A Novel pH-Tunable Secondary Conformation Containing Mixed Micellar System in Anticancer Treatment
Abstract
1. Introduction
2. Results
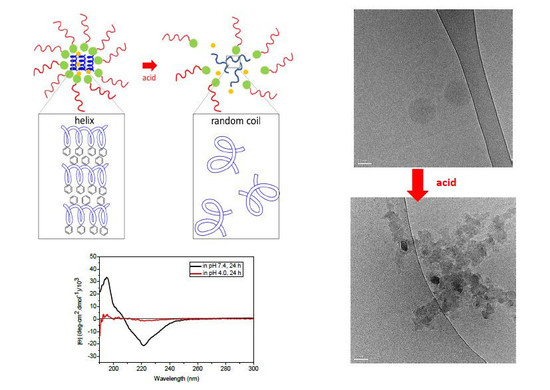
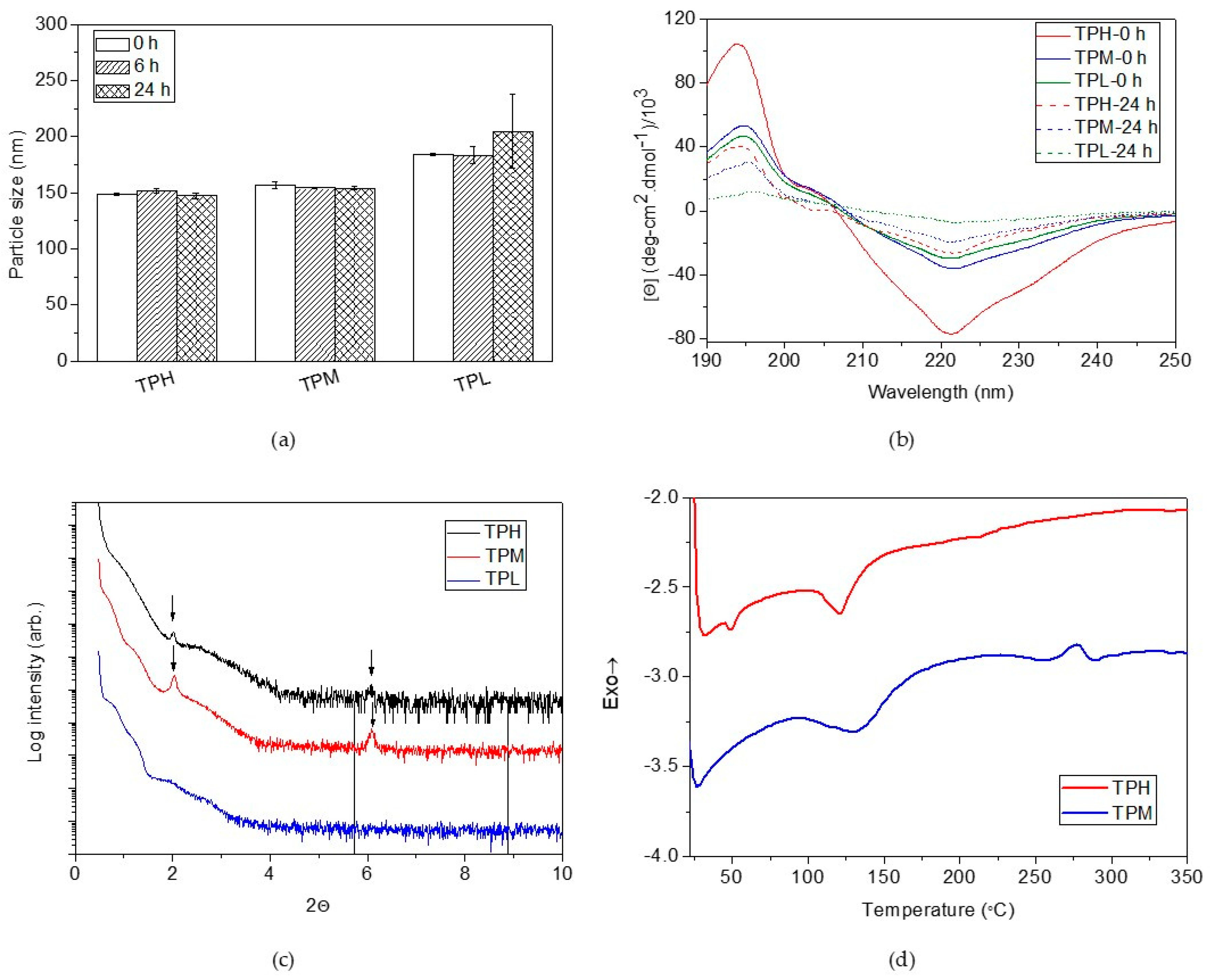
2.1. Preparation and Characterization of pH-Responsive Secondary Structure Contained Mixed Micelles
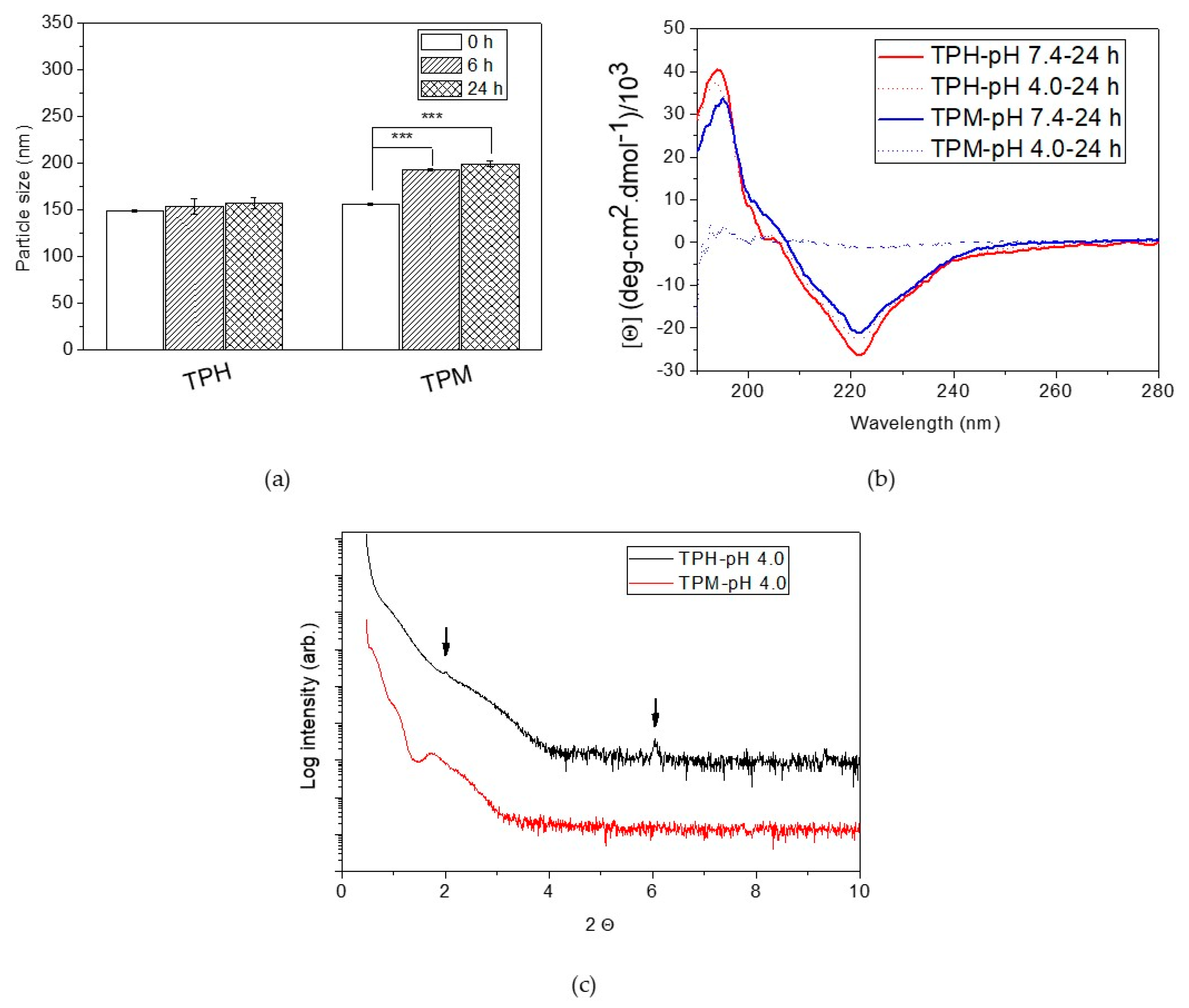
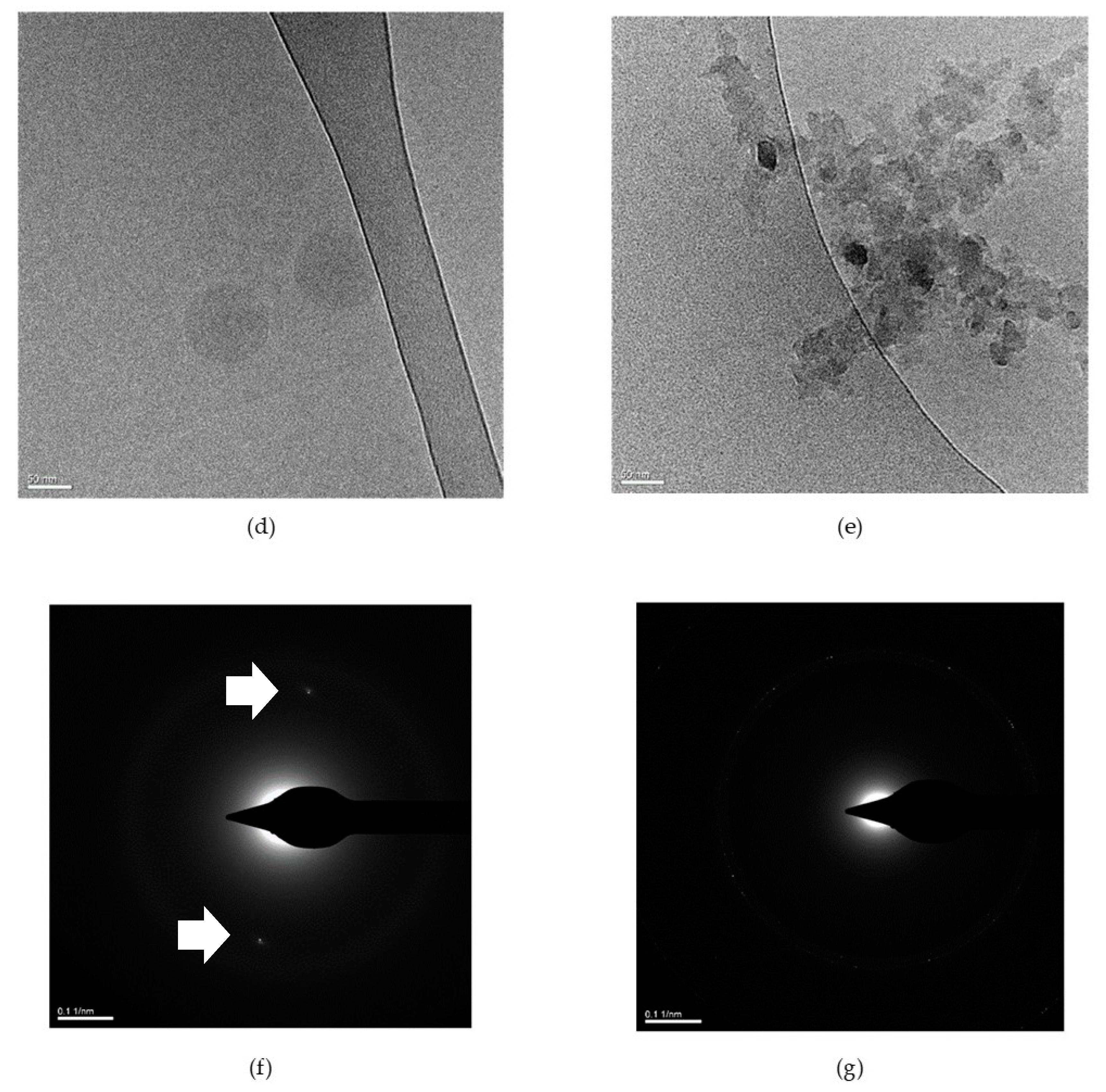
2.2. pH Responsiveness of the pH-Responsive Secondary Structure Contained Mixed Micelles
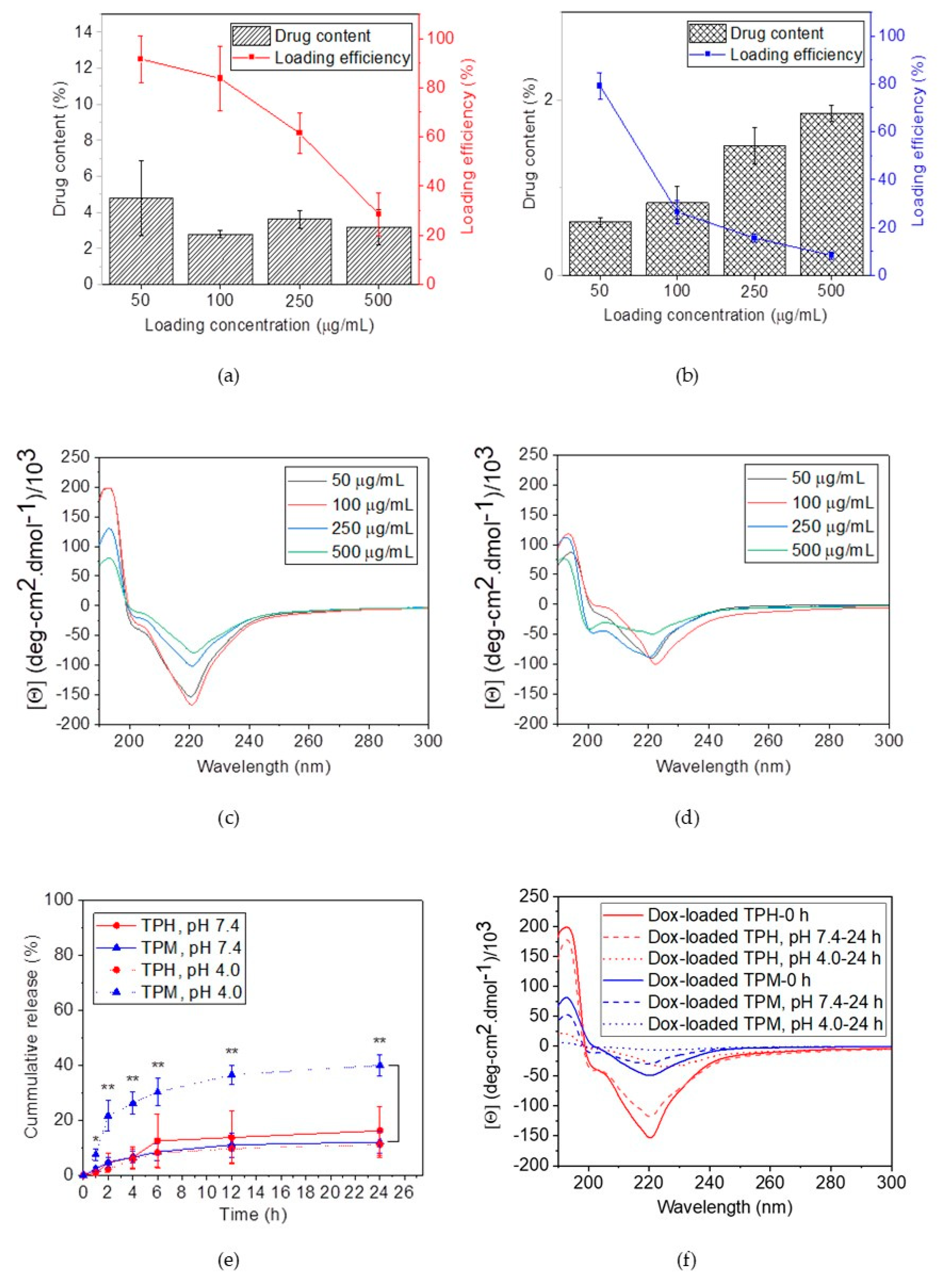
2.3. Doxorubicin-Loaded Secondary Conformation Contained Mixed Micelles Preparation and Drug-Releasing Behaviors
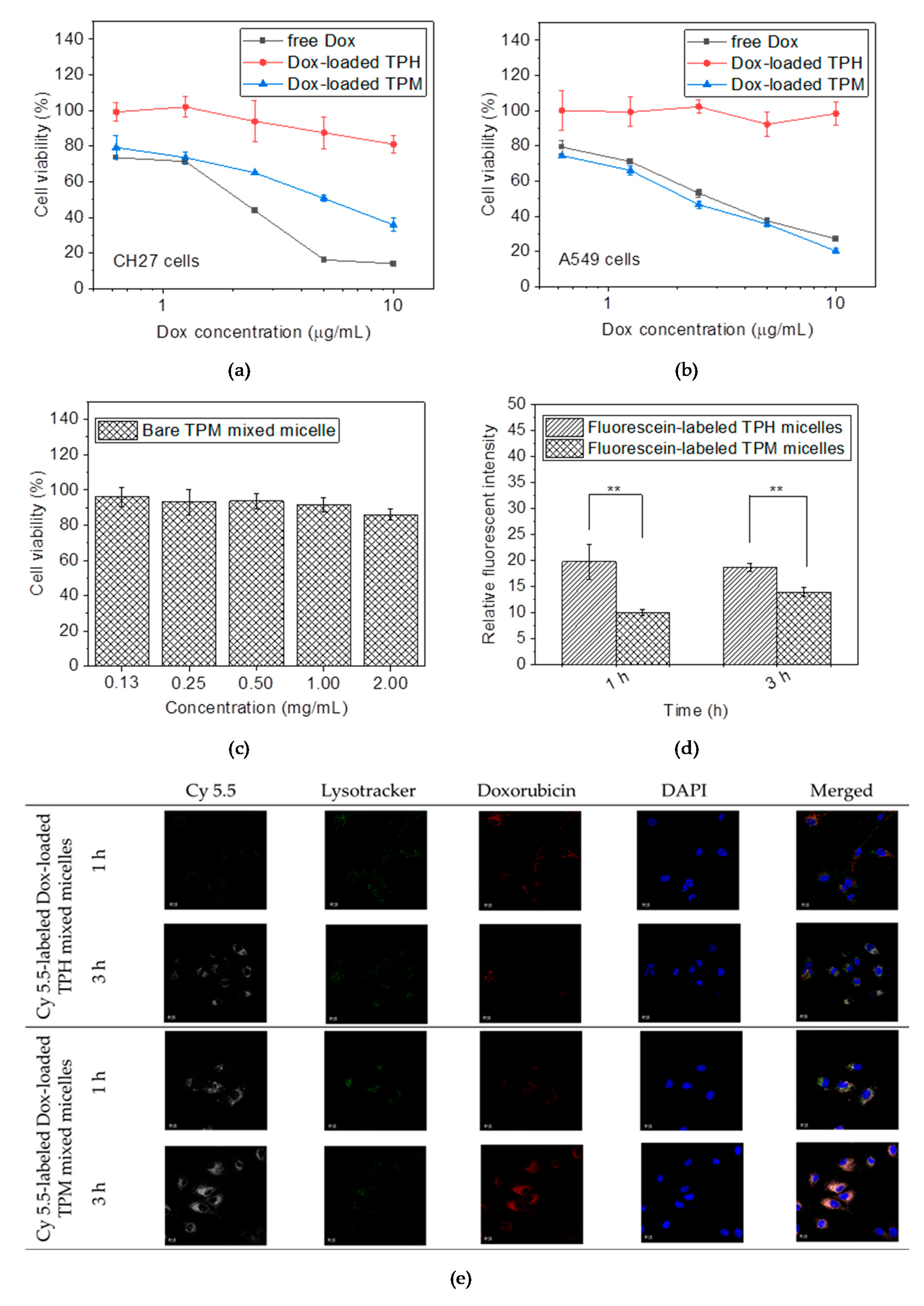
2.4. In Vitro Tests
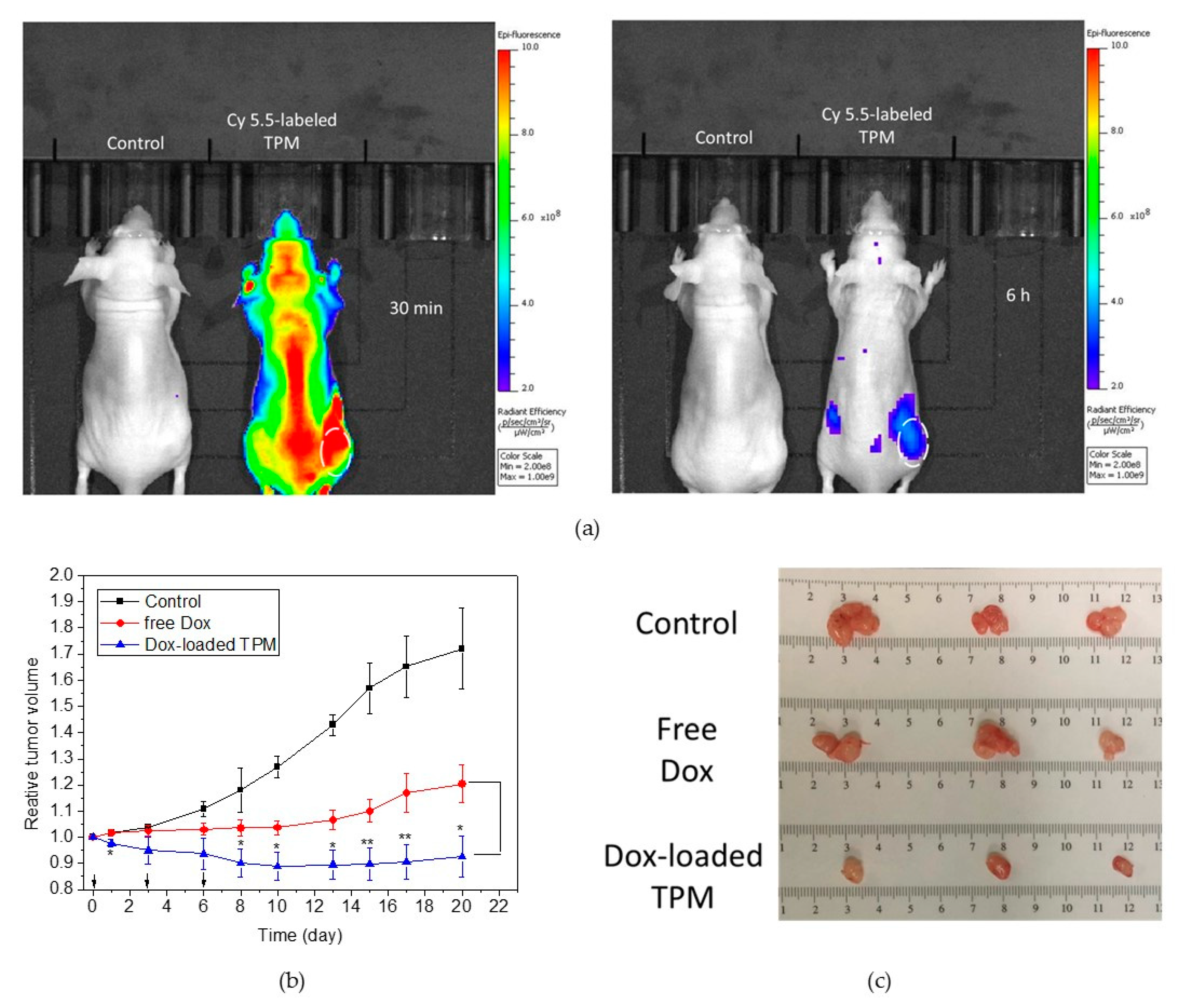
2.5. Tumor Accumulation and In Vivo Antitumor Efficacy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterizations of TPGS/PBLG Polymeric Mixed Micelles
4.3. Stability and pH Responsive Behaviors
4.4. Drug Loading and Releasing Behavior Study
4.5. In Vitro Cytotoxicity Assessment
4.6. Internalization and Intracellular Drug Releasing Observation
4.7. Tumor Deposit and In Vivo Antitumor Efficacy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PBLG | poly-γ-benzyl-l-glutamate |
| TPGS | d-α-tocopherol polyethylene glycol succinate |
| Dox | doxorubicin |
| DMAc | dimethylacetamide |
| DLS | dynamic laser scattering |
| CD spectrum | circular dichroism spectrum |
| DSC | differential scanning calorimetry |
| Tm | melting point |
| XRD | x-ray diffractometer |
| TEM | transmission electron microscopy |
| PTA | phosphotungstic acid |
| GPC | gel permeation chromatogram |
| UV–vis spectrometer | ultraviolet–visible light spectrometer |
| Fluorescein | 5,6-carboxyfluorescein succinimidyl ester |
| CLSM | confocal laser scanning microscopy |
References
- Tian, H.; Deng, C.; Lin, H.; Sun, J.; Deng, M.; Chen, X.; Jing, X. Biodegradable cationic PEG–PEI–PBLG hyperbranched block copolymer: Synthesis and micelle characterization. Biomaterials 2005, 26, 4209–4217. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, W.; Wei, J.; Wang, Y.; Chen, X.; Jing, X.; Zhu, Q. Gene transfection of hyperbranched PEI grafted by hydrophobic amino acid segment PBLG. Biomaterials 2007, 28, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, A.; Jiang, W.; Guan, Z. Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer 2008, 8, 103. [Google Scholar] [CrossRef]
- Vandermeulen, G.W.; Klok, H.A. Peptide/protein hybrid materials: Enhanced control of structure and improved performance through conjugation of biological and synthetic polymers. Macromol. Biosci. 2004, 4, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Floudas, G.; Klok, H.-A.; Schnell, I.; Pakula, T. Self-Assembly and Dynamics of Poly (γ-benzyl-L-glutamate) Peptides. Biomacromolecules 2004, 5, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.; Crane-Robinson, C.; Hartman, P. Effect of polydispersity on the nmr spectra of poly (γ-benzyl-l-glutamate) through the helix→ coil transition. Polymer 1973, 14, 543–548. [Google Scholar] [CrossRef]
- Novotná, P.; Urbanová, M. Vibrational circular dichroism study of solvent-and temperature-induced conformational changes in poly-γ-benzyl-l-glutamate and poly-β-benzyl-l-aspartate. Vib. Spectrosc. 2013, 66, 1–7. [Google Scholar] [CrossRef]
- Itoh, T.; Hatanaka, T.; Ihara, E.; Inoue, K. Helix–coil transformation of poly (γ-benzyl-L-glutamate) with polystyrene attached to the N or C terminus in trifluoroacetic acid–chloroform mixtures. Polym. J. 2012, 44, 189. [Google Scholar] [CrossRef]
- Inomata, K.; Itoh, M.; Nakanishi, E. Helix–Coil Transition and Micellar Structure of Poly (ethylene glycol)-block-Poly [N 5-(2-hydroxyethyl) L-glutamine] in Cyclohexanol/Water Mixed Solvents. Polym. J. 2005, 37, 404. [Google Scholar] [CrossRef][Green Version]
- Kuo, S.-W.; Chen, C.-J. Using hydrogen-bonding interactions to control the peptide secondary structures and miscibility behavior of poly (L-glutamate) s with phenolic resin. Macromolecules 2011, 44, 7315–7326. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chen, C.-J. Functional polystyrene derivatives influence the miscibility and helical peptide secondary structures of poly (γ-benzyl l-glutamate). Macromolecules 2012, 45, 2442–2452. [Google Scholar] [CrossRef]
- Niehoff, A.; Mantion, A.; McAloney, R.; Huber, A.; Falkenhagen, J.; Goh, C.M.; Thünemann, A.F.; Winnik, M.A.; Menzel, H. Elucidation of the structure of poly (γ-benzyl-L-glutamate) nanofibers and gel networks in a helicogenic solvent. Colloid Polym. Sci. 2013, 291, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.; Woo, C.-W. Liquid-crystalline properties and reentrance phenomena in PBLG solutions. Phys. Rev. A 1980, 21, 990. [Google Scholar] [CrossRef]
- Mochida, Y.; Cabral, H.; Miura, Y.; Albertini, F.; Fukushima, S.; Osada, K.; Nishiyama, N.; Kataoka, K. Bundled assembly of helical nanostructures in polymeric micelles loaded with platinum drugs enhancing therapeutic efficiency against pancreatic tumor. ACS Nano 2014, 8, 6724–6738. [Google Scholar] [CrossRef]
- Sallach, R.E.; Wei, M.; Biswas, N.; Conticello, V.P.; Lecommandoux, S.; Dluhy, R.A.; Chaikof, E.L. Micelle density regulated by a reversible switch of protein secondary structure. J. Am. Chem. Soc. 2006, 128, 12014–12019. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R.; Wei, J.; Cheng, C.; Zheng, Y.; Pan, Y.; He, X.; Ding, M.; Tan, H.; Fu, Q. Conformation-directed micelle-to-vesicle transition of cholesterol-decorated polypeptide triggered by oxidation. J. Am. Chem. Soc. 2018, 140, 6604–6610. [Google Scholar] [CrossRef]
- Choi, M.; Choi, D.; Hong, J. Multilayered controlled drug release silk fibroin nanofilm by manipulating secondary structure. Biomacromolecules 2018, 19, 3096–3103. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Mason, A.F.; Buddingh’, B.C.; Williams, D.S.; van Hest, J.C. Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J. Am. Chem. Soc. 2017, 139, 17309–17312. [Google Scholar] [CrossRef]
- McGill, M.; Holland, G.P.; Kaplan, D.L. Experimental methods for characterizing the secondary structure and thermal properties of silk proteins. Macromol. Rapid Commun. 2019, 40, 1800390. [Google Scholar] [CrossRef]
- Ndukwe, I.E.; Wang, X.; Pelczer, I.; Reibarkh, M.; Williamson, R.T.; Liu, Y.; Martin, G.E. PBLG as a versatile liquid crystalline medium for anisotropic NMR data acquisition. Chem. Commun. 2019, 55, 4327–4330. [Google Scholar] [CrossRef] [PubMed]
- Müller-Goymann, C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004, 58, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, W.; Wang, P.; Tian, Q.; Zhang, C.; Wang, C.; Yuan, Z.; Liu, M.; Wan, H.; Tang, H. Glycyrrhetinic acid-modified poly (ethylene glycol)–b-poly (γ-benzyl l-glutamate) micelles for liver targeting therapy. Acta Biomater. 2010, 6, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Sato, T.; Goto, R.; Nagao, Y.; Mitsuishi, M.; Nagano, S.; Matsui, J. In-plane oriented highly ordered lamellar structure formation of poly (N-dodecylacrylamide) induced by humid annealing. RSC Adv. 2017, 7, 6631–6635. [Google Scholar] [CrossRef]
- Wang, Z.; Sheng, R.; Luo, T.; Sun, J.; Cao, A. Synthesis and self-assembly of diblock glycopolypeptide analogues PMAgala-b-PBLG as multifunctional biomaterials for protein recognition, drug delivery and hepatoma cell targeting. Polym. Chem. 2017, 8, 472–484. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Malde, C.; Wang, R. Superoleophobic surface modification for robust membrane distillation performance. J. Membr. Sci. 2017, 541, 162–173. [Google Scholar] [CrossRef]
- Lu, P.-L.; Chen, Y.-C.; Ou, T.-W.; Chen, H.-H.; Tsai, H.-C.; Wen, C.-J.; Lo, C.-L.; Wey, S.-P.; Lin, K.-J.; Yen, T.-C. Multifunctional hollow nanoparticles based on graft-diblock copolymers for doxorubicin delivery. Biomaterials 2011, 32, 2213–2221. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Du, B.; Yu, M.; Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 2018, 3, 358–374. [Google Scholar] [CrossRef]
- Mondal, R.; Ghosh, N.; Paul, B.K.; Mukherjee, S. Triblock-copolymer-assisted mixed-micelle formation results in the refolding of unfolded protein. Langmuir 2017, 34, 896–903. [Google Scholar] [CrossRef]
- Atkinson, J.; Clarke, M.W.; Warnica, J.M.; Boddington, K.F.; Graether, S.P. Structure of an intrinsically disordered stress protein alone and bound to a membrane surface. Biophys. J. 2016, 111, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xiao, C.; Zhao, L.; Cheng, Y.; Ma, L.; Tang, Z.; Zhuang, X.; Chen, X. Poly (L-glutamic acid) grafted with oligo (2-(2-(2-methoxyethoxy) ethoxy) ethyl methacrylate): Thermal phase transition, secondary structure, and self-assembly. J. Polym. Sci. Part A 2011, 49, 2665–2676. [Google Scholar] [CrossRef]
- Funari, S.S.; Nuscher, B.; Rapp, G.; Beyer, K. Detergent-phospholipid mixed micelles with a crystalline phospholipid core. Proc. Natl. Acad. Sci. USA 2001, 98, 8938–8943. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Lee, H.-F.; Huang, W.-J.; Jeong, K.-U.; Chang, F.-C. Solid state and solution self-assembly of helical polypeptides tethered to polyhedral oligomeric silsesquioxanes. Macromolecules 2009, 42, 1619–1626. [Google Scholar] [CrossRef]
- Mao, J.; DeSantis, C.; Bong, D. Small molecule recognition triggers secondary and tertiary interactions in DNA folding and hammerhead ribozyme catalysis. J. Am. Chem. Soc. 2017, 139, 9815–9818. [Google Scholar] [CrossRef]
- Ryan, T.M.; Friedhuber, A.; Lind, M.; Howlett, G.J.; Masters, C.; Roberts, B.R. Small amphipathic molecules modulate secondary structure and amyloid fibril-forming kinetics of Alzheimer disease peptide Aβ1–42. J. Biol. Chem. 2012, 287, 16947–16954. [Google Scholar] [CrossRef]
- Cagel, M.; Bernabeu, E.; Gonzalez, L.; Lagomarsino, E.; Zubillaga, M.; Moretton, M.A.; Chiappetta, D.A. Mixed micelles for encapsulation of doxorubicin with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines versus Doxil®. Biomed. Pharmacother. 2017, 95, 894–903. [Google Scholar] [CrossRef]
- Mu, L.; Elbayoumi, T.; Torchilin, V. Mixed micelles made of poly (ethylene glycol)–phosphatidylethanolamine conjugate and d-α-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int. J. Pharm. 2005, 306, 142–149. [Google Scholar] [CrossRef]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, T.G. Comparative study on sustained release of human growth hormone from semi-crystalline poly (L-lactic acid) and amorphous poly (D, L-lactic-co-glycolic acid) microspheres: Morphological effect on protein release. J. Control. Release 2004, 98, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-C.; Lee, J.; Cho, K. Effects of crystalline microstructure on drug release behavior of poly (ε-caprolactone) microspheres. J. Control. Release 2003, 92, 249–258. [Google Scholar] [CrossRef]
- Seymour, L.W.; Ferry, D.R.; Kerr, D.J.; Rea, D.; Whitlock, M.; Poyner, R.; Boivin, C.; Hesslewood, S.; Twelves, C.; Blackie, R. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int. J. Oncol. 2009, 34, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Johnson, D.H.; Einhorn, L.H.; Schacter, L.P.; Cherng, N.C.; Cohen, H.J.; Crawford, J.; Randolph, J.A.; Goodlow, J.L.; Broun, G.O. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: A phase III trial of the Southeastern Cancer Study Group. J. Clin. Oncol. 1992, 10, 282–291. [Google Scholar] [CrossRef]
- Sliwinska, M.A.; Mosieniak, G.; Wolanin, K.; Babik, A.; Piwocka, K.; Magalska, A.; Szczepanowska, J.; Fronk, J.; Sikora, E. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech. Ageing Dev. 2009, 130, 24–32. [Google Scholar] [CrossRef]
- Gellman, S.H. Foldamers: A manifesto. Acc. Chem. Res. 1998, 31, 173–180. [Google Scholar] [CrossRef]
- Potocky, T.B.; Menon, A.K.; Gellman, S.H. Effects of conformational stability and geometry of guanidinium display on cell entry by β-peptides. J. Am. Chem. Soc. 2005, 127, 3686–3687. [Google Scholar] [CrossRef]
- Wada, S.-I.; Urase, T.; Hasegawa, Y.; Ban, K.; Sudani, A.; Kawai, Y.; Hayashi, J.; Urata, H. Aib-containing peptide analogs: Cellular uptake and utilization in oligonucleotide delivery. Bioorg. Med. Chem. 2014, 22, 6776–6780. [Google Scholar] [CrossRef]
- Oba, M.; Nagano, Y.; Kato, T.; Tanaka, M. Secondary structures and cell-penetrating abilities of arginine-rich peptide foldamers. Sci. Rep. 2019, 9, 1349. [Google Scholar] [CrossRef]
- Noguchi, Y.; Wu, J.; Duncan, R.; Strohalm, J.; Ulbrich, K.; Akaike, T.; Maeda, H. Early phase tumor accumulation of macromolecules: A great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998, 89, 307–314. [Google Scholar] [CrossRef]
- Seymour, L.; Ulbrich, K.; Steyger, P.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br. J. Cancer 1994, 70, 636. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-T.; Cheng, Y.-T.; Lu, C.-Y.; Yen, Y.-W.; Yu, L.-Y.; Yu, K.-S.; Lyu, S.-Y.; Yang, C.-Y.; Lo, C.-L. Polymer–liposome complexes with a functional hydrogen-bond cross-linker for preventing protein adsorption and improving tumor accumulation. Chem. Mater. 2013, 25, 4364–4372. [Google Scholar] [CrossRef]
- Banerjee, K.; Gautam, S.K.; Kshirsagar, P.; Ross, K.A.; Spagnol, G.; Sorgen, P.; Wannemuehler, M.J.; Narasimhan, B.; Solheim, J.C.; Kumar, S. Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells. Genes Cancer 2019, 10, 52. [Google Scholar] [PubMed]

| Code | Composition (wt%) | Size (nm) | PDI | CMC (mg/mL) | |
|---|---|---|---|---|---|
| TPGS | PBLG | ||||
| TPH | 25 | 75 | 148.73 ± 1.27 | 0.09 ± 0.03 | 5.68 × 10−4 |
| TPM | 50 | 50 | 157.03 ± 3.00 | 0.09 ± 0.02 | 3.22 × 10−3 |
| TPL | 75 | 25 | 184.00 ± 0.72 | 0.07 ± 0.04 | 1.29 × 10−3 |
| Code | Hepatic Function | Renal Function | ||
|---|---|---|---|---|
| GOT (U/L) | GPT (U/L) | BUN (mg/dL) | Creatinine (mg/dL) | |
| Control | 216 ± 43 | 91 ± 21 | 32 ± 3 | 0.42 ± 0.03 |
| Free Dox | 226 ± 50 | 95 ± 11 | 31 ± 3 | 0.39 ± 0.06 |
| Dox-loaded TPM micelle | 187 ± 42 | 90 ± 26 | 31 ± 2 | 0.36 ± 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, F.-Y.; Jiang, W.-P.; Lin, X.; Kuo, S.-C.; Huang, G.-J.; Hou, Y.-C.; Chang, C.-S.; Liu, Y.; Chiang, Y.-T. A Novel pH-Tunable Secondary Conformation Containing Mixed Micellar System in Anticancer Treatment. Cancers 2020, 12, 503. https://doi.org/10.3390/cancers12020503
Shih F-Y, Jiang W-P, Lin X, Kuo S-C, Huang G-J, Hou Y-C, Chang C-S, Liu Y, Chiang Y-T. A Novel pH-Tunable Secondary Conformation Containing Mixed Micellar System in Anticancer Treatment. Cancers. 2020; 12(2):503. https://doi.org/10.3390/cancers12020503
Chicago/Turabian StyleShih, Fu-Ying, Wen-Ping Jiang, Xiaojie Lin, Sheng-Chu Kuo, Guan-Jhong Huang, Yu-Chi Hou, Chih-Shiang Chang, Yang Liu, and Yi-Ting Chiang. 2020. "A Novel pH-Tunable Secondary Conformation Containing Mixed Micellar System in Anticancer Treatment" Cancers 12, no. 2: 503. https://doi.org/10.3390/cancers12020503
APA StyleShih, F.-Y., Jiang, W.-P., Lin, X., Kuo, S.-C., Huang, G.-J., Hou, Y.-C., Chang, C.-S., Liu, Y., & Chiang, Y.-T. (2020). A Novel pH-Tunable Secondary Conformation Containing Mixed Micellar System in Anticancer Treatment. Cancers, 12(2), 503. https://doi.org/10.3390/cancers12020503