Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
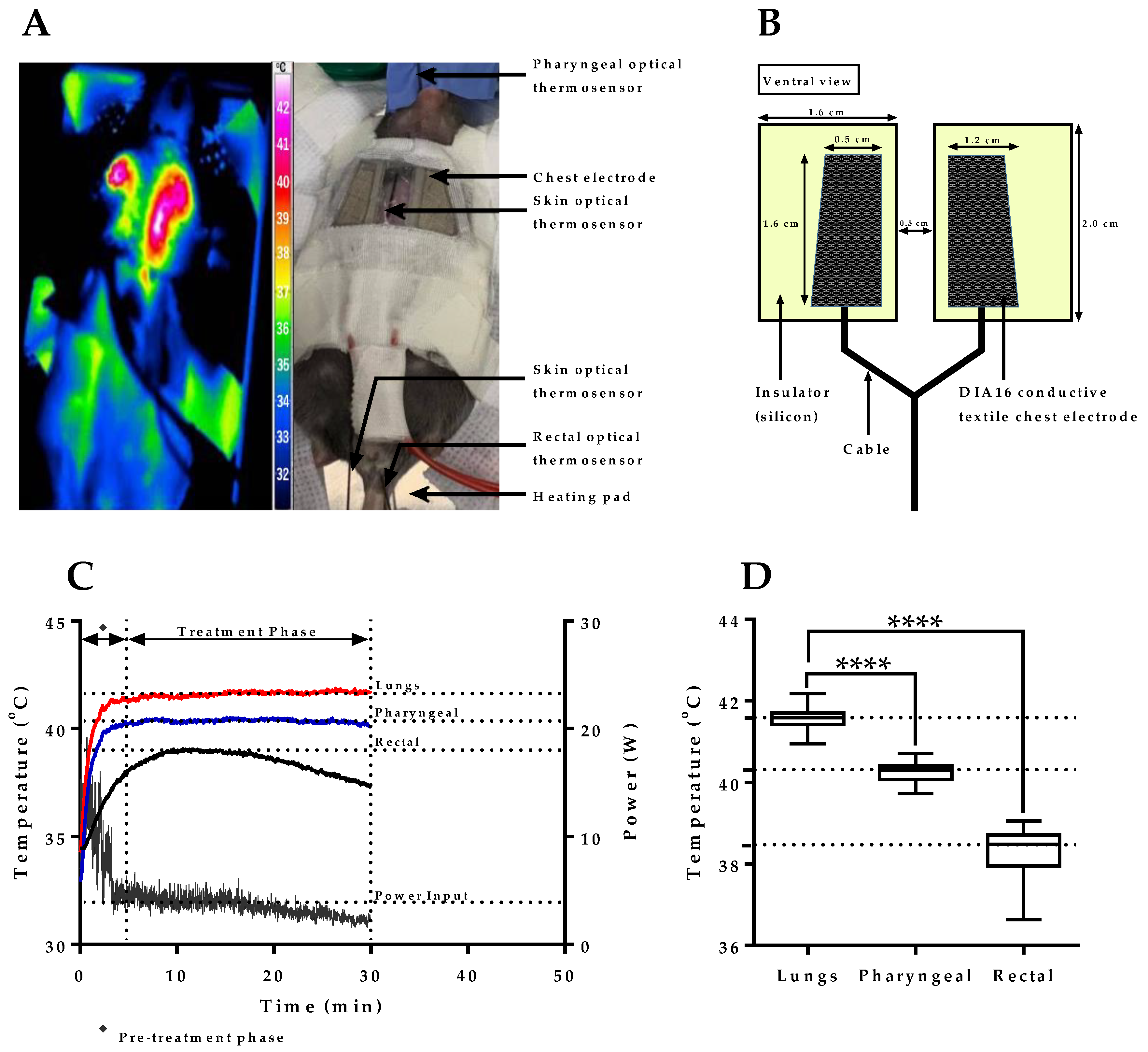
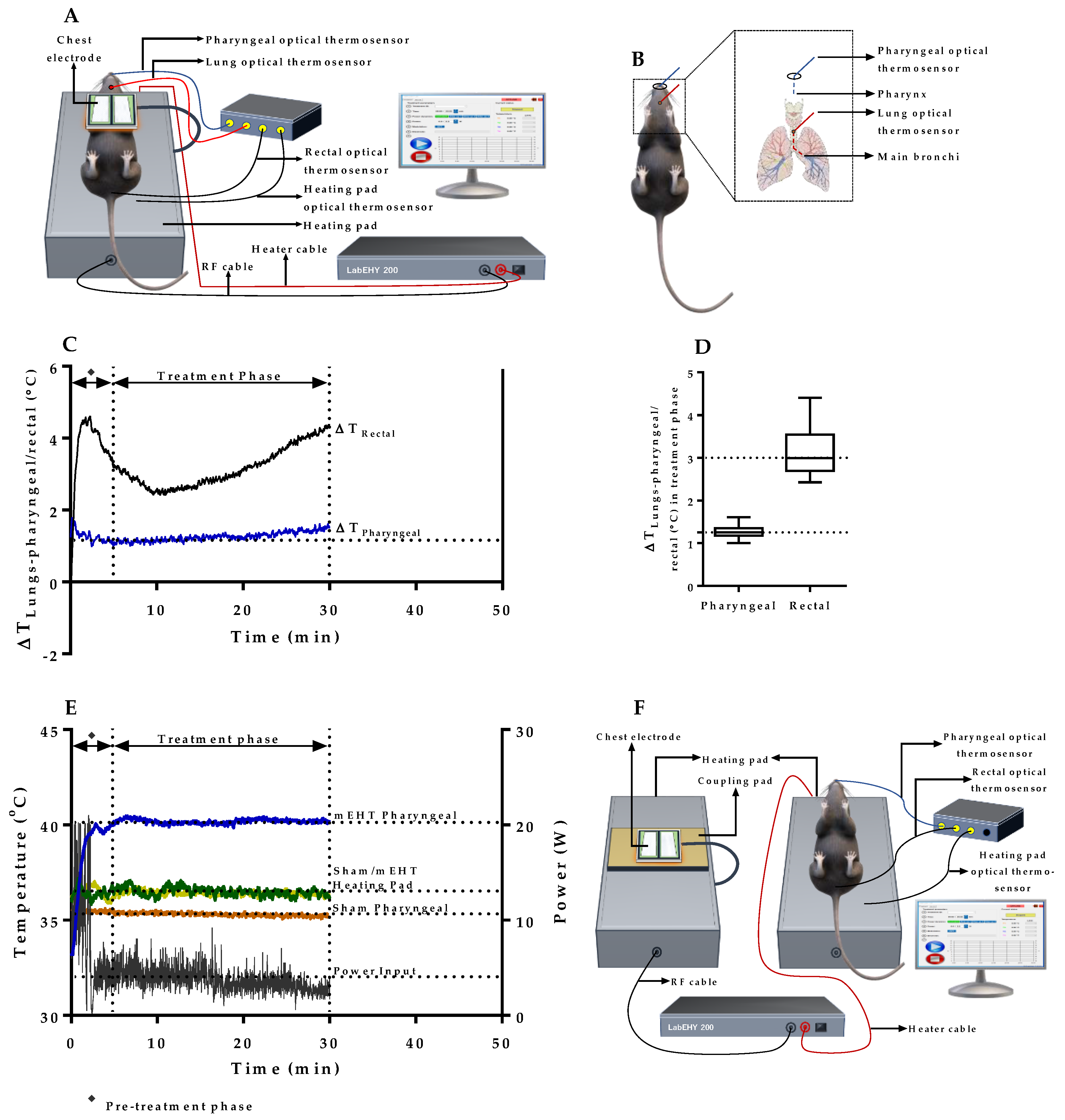
2.1. mEHT Induced Localized Increase in Lung Temperature
2.2. Verification of Pharyngeal Temperature Measurement as a Minimally Invasive Method of Estimating Lung Temperature during mEHT Treatment of Lung Tumors
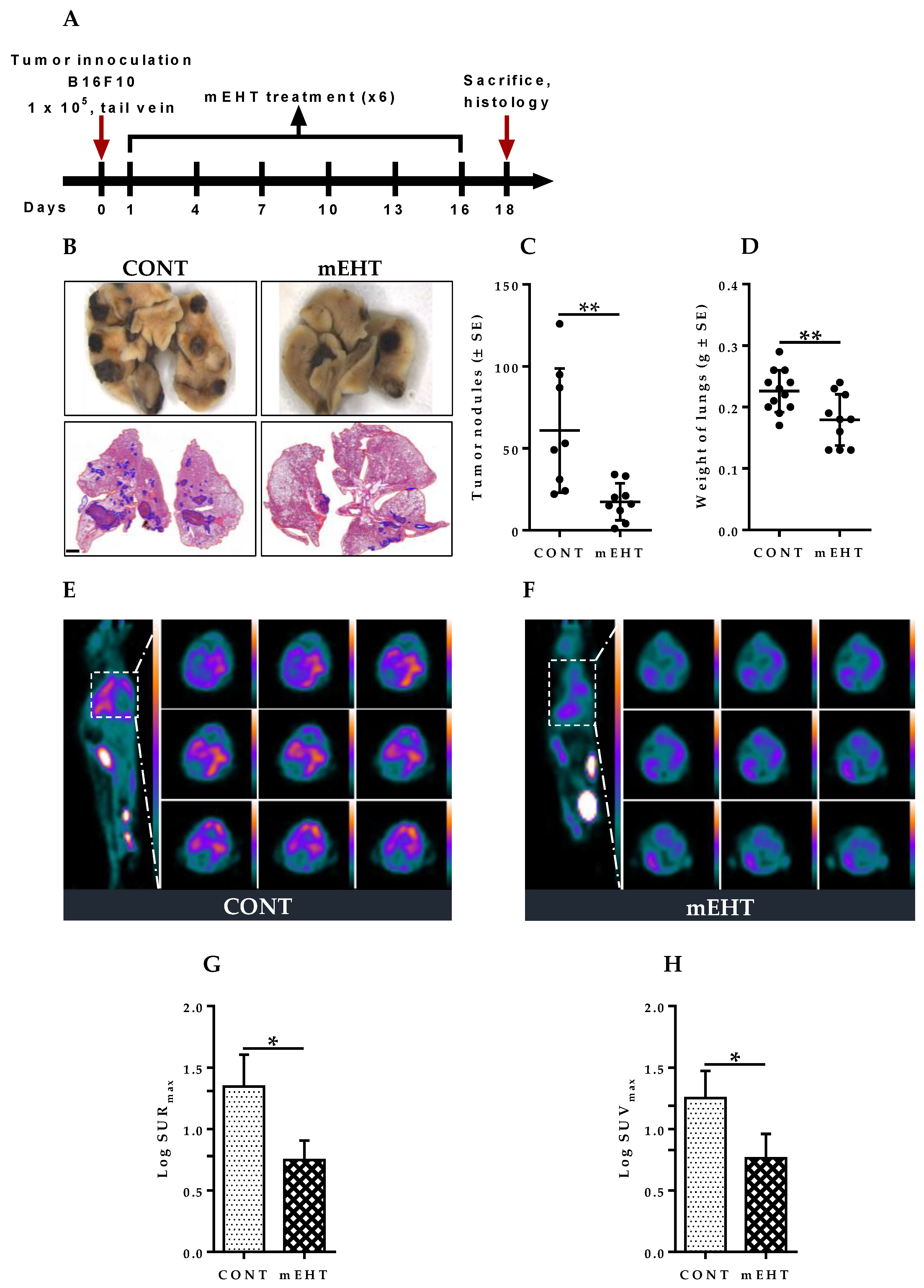
2.3. mEHT Induced Reduction of Metastatic Tumor Burden in Mice Lungs
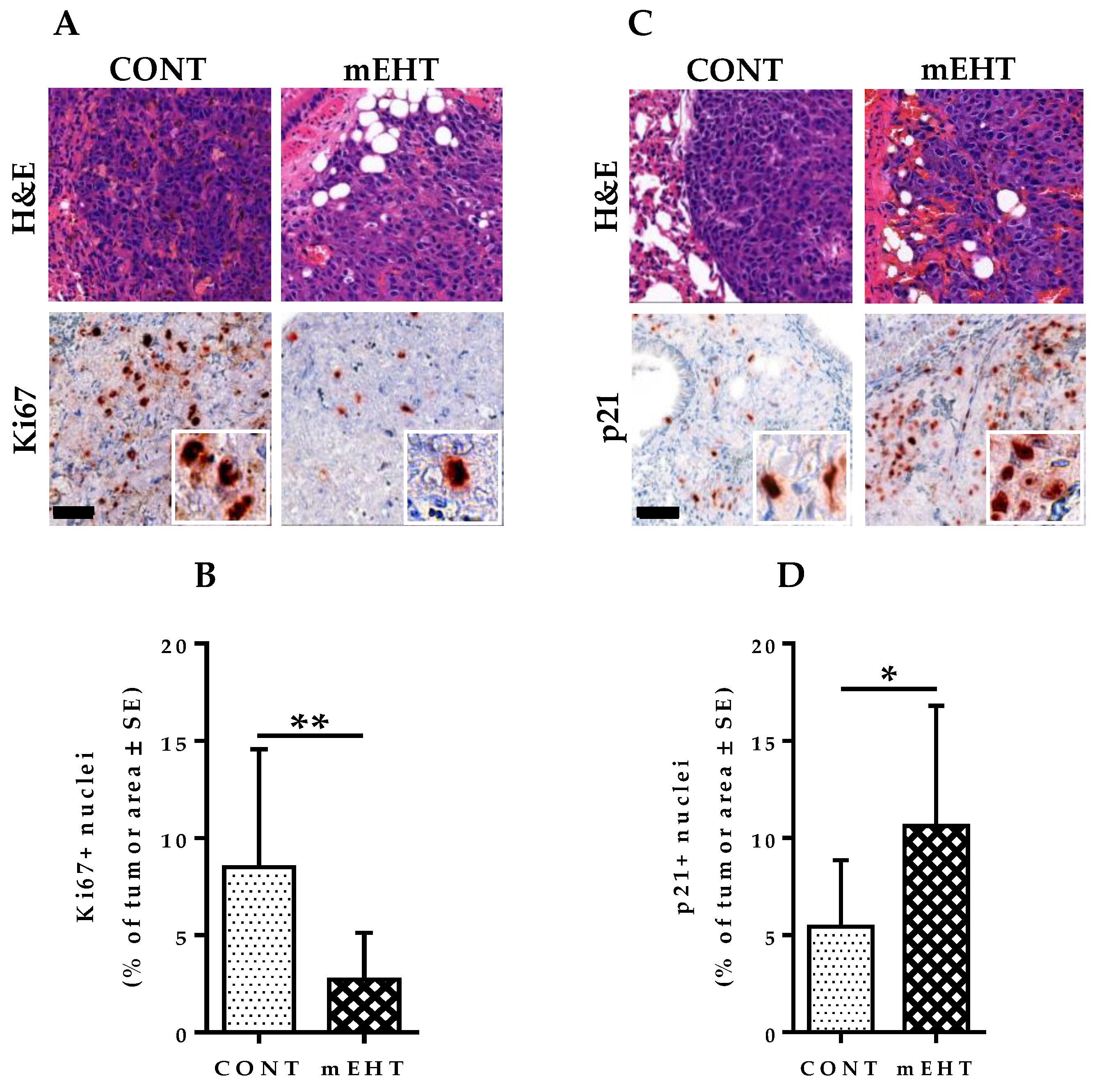
2.4. mEHT Suppresses Melanoma Proliferation in the Lungs by Tumor Growth Inhibition without Direct Treatment-Induced Tumor Necrosis
2.5. mEHT Induced Reduction in Ki67 Expression in B16F10 Melanoma Cells
2.6. mEHT Induced Cell Cycle Arrest in B16F10 Melanoma Cells
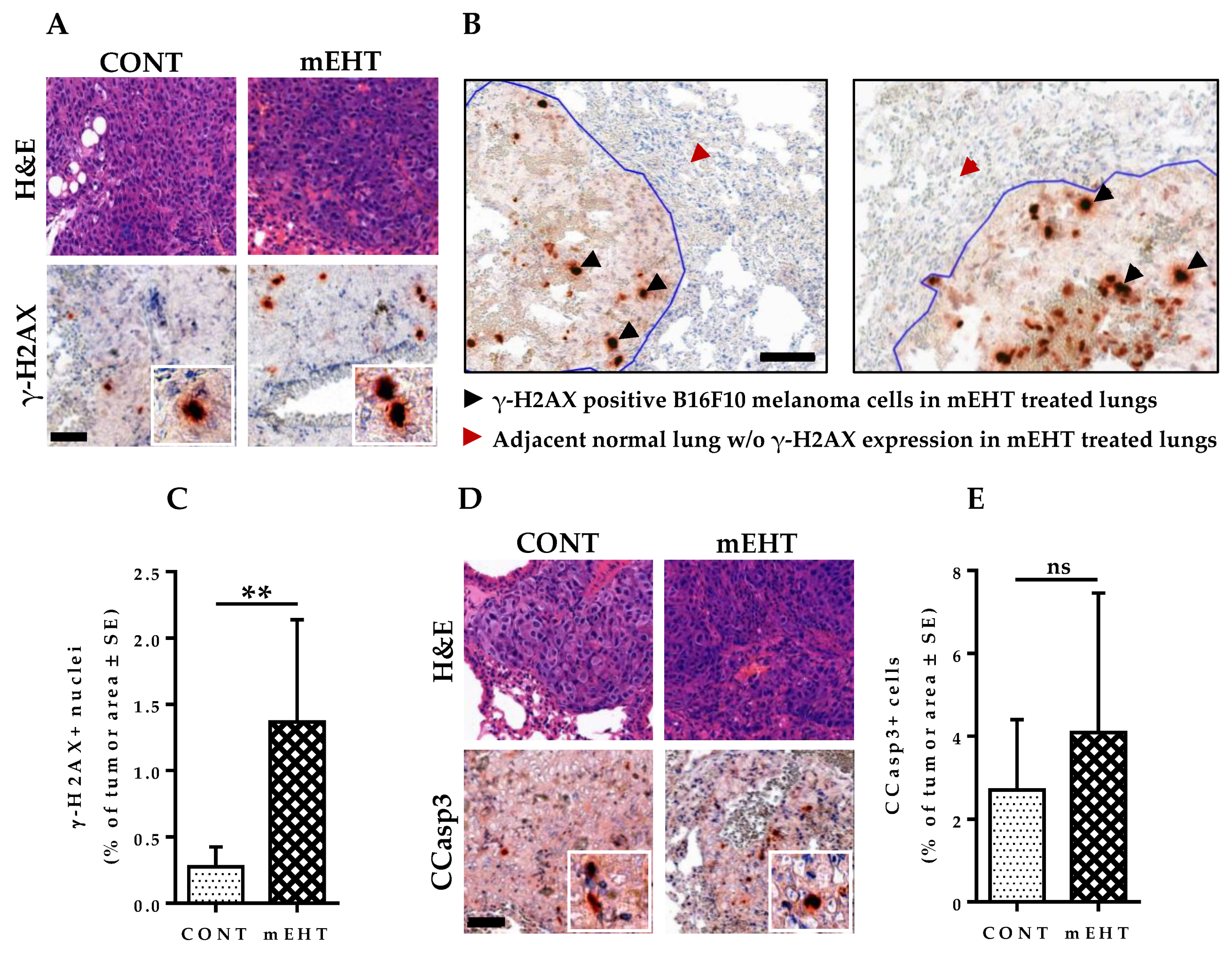
2.7. mEHT Induced DNA Damage Response in Proliferating B16F10 Melanoma Cells without Significant Apoptosis
2.8. mEHT Induced Cellular Death Is Dose-Dependent in B16F10 Melanoma Cells
2.9. T-Lymphocytes and Antigen-Presenting Cells Are Increased in mEHT-Treated Lungs and Tumors
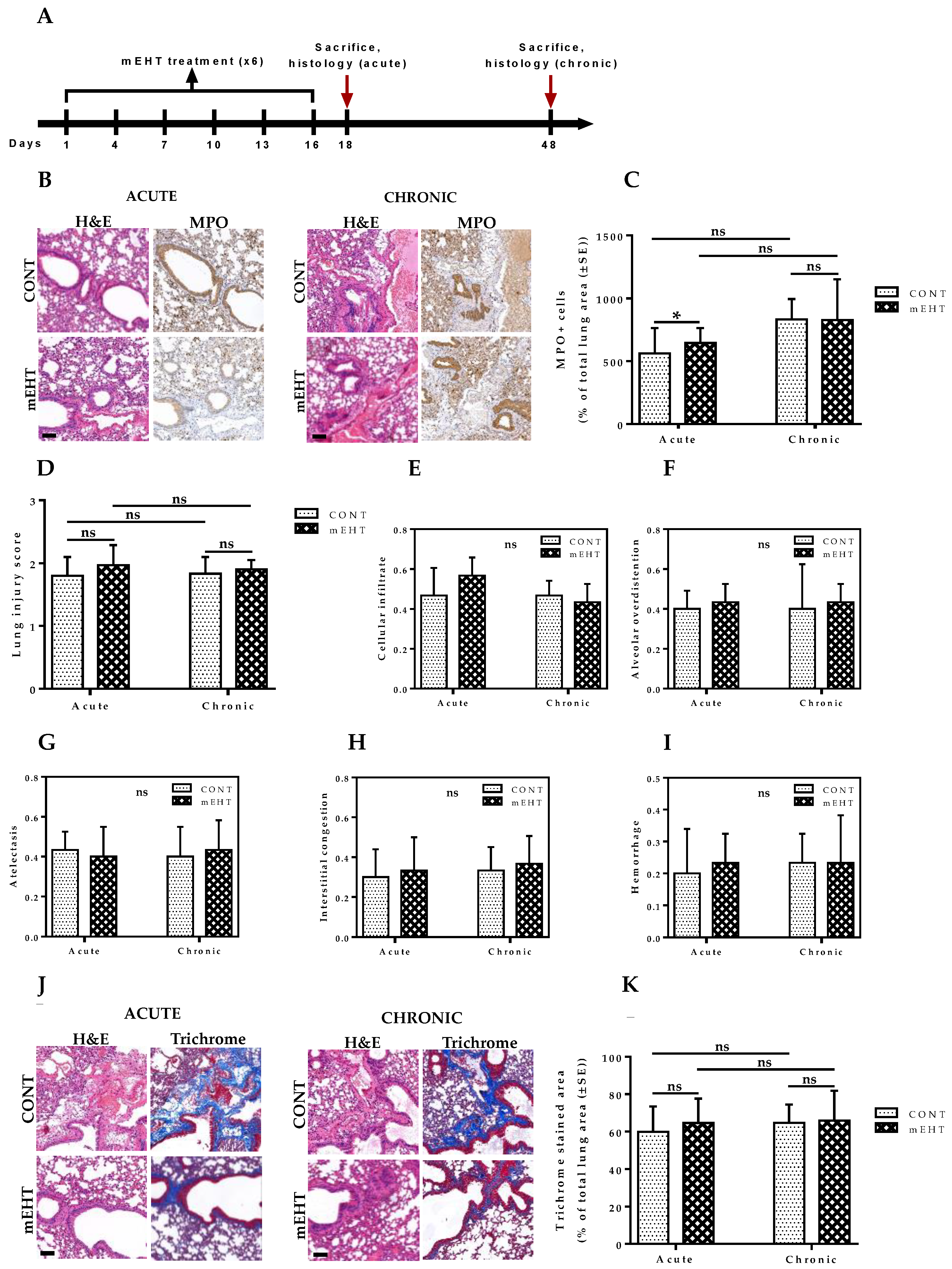
2.10. mEHT-Treated Lungs Showed No Auxiliary Lung Damage after Six-Time Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. PET Imaging and Data Analysis
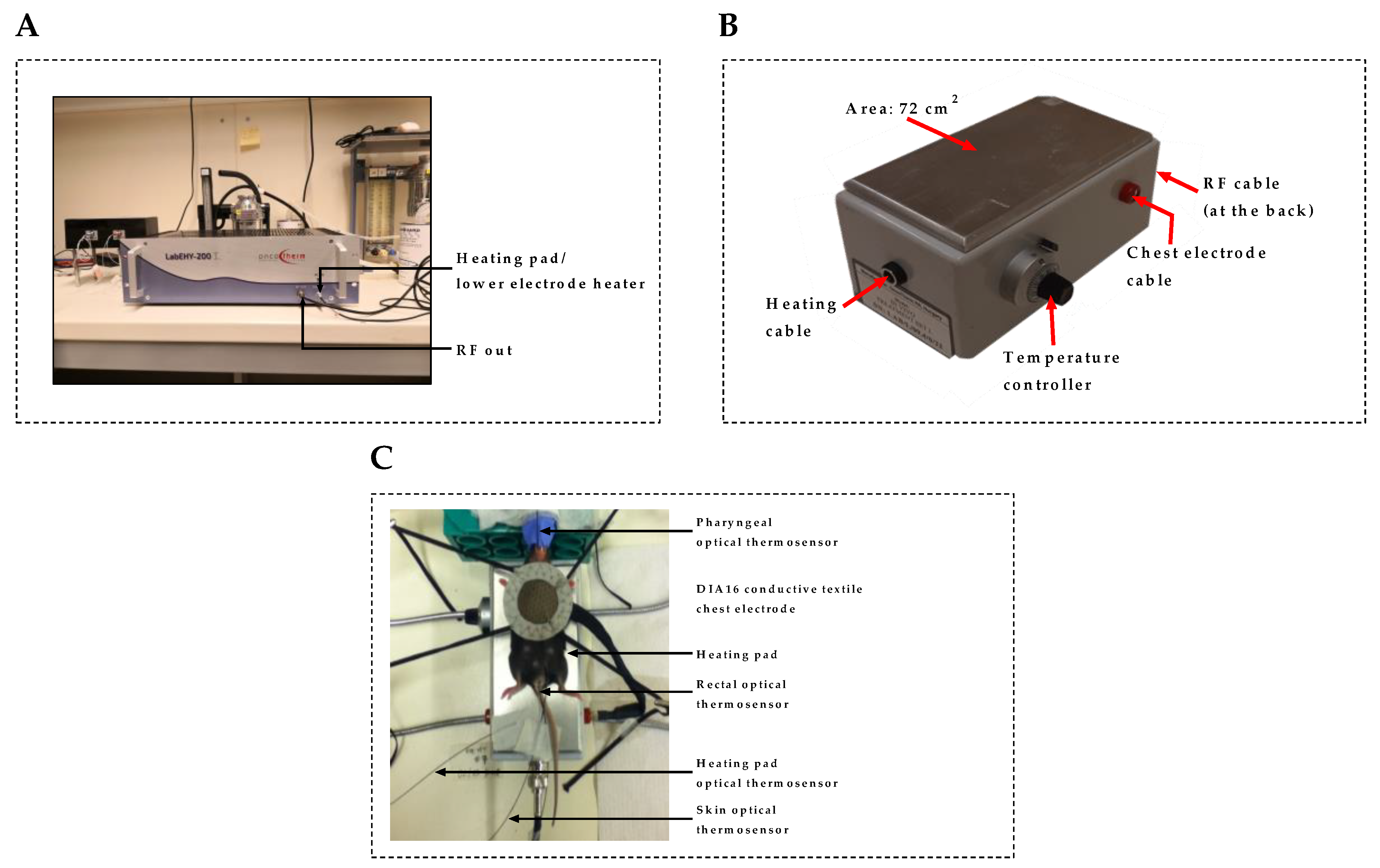
4.3. In Vivo mEHT Treatment
4.4. In Vitro mEHT Treatment
4.5. Immunohistochemistry
4.6. Flow Cytometry Analysis of Apoptotic and Necrotic Cell Death
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younes, R.; Abrao, F.C.; Gross, J. Pulmonary metastasectomy for malignant melanoma: Prognostic factors for long-term survival. Melanoma Res. 2013, 23, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tas, F. Metastatic behavior in melanoma: Timing, pattern, survival, and influencing factors. J. Oncol. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleef, R.; Moss, R.; Szasz, A.M.; Bohdjalian, A.; Bojar, H.; Bakacs, T. Complete clinical remission of stage iv triple-negative breast cancer lung metastasis administering low-dose immune checkpoint blockade in combination with hyperthermia and interleukin-2. Integr. Cancer Ther. 2018, 17, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roussakow, S.V. Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: A retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis. BMJ Open 2017, 7, e017387. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Kim, M.S.; Kim, H.J.; Lee, E.; Jeong, J.H.; Park, I.; Jeong, Y.K.; Jang, W.I. Role of HIF-1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int. J. Hyperth. 2018, 34, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Andocs, G.; Szasz, O.; Szasz, A. Oncothermia treatment of cancer: From the laboratory to clinic. Electromagn. Biol. Med. 2009, 28, 148–165. [Google Scholar] [CrossRef]
- Morimoto, T.; Kimura, S.; Konishi, Y.; Komaki, K.; Uyama, T.; Monden, Y.; Kinouchi, D.Y.; Iritani, D.T. A study of the electrical bio-impedance of tumors. J. Investig. Surg. 1993, 6, 25–32. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Khramtsov, V.V.; Gillies, R.J. Janus-faced tumor microenvironment and redox. Antioxid. Redox Signal. 2014, 21, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Szasz, A.; Szasz, N.; Szasz, O. Oncology–Treatments and Their Limits. In Oncothermia: Principles and Practices; Springer: Dordrecht, The Netherlands, 2010; pp. 1–15. [Google Scholar]
- Scholz, B.; Electromedica, R.A. On Electrical Impedance Scanning—Principles and Simulations. 2000. Available online: http://www.biophysicssite.com/Documents/Siemens_EIT.pdf (accessed on 23 February 2020).
- O’Rourke, A.P.; Lazebnik, M.; Bertram, J.M.; Converse, M.C.; Hagness, S.C.; Webster, J.G.; Mahvi, D.M. Dielectric properties of human normal, malignant and cirrhotic liver tissue: In vivo and ex vivo measurements from 0.5 to 20 GHz using a precision open-ended coaxial probe. Phys. Med. Biol. 2007, 52, 4707–4719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethig, R.; Gascoyne, P.R.C.; McLaughlin, J.A.; Szent-Gyorgyi, A. Interaction of the 2,6-dimethoxysemiquinone and ascorbic free radicals with Ehrlich ascites cells: A probe of cell-surface charge. Proc. Natl. Acad. Sci. USA 1984, 81, 2088–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Guo, Z. A review of electrical impedance techniques for breast cancer detection. Med. Eng. Phys. 2003, 25, 79–90. [Google Scholar] [CrossRef]
- Cha, J.; Jeon, T.-W.; Lee, C.G.; Oh, S.T.; Yang, H.B.; Choi, K.-J.; Seo, D.; Yun, I.; Baik, I.H.; Park, K.R.; et al. Electro-hyperthermia inhibits glioma tumorigenicity through the induction of E2F1-mediated apoptosis. Int. J. Hyperth. 2015, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Vancsik, T.; Forika, G.; Balogh, A.; Kiss, E.; Krenács, T. Modulated electro-hyperthermia induced p53 driven apoptosis and cell cycle arrest additively support doxorubicin chemotherapy of colorectal cancer in vitro. Cancer Med. 2019, 8, 4292–4303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vancsik, T.; Kovago, C.; Kiss, E.; Papp, E.; Forika, G.; Benyo, Z.; Meggyeshazi, N.; Krenács, T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J. Cancer 2018, 9, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Meggyesházi, N.; Andocs, G.; Balogh, L.; Balla, P.; Kiszner, G.; Teleki, I.; Jeney, A.; Krenács, T. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther. Onkol. 2014, 190, 815–822. [Google Scholar] [CrossRef]
- Danics, L.; Schvarcz, C.A.; Viana, P.; Vancsik, T.; Krenács, T.; Benyó, Z.; Kaucsár, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2000, 39. [Google Scholar] [CrossRef]
- Damsky, W.E.; Rosenbaum, L.E.; Bosenberg, M. Decoding Melanoma Metastasis. Cancers 2010, 3, 126–163. [Google Scholar] [CrossRef]
- Sun, X.; Bizhanova, A.; Matheson, T.D.; Yu, J.; Zhu, L.J.; Kaufman, P.D. Ki-67 Contributes to normal cell cycle progression and Inactive X heterochromatin in p21 checkpoint-proficient human cells. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragkos, M.; Jurvansuu, J.; Beard, P. H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol. Cell. Biol. 2009, 29, 2828–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besztercei, B.; Vancsik, T.; Benedek, A.; Major, E.; Thomas, M.J.; Schvarcz, C.A.; Krenács, T.; Benyó, Z.; Balogh, A. Stress-induced, p53-mediated tumor growth inhibition of melanoma by modulated electrohyperthermia in mouse models without major immunogenic effects. Int. J. Mol. Sci. 2019, 20, 4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Capetillo, O.; Lee, A.; Nussenzweig, M.; Nussenzweig, A. H2AX: The histone guardian of the genome. DNA Repair 2004, 3, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Capetillo, O.; Celeste, A.; Nussenzweig, A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle 2003, 2, 425–426. [Google Scholar] [CrossRef]
- Yang, K.L.; Huang, C.C.; Chi, M.S.; Chiang, H.C.; Wang, Y.S.; Hsia, C.C.; Andocs, G.; Wang, H.E.; Chi, K.H. In vitro comparison of conventional hyperthermia and modulated electro-hyperthermia. Oncotarget 2016, 7, 84082–84092. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef]
- Haegens, A.; Vernooy, J.H.J.; Heeringa, P.; Mossman, B.T.; Wouters, E.F.M. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008, 31, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Andocs, G.; Meggyeshazi, N.; Balogh, L.; Spisak, S.; Maros, M.E.; Balla, P.; Kiszner, G.; Teleki, I.; Kovago, C.; Krenacs, T. Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell Stress Chaperones 2015, 20, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, M.; Bessler, H. High temperature affects the phagocytic activity of human peripheral blood mononuclear cells. Scand. J. Clin. Lab. Investig. 2015, 75, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, F.; Li, Y.; Steffen, I.G.; Lin, Q.; Lili, C.; Hua, W.; van den Hoff, J.; Zschaeck, S. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in patients with esophageal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Pak, K.; Kim, I.J.; Kim, B.S.; Kim, S.J. Prognostic value of tumor-to-blood standardized uptake ratio in patients with resectable non-small-cell lung cancer. Nucl. Med. Mol. Imaging 2017, 51, 233–239. [Google Scholar] [CrossRef]
- Hofheinz, F.; Apostolova, I.; Oehme, L.; Kotzerke, J.; Van Den Hoff, J. Test–retest variability in lesion SUV and lesion SUR in18F-FDG PET: An analysis of data from two prospective multicenter trials. J. Nucl. Med. 2017, 58, 1770–1775. [Google Scholar] [CrossRef] [Green Version]
- Ehrentraut, H.; Clambey, E.T.; McNamee, E.N.; Brodsky, K.S.; Ehrentraut, S.F.; Poth, J.M.; Riegel, A.K.; Westrich, J.A.; Colgan, S.P.; Eltzschig, H.K. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 2013, 27, 2207–2219. [Google Scholar] [CrossRef] [Green Version]



| Antigen | Type | Reference No. | Dilution | Antigen Retrieval | Vendor |
|---|---|---|---|---|---|
| p-H2Axγ (Ser139) | Rabbit, mAb | #9718 | 1:200 | T-E | Cell Signaling |
| CCasp3 | Rabbit, pAb | #9664 | 1:100 | Citrate | Cell Signaling |
| P21waf1 | Rabbit, mAb | #ab188224 | 1:300 | T-E | Abcam |
| Ki67 (SP6) | Rabbit, mAb | #MA5-14520 | 1:100 | T-E | Thermo |
| F4/80 (D2S9R) XP | Rabbit, mAb | # 70076 | 1:300 | T-E | Cell Signaling |
| CD3 | Rabbit, pAb | #IS503 | 1:3 | T-E | Dako |
| CD8α (D4W2Z) XP | Rabbit, mAb | #98941 | 1:500 | T-E | Cell Signaling |
| MPO | Goat, pAb | #AF3667 | 1:300 | T-E | R&D Systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.J.; Major, E.; Benedek, A.; Horváth, I.; Máthé, D.; Bergmann, R.; Szász, A.M.; Krenács, T.; Benyó, Z. Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice. Cancers 2020, 12, 3872. https://doi.org/10.3390/cancers12123872
Thomas MJ, Major E, Benedek A, Horváth I, Máthé D, Bergmann R, Szász AM, Krenács T, Benyó Z. Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice. Cancers. 2020; 12(12):3872. https://doi.org/10.3390/cancers12123872
Chicago/Turabian StyleThomas, Mbuotidem Jeremiah, Enikő Major, Anett Benedek, Ildikó Horváth, Domokos Máthé, Ralf Bergmann, Attila Marcell Szász, Tibor Krenács, and Zoltán Benyó. 2020. "Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice" Cancers 12, no. 12: 3872. https://doi.org/10.3390/cancers12123872
APA StyleThomas, M. J., Major, E., Benedek, A., Horváth, I., Máthé, D., Bergmann, R., Szász, A. M., Krenács, T., & Benyó, Z. (2020). Suppression of Metastatic Melanoma Growth in Lung by Modulated Electro-Hyperthermia Monitored by a Minimally Invasive Heat Stress Testing Approach in Mice. Cancers, 12(12), 3872. https://doi.org/10.3390/cancers12123872







