [Google Scholar] [CrossRef]
- Tu, B.P. Biochemical Basis of Oxidative Protein Folding in the Endoplasmic Reticulum. Science 2000, 290, 1571–1574. [Google Scholar] [CrossRef]
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermöhlen, O.; Krönke, M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Buchala, B.; Plytycz, B. Riboflavin deprivation inhibits macrophage viability and activity – a study on the RAW 264.7 cell line. Br. J. Nutr. 2013, 110, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanches, S.C.; Ramalho, L.N.Z.; Mendes-Braz, M.; Terra, V.A.; Cecchini, R.; Augusto, M.J.; Ramalho, F.S. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
- Liu, D.; Zempleni, J. Low activity of LSD1 elicits a pro-inflammatory gene expression profile in riboflavin-deficient human T Lymphoma Jurkat cells. Genes Nutr. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.N.; Swaan, P.W. Involvement of a receptor-mediated component in cellular translocation of riboflavin. J. Pharmacol. Exp. Ther. 2000, 294, 117–125. [Google Scholar]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Jaeger, B.; Bosch, A.M. Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience. J. Inherit. Metab. Dis. 2016, 39, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Banares, F.; Abad-Lacruz, A.; Xiol, X.; Gine, J.J.; Dolz, C.; Cabre, E.; Esteve, M.; Gonzalez-Huix, F.; Gassull, M.A. Vitamin status in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1989, 84, 744–748. [Google Scholar]
- Rosenthal, W.S.; Adham, N.F.; Lopez, R.; Cooperman, J.M. Riboflavin deficiency in complicated chronic alcoholism. Am. J. Clin. Nutr. 1973, 26, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Kodentsova, V.M.; Vrzhesinskaia, O.A.; Trofimenko, E.V.; Sokol’nikov, A.A.; Beketova, N.A.; Blazheevich, N.V.; Isaeva, V.A.; Aleĭnik, S.I.; Trofimenko, L.S.; Dronova, V.I. Vitamin status of children with diabetes mellitus. Vopr. Med. Khim. 1994, 40, 45–48. [Google Scholar] [PubMed]
- Rhodes, M.B.; Bennett, N.; Feeney, R.E. The flavoprotein-apoprotein system of egg white. J. Biol. Chem. 1959, 234, 2054–2060. [Google Scholar] [PubMed]
- Ostrowski, W.; Skarzynski, B.; Zak, Z. Isolation and properties of flavoprotein from the egg yolk. Biochim. Biophys. Acta 1962, 59, 515–517. [Google Scholar] [CrossRef]
- Visweswariah, S.S.; Adiga, P.R. Isolation of riboflavin carrier proteins from pregnant human and umbilical cord serum: Similarities with chicken egg riboflavin carrier protein. Biosci. Rep. 1987, 7, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Kyogoku, Y. Flavin-Protein Interaction in Egg White Flavoprotein. J. Biochem. 1973, 73, 1233–1242. [Google Scholar] [CrossRef]
- Choi, J.D.; McCormick, D.B. The interaction of flavins with egg white riboflavin-binding protein. Arch. Biochem. Biophys. 1980, 204, 41–51. [Google Scholar] [CrossRef]
- Plantinga, A.; Witte, A.; Li, M.-H.; Harmon, A.; Choi, S.K.; Banaszak Holl, M.M.; Orr, B.G.; Baker, J.R.; Sinniah, K. Bioanalytical Screening of Riboflavin Antagonists for Targeted Drug Delivery—A Thermodynamic and Kinetic Study. ACS Med. Chem. Lett. 2011, 2, 363–367. [Google Scholar] [CrossRef]
- Monaco, H.L. Crystal structure of chicken riboflavin-binding protein. EMBO J. 1997, 16, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Natraj, U.; Kumar R, A.; Kadam, P. Termination of Pregnancy in Mice with Antiserum to Chicken Riboflavin-Carrier Protein. Biol. Reprod. 1987, 36, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Murty, C.; Adiga, P. Pregnancy suppression by active immunization against gestation-specific riboflavin carrier protein. Science 1982, 216, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N.; Levine, E.; Myers, M.O.; Prakash, V.; Watson, J.; Stolier, A.; Kopicko, J.J.; Kissinger, P.; Raj, S.G.; Raj, M.H.G. Elevation of Serum Riboflavin Carrier Protein in Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 985–990. [Google Scholar] [PubMed]
- Karande, A.A.; Sridhar, L.; Gopinath, K.S.; Adiga, P.R. Riboflavin carrier protein: A serum and tissue marker for breast carcinoma. Int. J. Cancer 2001, 95, 277–281. [Google Scholar] [CrossRef]
- Rao, P.N.; Crippin, J.; Levine, E.; Hunt, J.; Baliga, S.; Balart, L.; Anthony, L.; Mulekar, M.; Raj, M.H.G. Elevation of serum riboflavin carrier protein in hepatocellular carcinoma. Hepatol. Res. 2006, 35, 83–87. [Google Scholar] [CrossRef]
- Johnson, T.M.; Ouhtit, A.; Gaur, R.; Fernando, A.; Schwarzenberger, P.O.; Su, J.L.; Ismail, M.F.; El-Sayyad, H.I.H.; Karande, A.; Elmageed, Z.Y.A.; et al. Biochemical characterization of riboflavin carrier protein (RCP) in prostate cancer. Front. Biosci. 2009, 14, 3634–3640. [Google Scholar] [CrossRef] [Green Version]
- Hediger, M.A.; Romero, M.F.; Peng, J.-B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef]
- Fredriksson, R.; Nordström, K.J.V.; Stephansson, O.; Hägglund, M.G.A.; Schiöth, H.B. The solute carrier (SLC) complement of the human genome: Phylogenetic classification reveals four major families. FEBS Lett. 2008, 582, 3811–3816. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, A.; Masuda, S.; Katsura, T.; Inui, K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am. J. Physiol. Cell Physiol. 2008, 295, C632–C641. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Inoue, K.; Ohta, K.; Fukatsu, R.; Maeda, J.; Yoshida, Y.; Yuasa, H. Identification and functional characterization of rat riboflavin transporter 2. J. Biochem. 2009, 145, 437–443. [Google Scholar] [CrossRef]
- Yao, Y.; Yonezawa, A.; Yoshimatsu, H.; Masuda, S.; Katsura, T.; Inui, K.-I. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J. Nutr. 2010, 140, 1220–1226. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, Y. Riboflavin transporter is finally identified. J. Biochem. 2011, 150, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Sabui, S.; Subramanian, V.S.; Pham, Q.; Said, H.M. Identification of transmembrane protein 237 as a novel interactor with the intestinal riboflavin transporter-3 (RFVT-3): Role in functionality and cell biology. Am. J. Physiol. Cell Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Inui, K. Novel riboflavin transporter family RFVT/SLC52: Identification, nomenclature, functional characterization and genetic diseases of RFVT/SLC52. Mol. Asp. Med. 2013, 34, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Yamamoto, S.; Murata, T.; Yasujima, T.; Inoue, K.; Ohta, K.; Yuasa, H. Functional Characteristics of the Human Ortholog of Riboflavin Transporter 2 and Riboflavin-Responsive Expression of Its Rat Ortholog in the Small Intestine Indicate Its Involvement in Riboflavin Absorption. J. Nutr. 2010, 140, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- Beztsinna, N.; Solé, M.; Taib, N.; Bestel, I. Bioengineered riboflavin in nanotechnology. Biomaterials 2016, 80, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Ma, T.Y. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am. J. Physiol. 1994, 266, G15–G21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.K.; Yanagawa, N.; Ortiz, A.; Said, H.M. Mechanism and regulation of riboflavin uptake by human renal proximal tubule epithelial cell line HK-2. Am. J. Physiol. 1998, 274, F104–F110. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Swaan, P.W. Riboflavin uptake in human trophoblast-derived BeWo cell monolayers: cellular translocation and regulatory mechanisms. J. Pharmacol. Exp. Ther. 2001, 298, 264–271. [Google Scholar]
- Foraker, A.B.; Khantwal, C.M.; Swaan, P.W. Current perspectives on the cellular uptake and trafficking of riboflavin. Adv. Drug Deliv. Rev. 2003, 55, 1467–1483. [Google Scholar] [CrossRef]
- Bartmann, L.; Schumacher, D.; Von Stillfried, S.; Sternkopf, M.; Alampour-Rajabi, S.; van Zandvoort, M.; Kiessling, F.; Wu, Z. Evaluation of Riboflavin Transporters as Targets for Drug Delivery and Theranostics. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-R.; Yu, X.-Y.; Fan, J.-H.; Guo, L.; Zhu, C.; Jiang, W.; Lu, S.-H. RFT2 is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenesis by sustaining cell proliferation and protecting against cell death. Cancer Lett. 2014, 353, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Liu, Y.; Wang, Q.; Sun, Z.; Di, H.; Fan, W.; Liu, M.; Wang, J. Overexpression of riboflavin transporter 2 contributes toward progression and invasion of glioma. Neuroreport 2016, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Aili, A.; Hasim, A.; Kelimu, A.; Guo, X.; Mamtimin, B.; Abudula, A.; Upur, H. Association of the plasma and tissue riboflavin levels with C20orf54 expression in cervical lesions and its relationship to HPV16 infection. PLoS ONE 2013, 8, e79937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, L.; Pang, X.-X.; Lei, F.; Zhang, J.-S.; Wang, W.; Liao, L.-D.; Xu, X.-E.; He, J.-Z.; Wu, J.-Y.; Wu, Z.-Y.; et al. SLC52A3 expression is activated by NF-κB p65/Rel-B and serves as a prognostic biomarker in esophageal cancer. Cell. Mol. Life Sci. 2018, 75, 2643–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosal, A.; Said, H.M. Mechanism and regulation of vitamin B2 (riboflavin) uptake by mouse and human pancreatic β-cells/islets: physiological and molecular aspects. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1052–G1058. [Google Scholar] [CrossRef] [Green Version]
- Dyer, D.L.; Said, H.M. Riboflavin uptake by native Xenopus laevis oocytes. Biochim. Biophys. Acta 1995, 1234, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Said, H.M.; Ortiz, A.; Ma, T.Y.; McCloud, E. Riboflavin uptake by the human-derived liver cells Hep G2: Mechanism and regulation. J. Cell. Physiol. 1998, 176, 588–594. [Google Scholar] [CrossRef]
- Said, H.M.; Ortiz, A.; Moyer, M.P.; Yanagawa, N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am. J. Physiol. Cell Physiol. 2000, 278, C270–C276. [Google Scholar] [CrossRef] [Green Version]
- Zempleni, J.; Mock, D.M. Proliferation of Peripheral Blood Mononuclear Cells Increases Riboflavin Influx (44554). SAGE J. 2000, 1. [Google Scholar] [CrossRef]
- Kansara, V.; Pal, D.; Jain, R.; Mitra, A.K. Identification and functional characterization of riboflavin transporter in human-derived retinoblastoma cell line (Y-79): mechanisms of cellular uptake and translocation. J. Ocul. Pharmacol. Ther. 2005, 21, 275–287. [Google Scholar] [CrossRef]
- Said, H.M.; Wang, S.; Ma, T.Y. Mechanism of riboflavin uptake by cultured human retinal pigment epithelial ARPE-19 cells: possible regulation by an intracellular Ca2+-calmodulin-mediated pathway. J. Physiol. (Lond.) 2005, 566, 369–377. [Google Scholar] [CrossRef]
- Hariharan, S.; Janoria, K.G.; Gunda, S.; Zhu, X.; Pal, D.; Mitra, A.K. Identification and Functional Expression of a Carrier-Mediated Riboflavin Transport System on Rabbit Corneal Epithelium. Curr. Eye Res. 2006, 31, 811–824. [Google Scholar] [CrossRef]
- Bareford, L.M.; Phelps, M.A.; Foraker, A.B.; Swaan, P.W. Intracellular Processing of Riboflavin in Human Breast Cancer Cells. Mol. Pharm. 2008, 5, 839–848. [Google Scholar] [CrossRef]
- Patel, M.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Molecular and functional characterization of riboflavin specific transport system in rat brain capillary endothelial cells. Brain Res. 2012, 1468, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yoshimatsu, H.; Yonezawa, A.; Yao, Y.; Sugano, K.; Nakagawa, S.; Omura, T.; Matsubara, K. Functional involvement of RFVT3/ SLC52A3 in intestinal riboflavin absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G102–G110. [Google Scholar] [CrossRef]
- Wangensteen, O.D.; Bartlett, M.M.; James, J.K.; Yang, Z.F.; Low, P.S. Riboflavin-enhanced transport of serum albumin across the distal pulmonary epithelium. Pharm. Res. 1996, 13, 1861–1864. [Google Scholar] [CrossRef]
- Holladay, S.R.; Yang, Z.; Kennedy, M.D.; Leamon, C.P.; Lee, R.J.; Jayamani, M.; Mason, T.; Low, P.S. Riboflavin-mediated delivery of a macromolecule into cultured human cells. BBA-Gen. Subj. 1999, 1426, 195–204. [Google Scholar] [CrossRef]
- Horn, M.A.; Heinstein, P.F.; Low, P.S. Biotin-Mediated Delivery of Exogenous Macromolecules into Soybean Cells. Plant Physiol. 1990, 93, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. USA 1991, 88, 5572–5576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayapaul, J.; Arns, S.; Lederle, W.; Lammers, T.; Comba, P.; Gätjens, J.; Kiessling, F. Riboflavin carrier protein-targeted fluorescent USPIO for the assessment of vascular metabolism in tumors. Biomaterials 2012, 33, 8822–8829. [Google Scholar] [CrossRef]
- Jayapaul, J.; Hodenius, M.; Arns, S.; Lederle, W.; Lammers, T.; Comba, P.; Kiessling, F.; Gaetjens, J. FMN-coated fluorescent iron oxide nanoparticles for RCP-mediated targeting and labeling of metabolically active cancer and endothelial cells. Biomaterials 2011, 32, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Jayapaul, J.; Arns, S.; Bunker, M.; Weiler, M.; Rutherford, S.; Comba, P.; Kiessling, F. In vivo evaluation of riboflavin receptor targeted fluorescent USPIO in mice with prostate cancer xenografts. Nano. Res. 2016, 9, 1319–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsvetkova, Y.; Beztsinna, N.; Jayapaul, J.; Weiler, M.; Arns, S.; Shi, Y.; Lammers, T.; Kiessling, F. Refinement of adsorptive coatings for fluorescent riboflavin-receptor-targeted iron oxide nanoparticles. Contrast Media Mol. Imaging 2016, 11, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.P.; Choi, S.K.; Li, M.-H.; Kotlyar, A.; Baker, J.R. Design of riboflavin-presenting PAMAM dendrimers as a new nanoplatform for cancer-targeted delivery. Bioorg. Med. Chem. Lett. 2010, 20, 5191–5194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.T.; Sinniah, K.; Choi, S.K. Riboflavin-Conjugated Multivalent Dendrimer Platform for Cancer-Targeted Drug and Gene Delivery. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J.L., Eds.; Springer: Singapore, 2017; pp. 145–171. [Google Scholar]
- Witte, A.B.; Leistra, A.N.; Wong, P.T.; Bharathi, S.; Refior, K.; Smith, P.; Kaso, O.; Sinniah, K.; Choi, S.K. Atomic Force Microscopy Probing of Receptor–Nanoparticle Interactions for Riboflavin Receptor Targeted Gold–Dendrimer Nanocomposites. J. Phys. Chem. B 2014, 118, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.B.; Timmer, C.M.; Gam, J.J.; Choi, S.K.; Banaszak Holl, M.M.; Orr, B.G.; Baker, J.R.; Sinniah, K. Biophysical Characterization of a Riboflavin-conjugated Dendrimer Platform for Targeted Drug Delivery. Biomacromolecules 2012, 13, 507–516. [Google Scholar] [CrossRef]
- Leistra, A.N.; Han, J.H.; Tang, S.; Orr, B.G.; Banaszak Holl, M.M.; Choi, S.K.; Sinniah, K. Force Spectroscopy of Multivalent Binding of Riboflavin-Conjugated Dendrimers to Riboflavin Binding Protein. J. Phys. Chem. B 2015, 119, 5785–5792. [Google Scholar] [CrossRef]
- Beztsinna, N.; Tsvetkova, Y.; Bartneck, M.; Lammers, T.; Kiessling, F.; Bestel, I. Amphiphilic Phospholipid-Based Riboflavin Derivatives for Tumor Targeting Nanomedicines. Bioconjug. Chem. 2016, 27, 2048–2061. [Google Scholar] [CrossRef]
- Bareford, L.M.; Avaritt, B.R.; Ghandehari, H.; Nan, A.; Swaan, P.W. Riboflavin-Targeted Polymer Conjugates for Breast Tumor Delivery. Pharm. Res. 2013, 30, 1799–1812. [Google Scholar] [CrossRef]
- Tsvetkova, Y.; Beztsinna, N.; Baues, M.; Klein, D.; Rix, A.; Golombek, S.K.; Al Rawashdeh, W.; Gremse, F.; Barz, M.; Koynov, K.; et al. Balancing Passive and Active Targeting to Different Tumor Compartments Using Riboflavin-Functionalized Polymeric Nanocarriers. Nano. Lett. 2017, 17, 4665–4674. [Google Scholar] [CrossRef]
- Miller, M.A.; Zheng, Y.-R.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B.; et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cell Line | Km in µM | Vmax in pmol/min/mg Protein |
|---|---|---|
| Human intestinal epithelial cells (Caco-2) [46] | 0.30 ± 0.03 | 69.97 ± 8.13 |
| Xenopus laevis oocytes [56] | 0.41 ± 0.02 | 0.00005 ± 0.0000007 |
| Human liver cells (Hep G2) [57] | 0.41 ± 0.08 | 1.19 ± 0.08 |
| Human renal proximal tubule epithelial cells (HK-2) [47] | 0.67 ± 0.21 | 3.35 ± 0.29 |
| Human colonic epithelial cells (NCM460) [58] | 0.14 ± 0.004 | 1.10 ± 0.19 |
| Peripheral blood mononuclear cells (PBMC) [59] | 0.955 ± 0.344 | 0.04 ± 0.02 |
| Human trophoblast cells (BeWo) [48] | 0.0013 ± 0.00068 | 0.01 ± 0.001 |
| Human retinoblastoma cells (Y-79) [60] | 0.019 ± 0.00037 | 6.98 ± 0.30 |
| Human retinal pigment epithelial cells (ARPE-19) [61] | 0.08 ± 0.014 | 0.45 ± 0.03 |
| Rabbit corneal epithelial cells (rPCEC) [62] | 2.05 | 3.99 |
| Human embryonic kidney cells (HEK293) [38] | 0.0350 ± 0.0041 | 0.17 ± 0.16 |
| Human breast adenocarcinoma cells (MCF-7) [63] | 0.106 ± 0.009 | 0.52 |
| Human pancreatic cells (β-TC-6) [55] | 0.17 ± 0.02 | 4.45 ± 0.16 |
| Rat brain capillary endothelial cells (BRE4) [64] | 19 ± 3 | 0.24 ± 0.01 |
| Human colorectal carcinoma cells (T84) [65] | 0.0532 ± 0.0216 | 0.36 ± 0.08 |
| Cell Line | RF | FMN | FAD | Lumiflavin | Lumichrome | Lumazine | D-Ribose |
|---|---|---|---|---|---|---|---|
| Caco-2 [46] | + | nt | nt | + | + | - | - |
| Oocytes [56] | + | nt | - | + | + | - | - |
| Hep G2 [57] | nt | nt | nt | + | + | - | nt |
| HK-2 [47] | + | nt | nt | + | + | - | - |
| NCM460 [58] | nt | nt | nt | + | + | nt | nt |
| PBMC [59] | + | - | - | nt | + | nt | - |
| BeWo [48] | + | + | + | + | + | nt | - |
| Y-79 [60] | + | nt | nt | + | + | nt | nt |
| ARPE-19 [61] | + | nt | nt | + | + | - | nt |
| rPCEC [62] | + | nt | nt | + | + | nt | - |
| HEK293 [38] | nt | + | + | + | nt | nt | nt |
| β-TC-6 [55] | + | nt | nt | + | + | nt | nt |
| BRE4 [64] | + | + | + | + | + | nt | - |
| Cell Line | Ouabain | Sodium Azide | DNP | p-CMPS | Iodoacetate | Calmidazolium |
|---|---|---|---|---|---|---|
| Caco-2 [46] | nt | + | + | nt | nt | nt |
| Oocytes [56] | - | nt | + | + | nt | nt |
| Hep G2 [57] | - | + | + | + | + | + |
| HK-2 [47] | nt | + | + | + | + | + |
| NCM460 [58] | - | + | + | + | nt | + |
| PBMC [59] | - | nt | + | nt | - | nt |
| BeWo [48] | nt | + | nt | nt | nt | + |
| Y-79 [60] | - | + | + | nt | nt | + |
| ARPE-19 [61] | nt | nt | + | + | + | + |
| rPCEC [62] | - | + | + | nt | nt | nt |
| β-TC-6 [55] | nt | nt | nt | nt | nt | + |
| BRE4 [64] | + | + | + | nt | nt | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darguzyte, M.; Drude, N.; Lammers, T.; Kiessling, F. Riboflavin-Targeted Drug Delivery. Cancers 2020, 12, 295. https://doi.org/10.3390/cancers12020295
Darguzyte M, Drude N, Lammers T, Kiessling F. Riboflavin-Targeted Drug Delivery. Cancers. 2020; 12(2):295. https://doi.org/10.3390/cancers12020295
Chicago/Turabian StyleDarguzyte, Milita, Natascha Drude, Twan Lammers, and Fabian Kiessling. 2020. "Riboflavin-Targeted Drug Delivery" Cancers 12, no. 2: 295. https://doi.org/10.3390/cancers12020295
APA StyleDarguzyte, M., Drude, N., Lammers, T., & Kiessling, F. (2020). Riboflavin-Targeted Drug Delivery. Cancers, 12(2), 295. https://doi.org/10.3390/cancers12020295







