Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Birinapant Is Synergistic with Specific Classes of Drugs
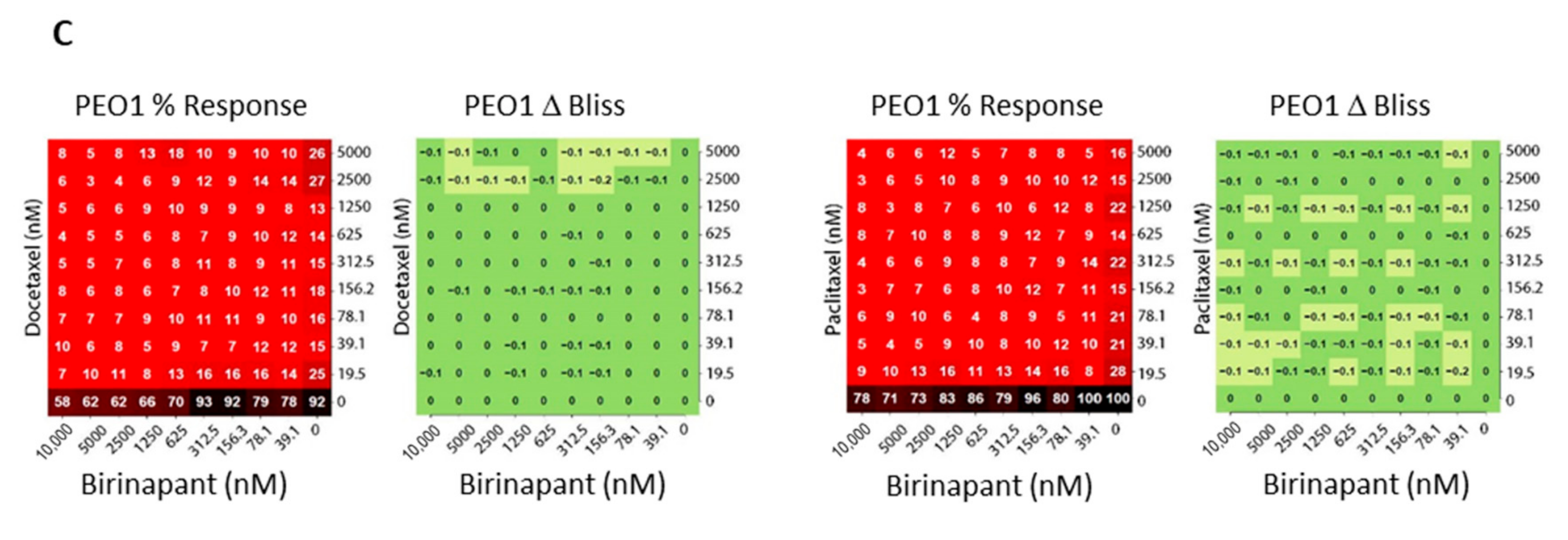
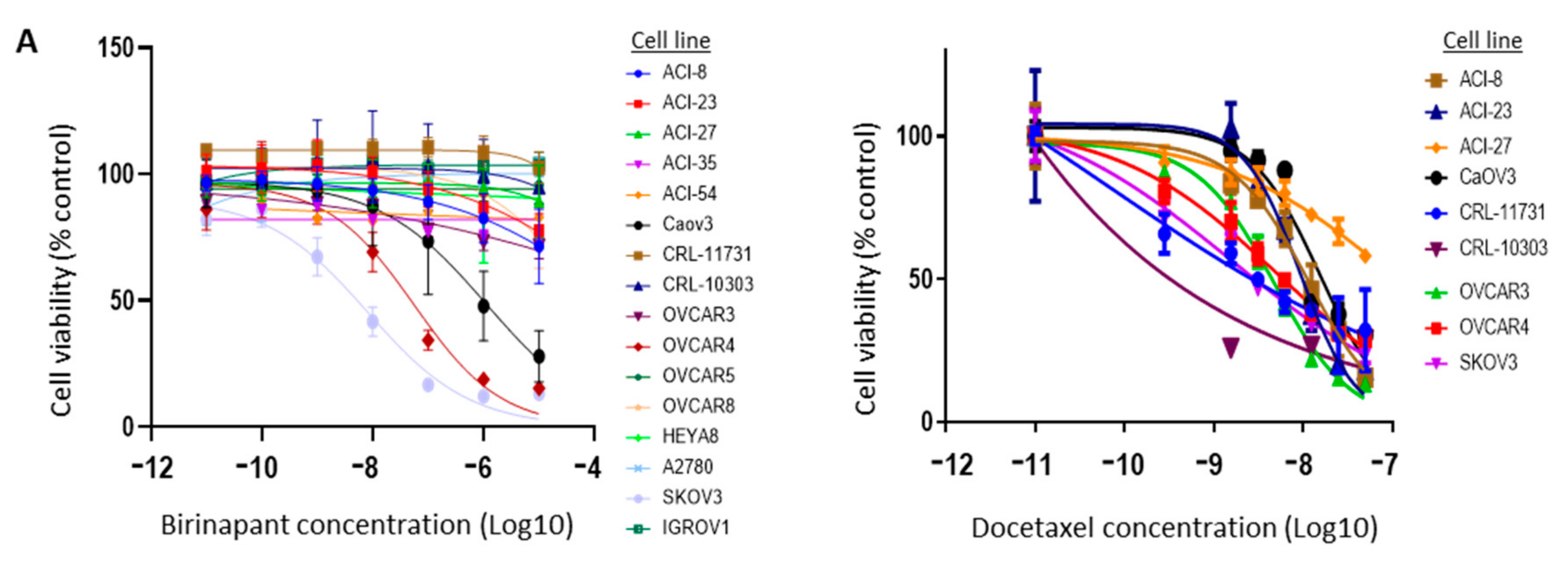
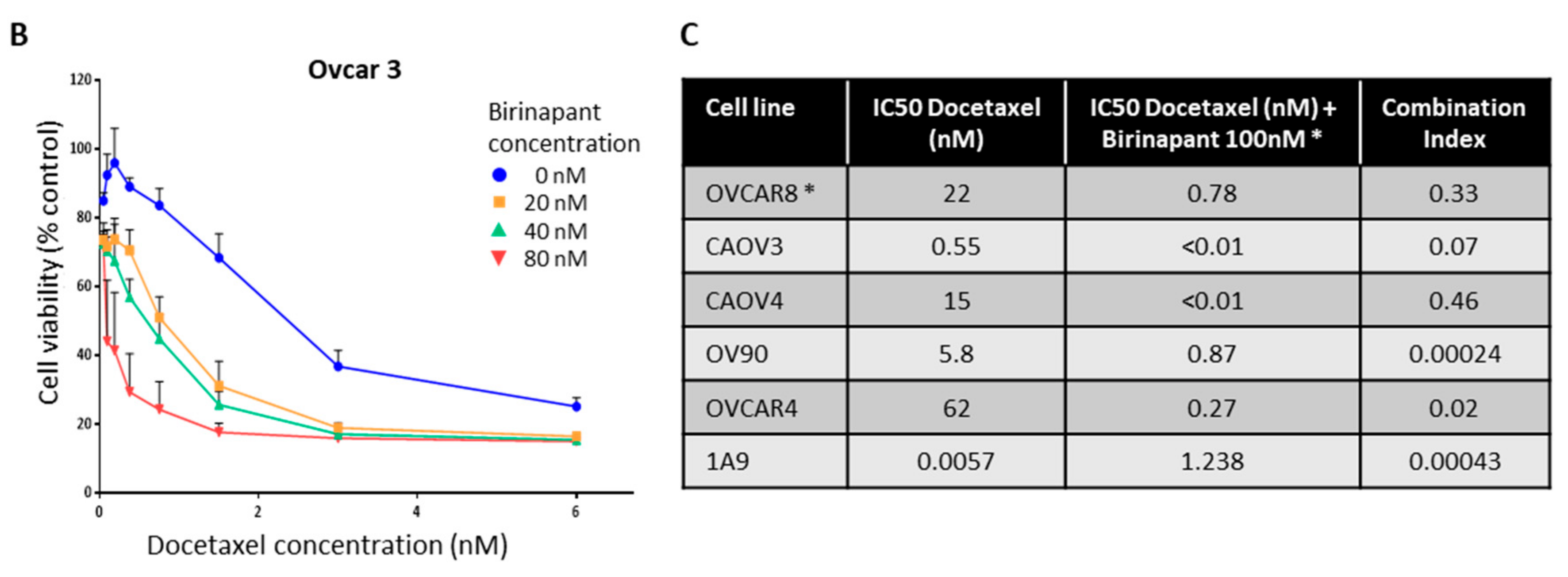
2.2. Birinapant Is Synergistic in Combination with Docetaxel in Killing Ovarian Cancer Cells
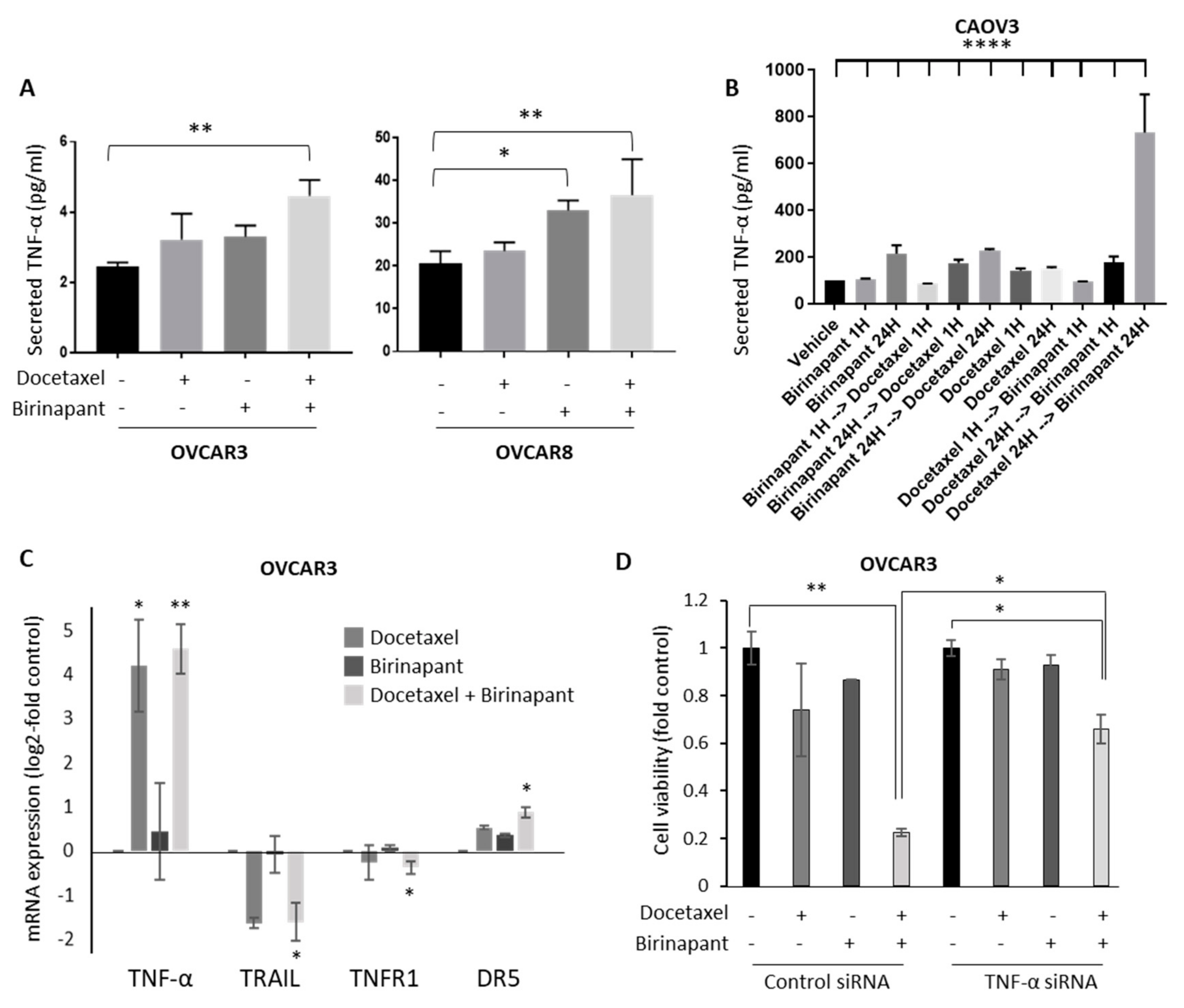
2.3. Docetaxel-Induced TNF-α Production in Tumor Cells Is Important for Synergy with Birinapant
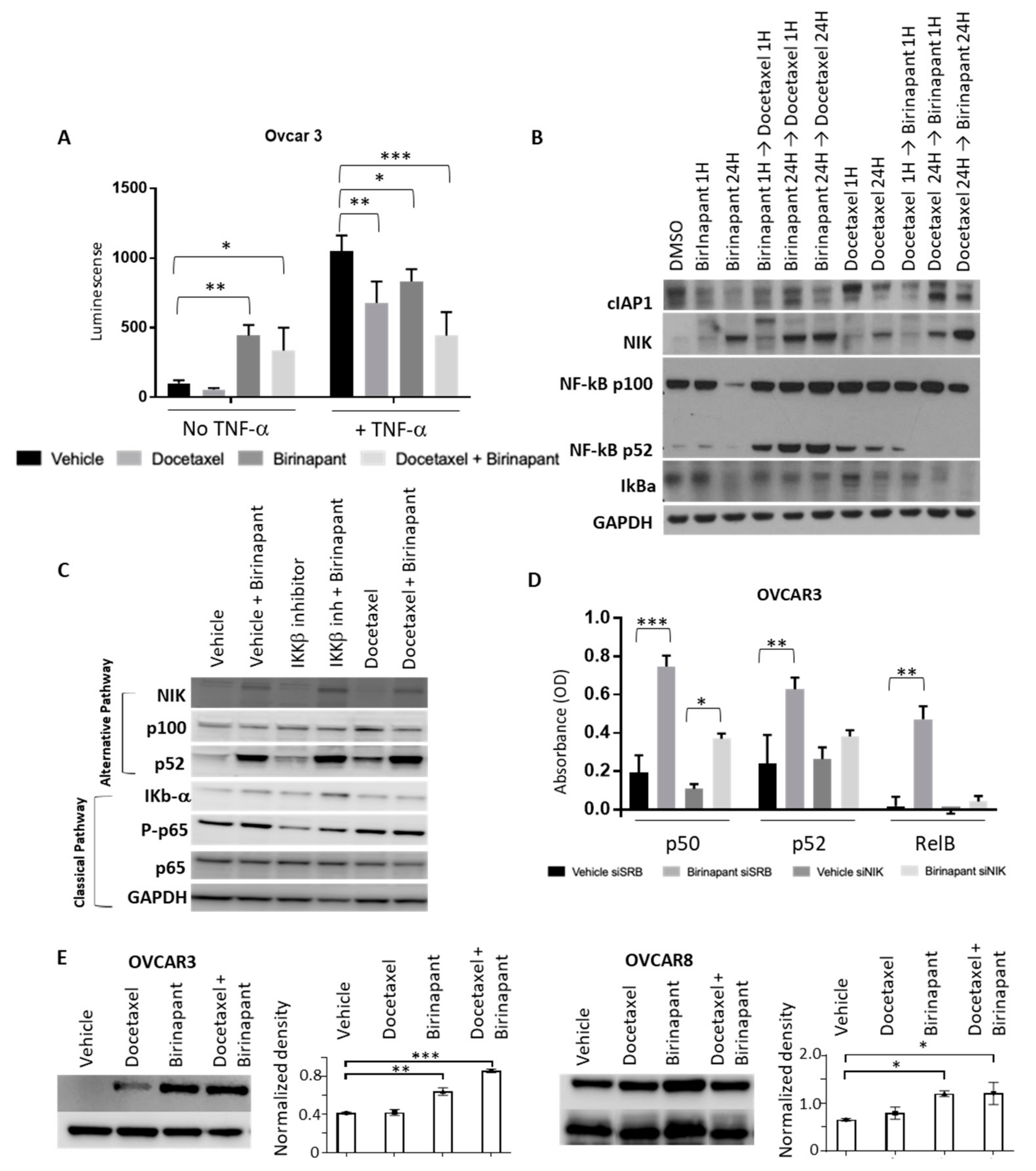
2.4. Birinapant Suppresses the Classical NF-kB Pathway and Enhances the Alternative NF-kB Pathway
2.5. Birinapant Stabilizes Microtubules to Enhance Docetaxel Activity
2.6. Docetaxel Achieves Better Cancer Control in Combination with Birinapant In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drug Screen
4.3. XTT Viability Assay
4.4. Caspase Activity Assay
4.5. NF-kB Reporter Assay
4.6. TransAM Assay
4.7. Western Blot
4.8. Cytokine Assay
4.9. In Vivo Survival Studies
4.10. Simple Western
4.11. RNAi Experiments
4.12. Quantitative Real-Time PCR
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Binju, M.; Amaya-Padilla, M.A.; Wan, G.; Gunosewoyo, H.; Suryo Rahmanto, Y.; Yu, Y. Therapeutic Inducers of Apoptosis in Ovarian Cancer. Cancers 2019, 11, 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Wang, X.Y.; Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Potency and Selectivity of SMAC/DIABLO Mimetics in Solid Tumor Therapy. Cells 2020, 9, 1012. [Google Scholar] [CrossRef] [Green Version]
- Hunter, A.M.; LaCasse, E.C.; Korneluk, R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007, 12, 1543–1568. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Busselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Fulda, S. Smac Mimetics to Therapeutically Target IAP Proteins in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 157–169. [Google Scholar] [CrossRef]
- Benetatos, C.A.; Mitsuuchi, Y.; Burns, J.M.; Neiman, E.M.; Condon, S.M.; Yu, G.; Seipel, M.E.; Kapoor, G.S.; Laporte, M.G.; Rippin, S.R.; et al. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Mol. Cancer Ther. 2014, 13, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Noonan, A.M.; Bunch, K.P.; Chen, J.Q.; Herrmann, M.A.; Lee, J.M.; Kohn, E.C.; O’Sullivan, C.C.; Jordan, E.; Houston, N.; Takebe, N.; et al. Pharmacodynamic markers and clinical results from the phase 2 study of the SMAC mimetic birinapant in women with relapsed platinum-resistant or -refractory epithelial ovarian cancer. Cancer 2016, 122, 588–597. [Google Scholar] [CrossRef]
- Sagher, E.; Hernandez, L.; Heywood, C.; Pauly, G.T.; Young, M.R.; Schneider, J.; Colburn, N.H.; Annunziata, C.M. The small molecule NSC676914A is cytotoxic and differentially affects NFkappaB signaling in ovarian cancer cells and HEK293 cells. Cancer Cell Int. 2014, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escarcega, R.O.; Fuentes-Alexandro, S.; Garcia-Carrasco, M.; Gatica, A.; Zamora, A. The transcription factor nuclear factor-kappa B and cancer. Clin. Oncol. 2007, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Morgan, M.; Kim, D.G.; Lee, J.Y.; Bai, L.; Lin, Y.; Liu, Z.G.; Kim, Y.S. TNFalpha induced noncanonical NF-kappaB activation is attenuated by RIP1 through stabilization of TRAF2. J. Cell Sci. 2011, 124, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piura, B.; Medina, L.; Rabinovich, A.; Dyomin, V.; Levy, R.S.; Huleihel, M. Distinct expression and localization of TNF system in ovarian carcinoma tissues: Possible involvement of TNF-alpha in morphological changes of ovarian cancerous cells. Anticancer Res. 2014, 34, 745–752. [Google Scholar] [PubMed]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-alpha expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Janzen, D.M.; Tiourin, E.; Salehi, J.A.; Paik, D.Y.; Lu, J.; Pellegrini, M.; Memarzadeh, S. An apoptosis-enhancing drug overcomes platinum resistance in a tumour-initiating subpopulation of ovarian cancer. Nat. Commun. 2015, 6, 7956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La, V.; Fujikawa, R.; Janzen, D.M.; Nunez, M.; Bainvoll, L.; Hwang, L.; Faull, K.; Lawson, G.; Memarzadeh, S. Birinapant sensitizes platinum-resistant carcinomas with high levels of cIAP to carboplatin therapy. NPJ Precis Oncol. 2017, 1, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mathews Griner, L.A.; Guha, R.; Shinn, P.; Young, R.M.; Keller, J.M.; Liu, D.; Goldlust, I.S.; Yasgar, A.; McKnight, C.; Boxer, M.B.; et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc. Natl. Acad. Sci. USA 2014, 111, 2349–2354. [Google Scholar] [CrossRef] [Green Version]
- Amaravadi, R.K.; Senzer, N.N.; Martin, L.P.; Schilder, R.J.; LoRusso, P.; Papadopoulos, K.P.; Weng, D.E.; Graham, M.; Adjei, A.A. A phase I study of birinapant (TL32711) combined with multiple chemotherapies evaluating tolerability and clinical activity for solid tumor patients. J. Clin. Oncol. 2013, 31, 2504. [Google Scholar] [CrossRef]
- Mott, B.T.; Eastman, R.T.; Guha, R.; Sherlach, K.S.; Siriwardana, A.; Shinn, P.; McKnight, C.; Michael, S.; Lacerda-Queiroz, N.; Patel, P.R.; et al. High-throughput matrix screening identifies synergistic and antagonistic antimalarial drug combinations. Sci. Rep. 2015, 5, 13891. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, H.; Shinn, P.; Ngan, D.; Ye, L.; Thakur, A.; Grewal, G.; Zhao, T.; Southall, N.; Hall, M.D.; et al. The NCATS Pharmaceutical Collection: A 10-year update. Drug Discov. Today 2019, 24, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Guha, R.; Mathews Griner, L.A.; Keller, J.M.; Zhang, X.; Fitzgerald, D.; Antignani, A.; Pastan, I.; Thomas, C.J.; Ferrer, M. Ranking Differential Drug Activities from Dose-Response Synthetic Lethality Screens. J. Biomol. Screen 2016, 21, 942–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.L.; Duska, L.R.; Ramirez, P.T.; Heymach, J.V.; Kamat, A.A.; Modesitt, S.C.; Schmeler, K.M.; Iyer, R.B.; Garcia, M.E.; Miller, D.L.; et al. Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011, 12, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Eytan, D.F.; Snow, G.E.; Carlson, S.G.; Schiltz, S.; Chen, Z.; Van Waes, C. Combination effects of SMAC mimetic birinapant with TNFalpha, TRAIL, and docetaxel in preclinical models of HNSCC. Laryngoscope 2015, 125, E118–E124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Kanno, Y.; Sakurai, D.; Hase, H.; Kojima, H.; Kobata, T. TACI induces cIAP1-mediated ubiquitination of NIK by TRAF2 and TANK to limit non-canonical NF-kappaB signaling. J. Recept Signal Transduct. Res. 2010, 30, 121–132. [Google Scholar] [CrossRef]
- Yang, H.; Mao, W.; Rodriguez-Aguayo, C.; Mangala, L.S.; Bartholomeusz, G.; Iles, L.R.; Jennings, N.B.; Ahmed, A.A.; Sood, A.K.; Lopez-Berestein, G.; et al. Paclitaxel Sensitivity of Ovarian Cancer Can be Enhanced by Knocking Down Pairs of Kinases that Regulate MAP4 Phosphorylation and Microtubule Stability. Clin. Cancer Res. 2018, 24, 5072–5084. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Wang, X.; Lu, Z.; Goldsmith, J.; Le, X.F.; Grandjean, G.; Bartholomeusz, G.; Broom, B.; Bast, R.C., Jr. Modulating microtubule stability enhances the cytotoxic response of cancer cells to Paclitaxel. Cancer Res. 2011, 71, 5806–5817. [Google Scholar] [CrossRef] [Green Version]
- Kaye, S.B.; Piccart, M.; Aapro, M.; Francis, P.; Kavanagh, J. Phase II trials of docetaxel (Taxotere) in advanced ovarian cancer--an updated overview. Eur. J. Cancer 1997, 33, 2167–2170. [Google Scholar] [CrossRef]
- Vince, J.E.; Wong, W.W.L.; Khan, N.; Feltham, R.; Chau, D.; Ahmed, A.U.; Benetatos, C.A.; Chunduru, S.K.; Condon, S.M.; McKinlay, M.; et al. IAP antagonists target cIAP1 to induce TNF alpha- dependent apoptosis. Cell 2007, 131, 682–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, S.L.; Wang, L.; Yalcin-Chin, A.; Li, L.; Peyton, M.; Minna, J.; Harran, P.; Wang, X. Autocrine TNF alpha signaling renders human cancer cells susceptible to smac-mimetic-induced apoptosis. Cancer Cell 2007, 12, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; McEachern, D.; Sun, H.; Bai, L.; Peng, Y.; Qiu, S.; Miller, R.; Liao, J.; Yi, H.; Liu, M.; et al. Therapeutic potential and molecular mechanism of a novel, potent, nonpeptide, Smac mimetic SM-164 in combination with TRAIL for cancer treatment. Mol. Cancer Ther. 2011, 10, 902–914. [Google Scholar] [CrossRef] [Green Version]
- Perimenis, P.; Galaris, A.; Voulgari, A.; Prassa, M.; Pintzas, A. IAP antagonists Birinapant and AT-406 efficiently synergise with either TRAIL, BRAF, or BCL-2 inhibitors to sensitise BRAFV600E colorectal tumour cells to apoptosis. BMC Cancer 2016, 16, 624–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalaoui, N.; Merino, D.; Giner, G.; Vaillant, F.; Chau, D.; Liu, L.; Kratina, T.; Pal, B.; Whittle, J.R.; Etemadi, N.; et al. Targeting triple-negative breast cancers with the Smac-mimetic birinapant. Cell Death Differ. 2020, 27, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Lalaoui, N.; Freeman, A.J.; Ramsbottom, K.M.; Silke, J.; Oliaro, J. PD-L1 and IAPs co-operate to protect tumors from cytotoxic lymphocyte-derived TNF. Cell Death Differ. 2017, 24, 1705–1716. [Google Scholar] [CrossRef] [Green Version]
- Beug, S.T.; Beauregard, C.E.; Healy, C.; Sanda, T.; St-Jean, M.; Chabot, J.; Walker, D.E.; Mohan, A.; Earl, N.; Lun, X.; et al. Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Allen, C.T.; Tran, L.; Patel, P.; Park, S.J.; Chen, Z.; Van Waes, C.; Schmitt, N.C. Antagonist of cIAP1/2 and XIAP enhances anti-tumor immunity when combined with radiation and PD-1 blockade in a syngeneic model of head and neck cancer. Oncoimmunology 2018, 7. [Google Scholar] [CrossRef]
- Yu, Y.; Gaillard, S.; Phillip, J.M.; Huang, T.C.; Pinto, S.M.; Tessarollo, N.G.; Zhang, Z.; Pandey, A.; Wirtz, D.; Ayhan, A.; et al. Inhibition of Spleen Tyrosine Kinase Potentiates Paclitaxel-Induced Cytotoxicity in Ovarian Cancer Cells by Stabilizing Microtubules. Cancer Cell 2015, 28, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Seigner, J.; Basilio, J.; Resch, U.; de Martin, R. CD40L and TNF both activate the classical NF-kappaB pathway, which is not required for the CD40L induced alternative pathway in endothelial cells. Biochem. Biophys. Res. Commun. 2018, 495, 1389–1394. [Google Scholar] [CrossRef]
- Workman, L.M.; Habelhah, H. TNFR1 signaling kinetics: Spatiotemporal control of three phases of IKK activation by posttranslational modification. Cell Signal 2013, 25, 1654–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium Formazan Assay for Cell-Growth and Drug Sensitivity in Culture Using Human and Other Tumor-Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Maracle, C.X.; Kucharzewska, P.; Helder, B.; van der Horst, C.; Correa de Sampaio, P.; Noort, A.R.; van Zoest, K.; Griffioen, A.W.; Olsson, H.; Tas, S.W. Targeting non-canonical nuclear factor-kappaB signalling attenuates neovascularization in a novel 3D model of rheumatoid arthritis synovial angiogenesis. Rheumatology 2017, 56, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noonan, A.M.; Cousins, A.; Anderson, D.; Zeligs, K.P.; Bunch, K.; Hernandez, L.; Shibuya, Y.; Goldlust, I.S.; Guha, R.; Ferrer, M.; et al. Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer. Cancers 2020, 12, 3784. https://doi.org/10.3390/cancers12123784
Noonan AM, Cousins A, Anderson D, Zeligs KP, Bunch K, Hernandez L, Shibuya Y, Goldlust IS, Guha R, Ferrer M, et al. Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer. Cancers. 2020; 12(12):3784. https://doi.org/10.3390/cancers12123784
Chicago/Turabian StyleNoonan, Anne M., Amanda Cousins, David Anderson, Kristen P. Zeligs, Kristen Bunch, Lidia Hernandez, Yusuke Shibuya, Ian S. Goldlust, Rajarshi Guha, Marc Ferrer, and et al. 2020. "Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer" Cancers 12, no. 12: 3784. https://doi.org/10.3390/cancers12123784
APA StyleNoonan, A. M., Cousins, A., Anderson, D., Zeligs, K. P., Bunch, K., Hernandez, L., Shibuya, Y., Goldlust, I. S., Guha, R., Ferrer, M., Thomas, C. J., & Annunziata, C. M. (2020). Matrix Drug Screen Identifies Synergistic Drug Combinations to Augment SMAC Mimetic Activity in Ovarian Cancer. Cancers, 12(12), 3784. https://doi.org/10.3390/cancers12123784




