Novel Thiosemicarbazones Sensitize Pediatric Solid Tumor Cell-Types to Conventional Chemotherapeutics through Multiple Molecular Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
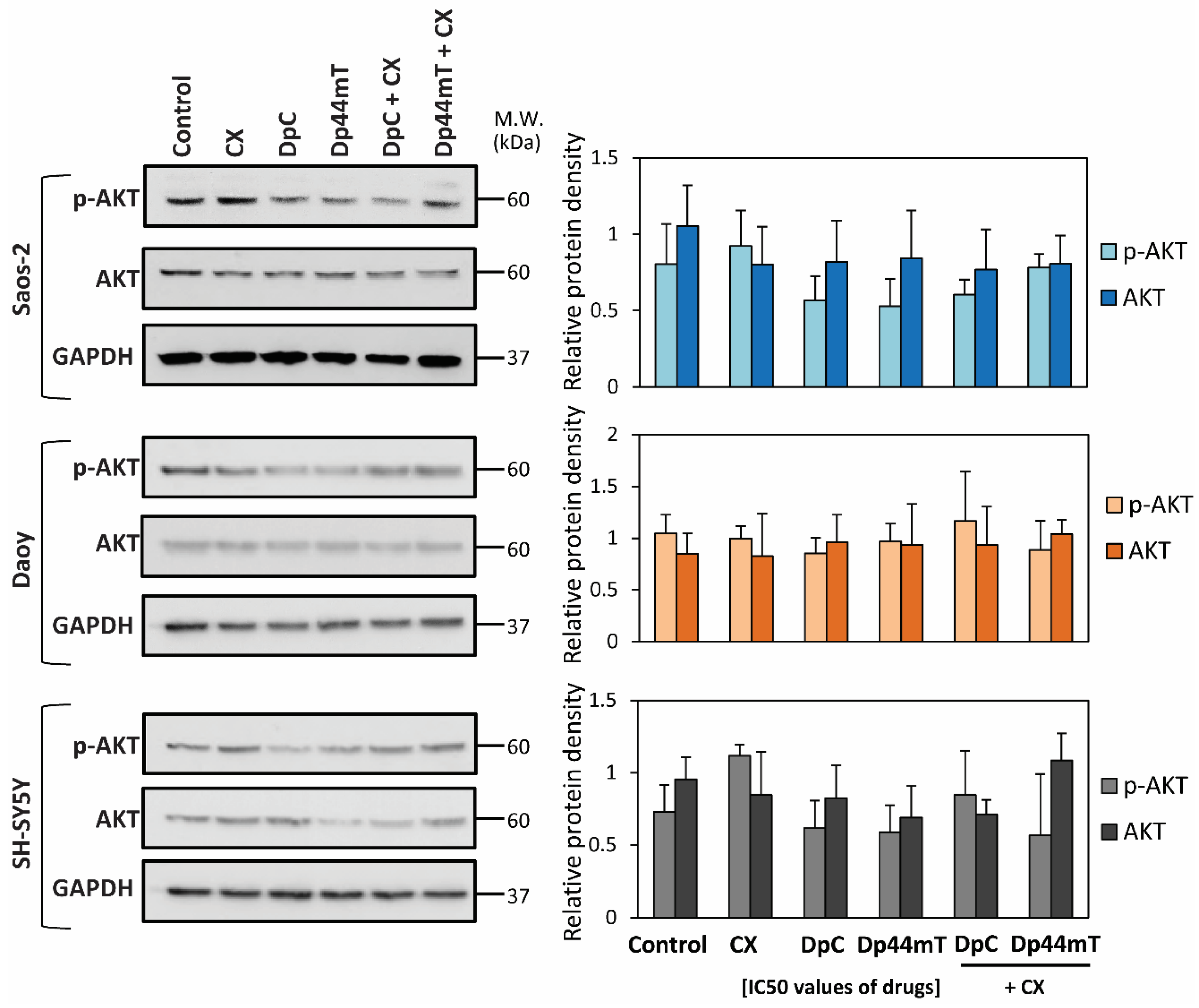
2.1. Synergism between Thiosemicarbazones and Established Chemotherapeutics Is Marked with CX
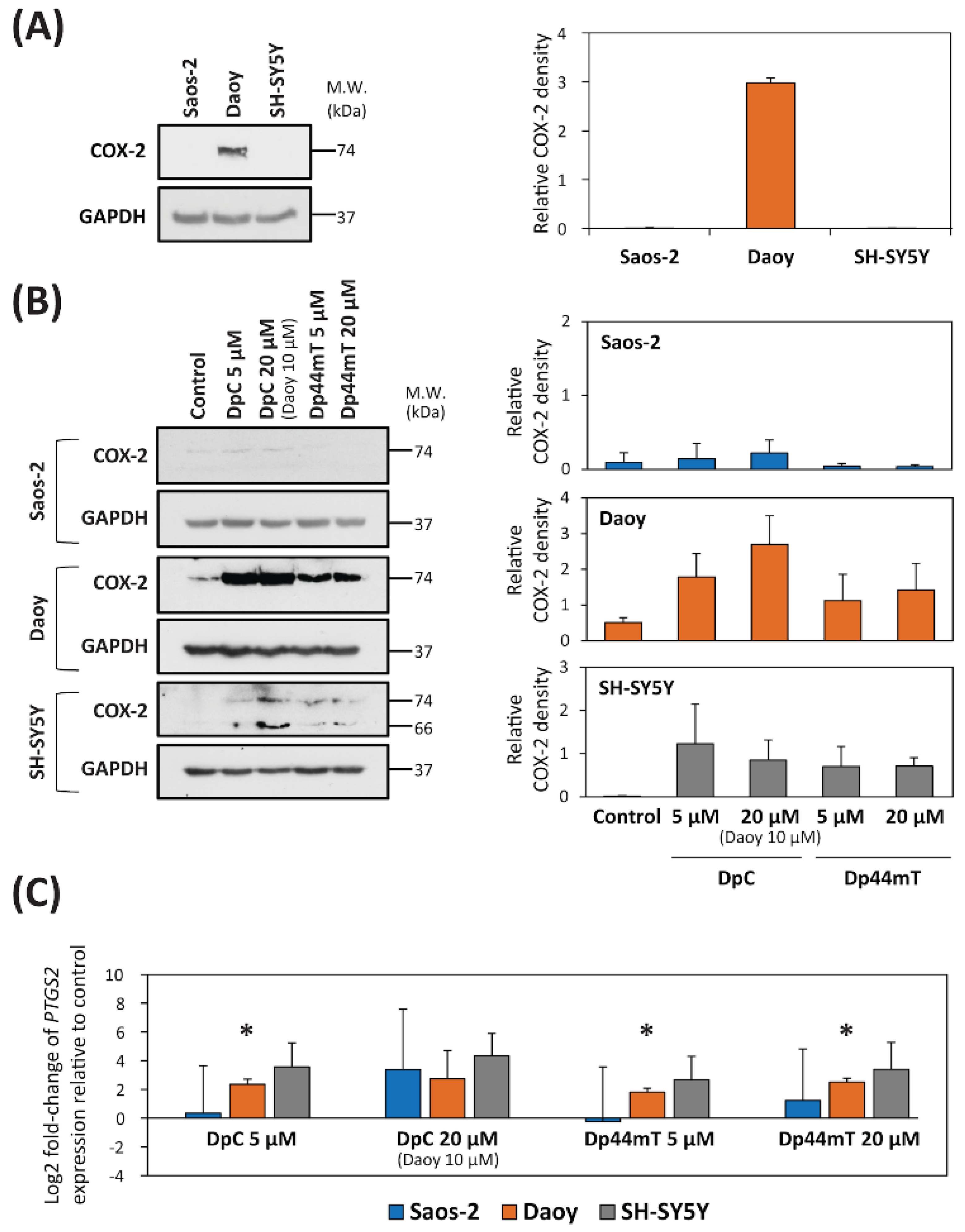
2.2. DpC and Dp44mT Up-Regulate COX-2 Expression
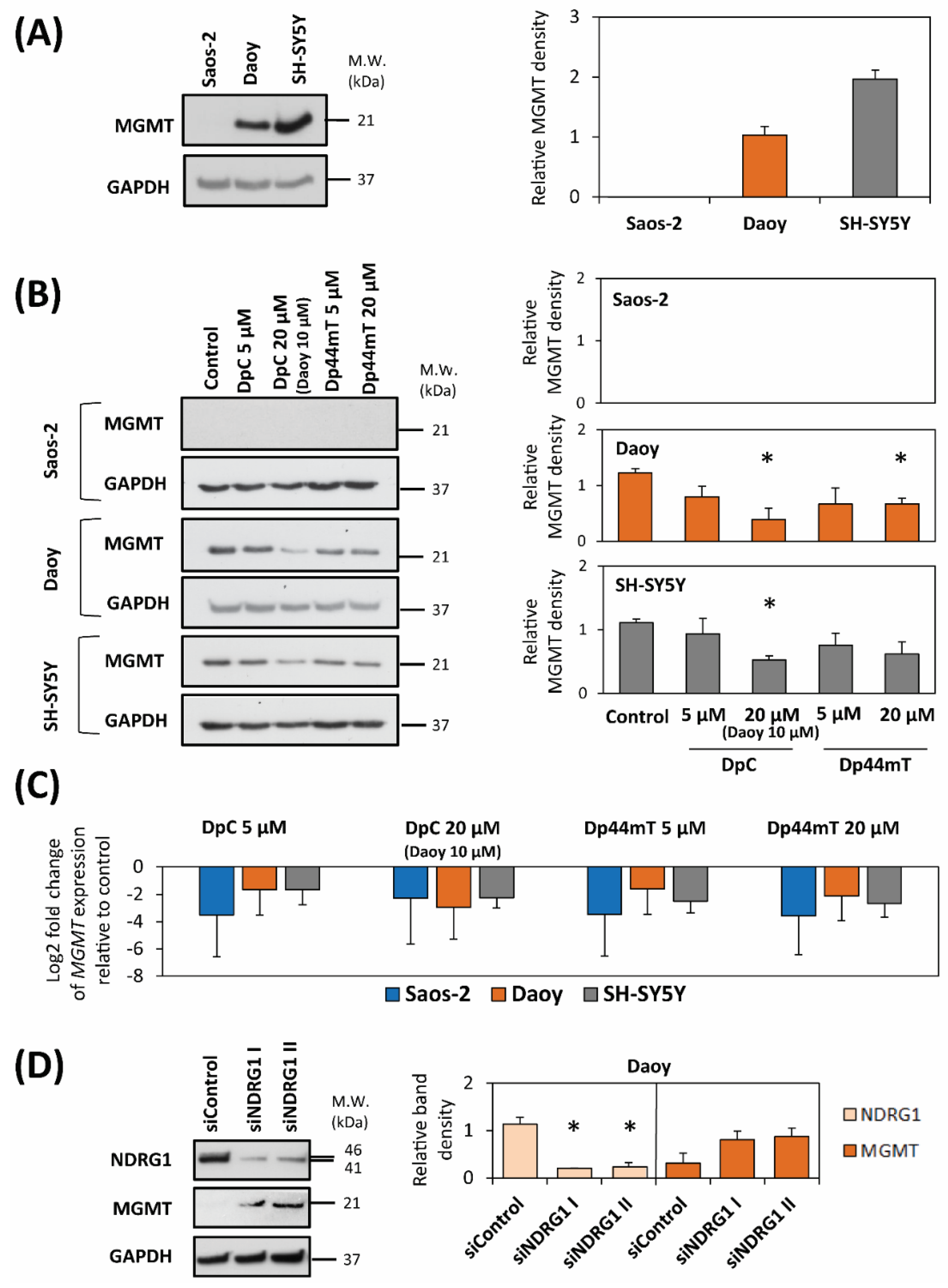
2.3. DpC and Dp44mT Down-Regulate MGMT Expression
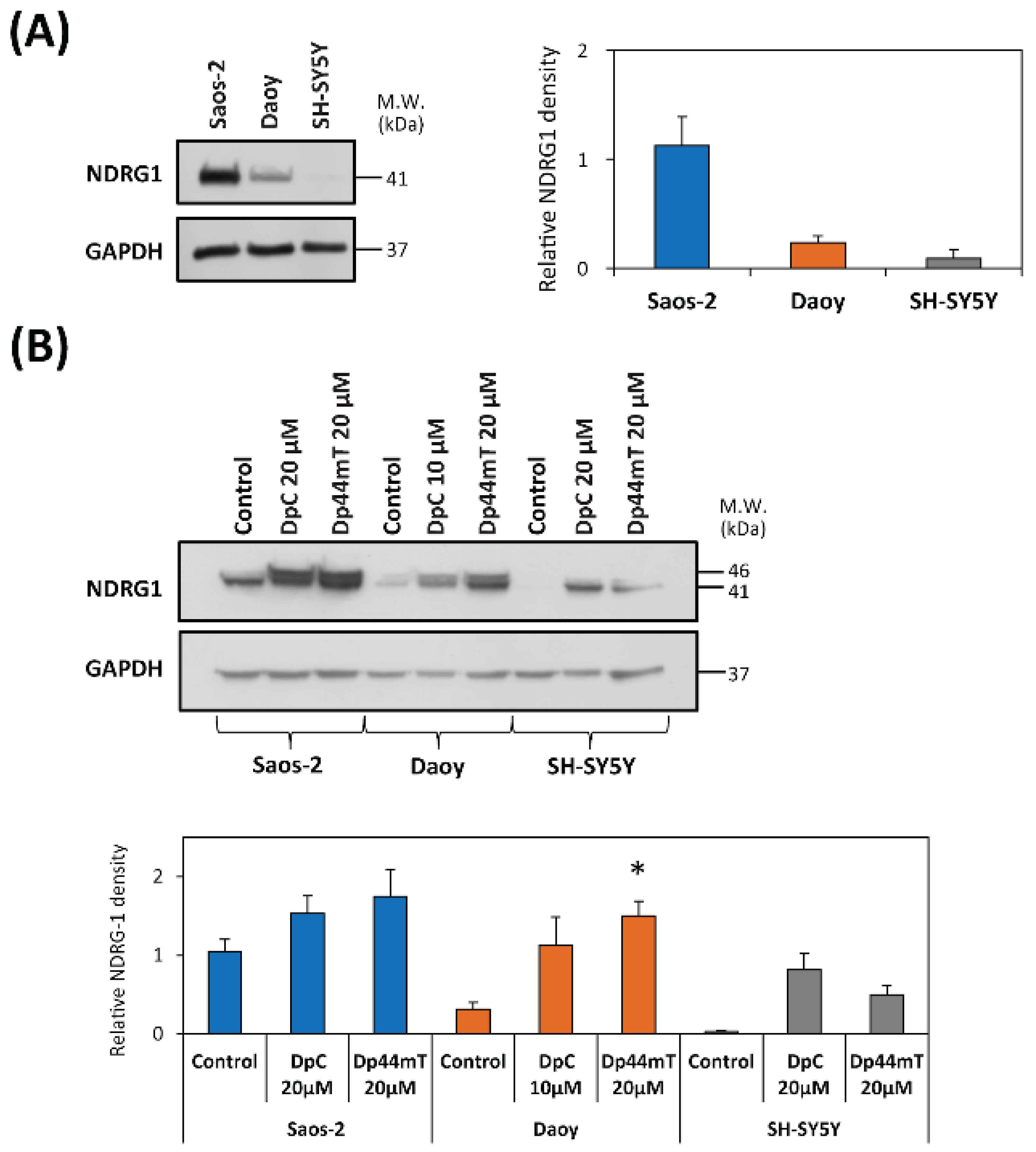
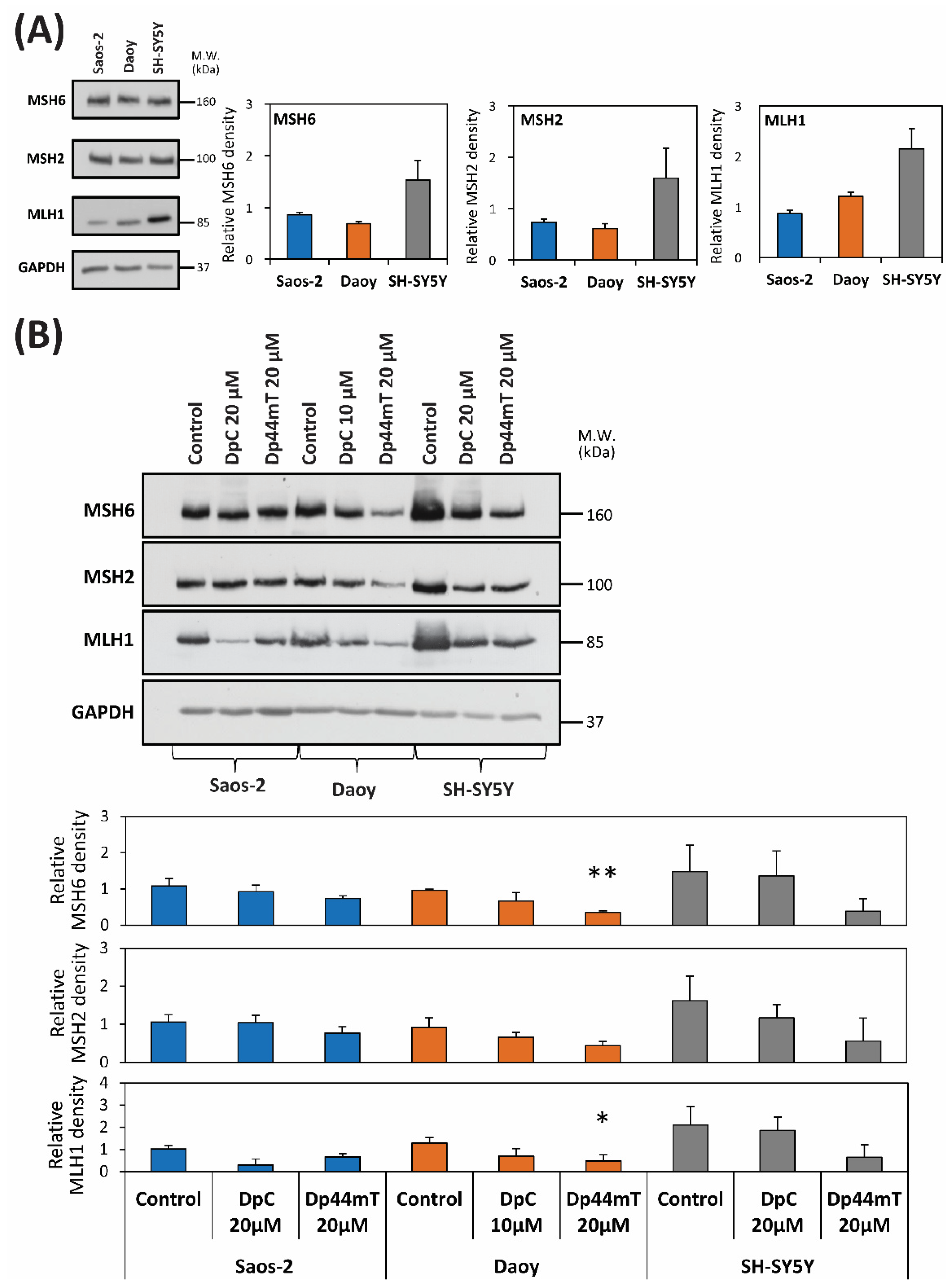
2.4. DpC and Dp44mT Generally Down-Regulate the Mismatch Repair (MMR) Proteins, MSH2, MSH6 and MLH1, Which May Also Affect Cellular Sensitivity to TMZ
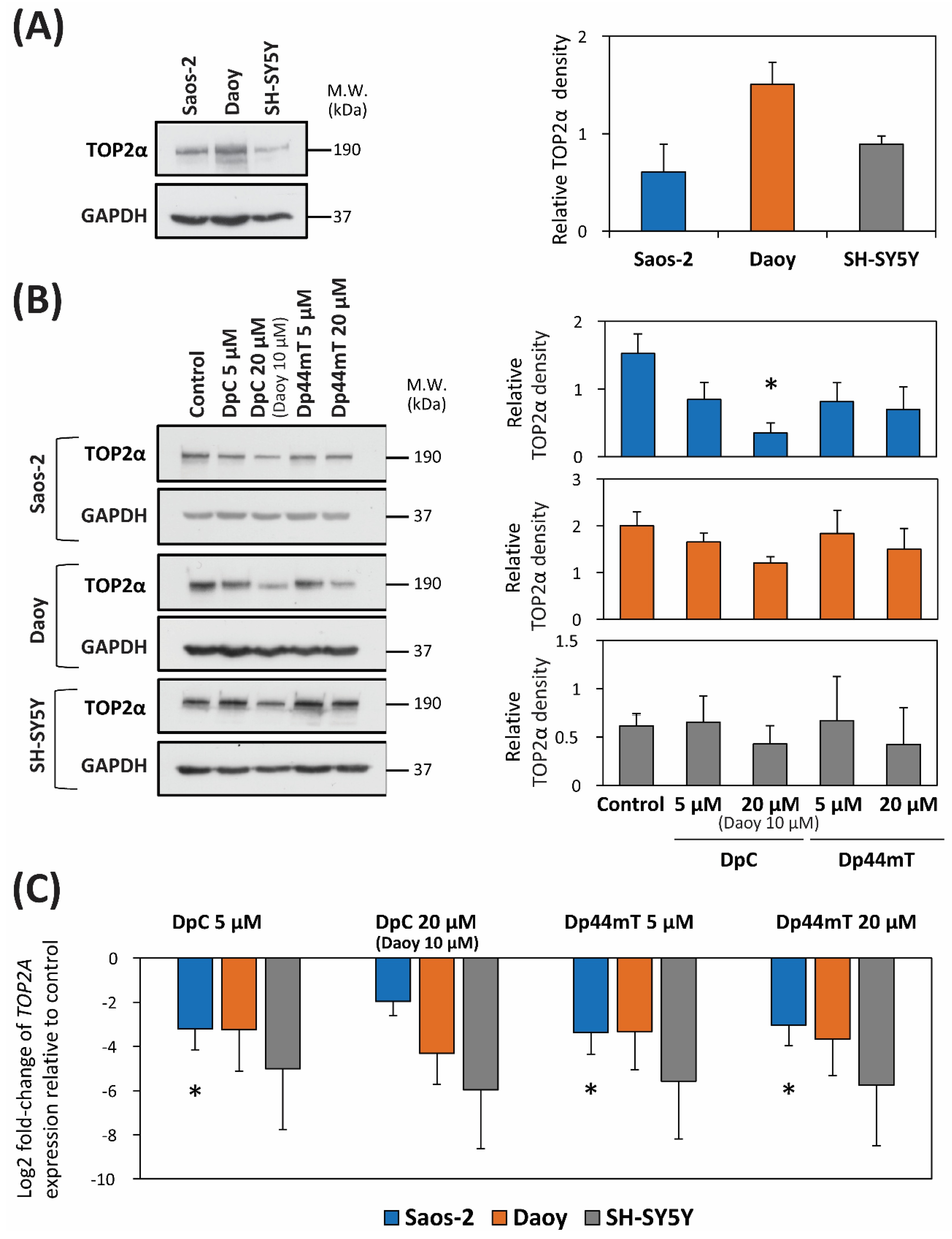
2.5. DpC and Dp44mT Down-Regulate TOP2α Expression
3. Discussion
3.1. Combination of the Novel Thiosemicarbazones and CX
3.2. Combination of Novel Thiosemicarbazones and TMZ
3.3. Combination of Novel Thiosemicarbazones and ETO
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. siRNA
4.4. Treatment Protocol
4.5. Cell Proliferation
4.6. Calculation of CI
4.7. RT-qPCR
4.8. Immunoblotting Assay
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA. Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Persson, L.; Xu, X. Molecular pharmacology of the interaction of anthracyclines with iron. Mol. Pharmacol. 2005, 68, 1–11. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A. Chronic health conditions in adult survivors of childhood cancer. Oncol. Times 2007, 29, 26. [Google Scholar] [CrossRef]
- Sterba, J.; Valik, D.; Mudry, P.; Kepak, T.; Pavelka, Z.; Bajciova, V.; Zitterbart, K.; Kadlecova, V.; Mazanek, P. Combined biodifferentiating and antiangiogenic oral metronomic therapy is feasible and effective in relapsed solid tumors in children: Single-center pilot study. Onkologie 2006, 29, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Zapletalova, D.; André, N.; Deak, L.; Kyr, M.; Bajciova, V.; Mudry, P.; Dubska, L.; Demlova, R.; Pavelka, Z.; Zitterbart, K.; et al. Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: A multicenter experience. Oncology 2012, 82, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lovejoy, D.B.; Richardson, D.R. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: In vitro and in vivo assessment. Blood 2004, 104, 1450–1458. [Google Scholar] [CrossRef]
- Whitnall, M.; Howard, J.; Ponka, P.; Richardson, D.R. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc. Natl. Acad. Sci. USA 2006, 103, 14901–14906. [Google Scholar] [CrossRef]
- Richardson, D.R.; Tran, E.H.; Ponka, P. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood 1995, 86, 4295–4306. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Richardson, D.R. Novel “hybrid” iron chelators derived from aroylhydrazones and thiosemicarbazones demonstrate selective antiproliferative activity against tumor cells. Blood 2002, 100, 666–676. [Google Scholar] [CrossRef]
- Liu, W.; Xing, F.; Iiizumi-Gairani, M.; Okuda, H.; Watabe, M.; Pai, S.K.; Pandey, P.R.; Hirota, S.; Kobayashi, A.; Mo, Y.-Y.; et al. N-myc downstream regulated gene 1 modulates Wnt-β-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol. Med. 2012, 4, 93–108. [Google Scholar] [CrossRef]
- Jansson, P.J.; Yamagishi, T.; Arvind, A.; Seebacher, N.; Gutierrez, E.; Stacy, A.; Maleki, S.; Sharp, D.; Sahni, S.; Richardson, D.R. Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp). J. Biol. Chem. 2015, 290, 9588–9603. [Google Scholar] [CrossRef]
- Guo, Z.-L.; Richardson, D.R.; Kalinowski, D.S.; Kovacevic, Z.; Tan-Un, K.C.; Chan, G.C.-F. The novel thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma growth in vitro and in vivo via multiple mechanisms. J. Hematol. Oncol. J. Hematol. Oncol. 2016, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Chiang, K.-C.; Feng, T.-H.; Chen, Y.-J.; Chuang, S.-T.; Tsui, K.-H.; Chung, L.-C.; Juang, H.-H. The iron chelator, Dp44mT, effectively inhibits human oral squamous cell carcinoma cell growth in vitro and in vivo. Int. J. Mol. Sci. 2016, 17, 1435. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, X.; Shou, K.; Niu, Y.; Jian, C.; Zhao, Y.; Yi, W.; Hu, X.; Yu, A. The iron chelator Dp44mT suppresses osteosarcoma’s proliferation, invasion and migration: In vitro and in vivo. Am. J. Transl. Res. 2016, 8, 5370–5385. [Google Scholar] [PubMed]
- Kovacevic, Z.; Chikhani, S.; Lovejoy, D.B.; Richardson, D.R. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: A new strategy for the treatment of pancreatic cancer. Mol. Pharmacol. 2011, 80, 598–609. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Sharp, D.M.; Seebacher, N.; Obeidy, P.; Prichard, T.; Stefani, C.; Basha, M.T.; Sharpe, P.C.; Jansson, P.J.; Kalinowski, D.S.; et al. Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J. Med. Chem. 2012, 55, 7230–7244. [Google Scholar] [CrossRef]
- Le, N.T.V.; Richardson, D.R. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: A link between iron metabolism and proliferation. Blood 2004, 104, 2967–2975. [Google Scholar] [CrossRef]
- Richardson, D.R.; Sharpe, P.C.; Lovejoy, D.B.; Senaratne, D.; Kalinowski, D.S.; Islam, M.; Bernhardt, P.V. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J. Med. Chem. 2006, 49, 6510–6521. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Jansson, P.J.; Brunk, U.T.; Wong, J.; Ponka, P.; Richardson, D.R. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011, 71, 5871–5880. [Google Scholar] [CrossRef]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)–thiosemicarbazone complexes are localized to the lysosomal compartment where they transmetallate with copper ions to induce cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef]
- Seebacher, N.A.; Richardson, D.R.; Jansson, P.J. A mechanism for overcoming P-glycoprotein-mediated drug resistance: Novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization using Dp44mT or DpC. Cell Death Dis. 2016, 7, e2510. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, Z.; Menezes, S.V.; Sahni, S.; Kalinowski, D.S.; Bae, D.-H.; Lane, D.J.R.; Richardson, D.R. The metastasis suppressor, N-MYC downstream-regulated gene-1 (NDRG1), down-regulates the ErbB family of receptors to inhibit downstream oncogenic signaling pathways. J. Biol. Chem. 2016, 291, 1029–1052. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.V.; Kovacevic, Z.; Richardson, D.R. The metastasis suppressor NDRG1 down-regulates the epidermal growth factor receptor via a lysosomal mechanism by up-regulating mitogen-inducible gene 6. J. Biol. Chem. 2019, 294, 4045–4064. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Geleta, B.; Leck, L.Y.W.; Paluncic, J.; Chiang, S.; Jansson, P.J.; Kovacevic, Z.; Richardson, D.R. Thiosemicarbazones suppress expression of the c-Met oncogene by mechanisms involving lysosomal degradation and intracellular shedding. J. Biol. Chem. 2020, 295, 481–503. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Chikhani, S.; Lui, G.Y.L.; Sivagurunathan, S.; Richardson, D.R. The iron-regulated metastasis suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the PI3K and Ras signaling pathways. Antioxid. Redox Signal. 2013, 18, 874–887. [Google Scholar] [CrossRef]
- Lui, G.Y.L.; Kovacevic, Z.; Menezes, S.; Kalinowski, D.S.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Novel thiosemicarbazones regulate the signal transducer and activator of transcription 3 (STAT3) pathway: Inhibition of constitutive and interleukin 6 (IL6)-induced activation by iron depletion. Mol. Pharmacol. 2015. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Yue, F.; Zheng, M.; Kovacevic, Z.; Richardson, D.R. The iron chelators Dp44mT and DFO inhibit TGF-β-induced epithelial-mesenchymal transition via up-regulation of N-Myc downstream-regulated gene 1 (NDRG1). J. Biol. Chem. 2012, 287, 17016–17028. [Google Scholar] [CrossRef]
- Menezes, S.V.; Fouani, L.; Huang, M.L.H.; Geleta, B.; Maleki, S.; Richardson, A.; Richardson, D.R.; Kovacevic, Z. The metastasis suppressor, NDRG1, attenuates oncogenic TGF-β and NF-κB signaling to enhance membrane E-cadherin expression in pancreatic cancer cells. Carcinogenesis 2019, 40, 805–818. [Google Scholar] [CrossRef]
- Jin, R.; Liu, W.; Menezes, S.; Yue, F.; Zheng, M.; Kovacevic, Z.; Richardson, D.R. The metastasis suppressor NDRG1 modulates the phosphorylation and nuclear translocation of β-catenin through mechanisms involving FRAT1 and PAK4. J. Cell Sci. 2014, 127, 3116–3130. [Google Scholar] [CrossRef]
- Gutierrez, E.; Richardson, D.R.; Jansson, P.J. The anticancer agent di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes prosurvival autophagy by two mechanisms: Persistent induction of autophagosome synthesis and impairment of lysosomal integrity. J. Biol. Chem. 2014, 289, 33568–33589. [Google Scholar] [CrossRef]
- Potuckova, E.; Jansova, H.; Machacek, M.; Vavrova, A.; Haskova, P.; Tichotova, L.; Richardson, V.; Kalinowski, D.S.; Richardson, D.R.; Simunek, T. Quantitative analysis of the anti-proliferative activity of combinations of selected iron-chelating agents and clinically used anti-neoplastic drugs. PLoS ONE 2014, 9, e88754. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, S.N.; Lim, S.C.; Park, K.C.; Hanif, R.; Richardson, D.R.; Jansson, P.J.; Kovacevic, Z. Overcoming tamoxifen resistance in oestrogen receptor-positive breast cancer using the novel thiosemicarbazone anti-cancer agent, DpC. Br. J. Pharmacol. 2020, 177, 2365–2380. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, V. Targeting apoptosis pathways by celecoxib in cancer. Cancer Lett. 2013, 332, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Tanaka, M.; Trepel, J.; Reinhold, W.C.; Rajapakse, V.N.; Pommier, Y. Temozolomide in the era of precision medicine. Cancer Res. 2017, 77, 823–826. [Google Scholar] [CrossRef]
- Relling, M.V.; Boyett, J.M.; Blanco, J.G.; Raimondi, S.; Behm, F.G.; Sandlund, J.T.; Rivera, G.K.; Kun, L.E.; Evans, W.E.; Pui, C.-H. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood 2003, 101, 3862–3867. [Google Scholar] [CrossRef][Green Version]
- Walker, J.V.; Nitiss, J.L. DNA topoisomerase II as a target for cancer chemotherapy. Cancer Investig. 2002, 20, 570–589. [Google Scholar] [CrossRef]
- Schwartzman, M.L.; Bonazzi, A.; Mieyal, P.; Mezentsev, A.; Abraham, N.G.; Dunn, M.W. COX-2 lack of function in hypoxia-induced ocular surface inflammation. Thromb. Res. 2003, 110, 293–298. [Google Scholar] [CrossRef]
- Hausmann, A.; Lee, J.; Pantopoulos, K. Redox control of iron regulatory protein 2 stability. FEBS Lett. 2011, 585, 687–692. [Google Scholar] [CrossRef]
- Saxena, N.; Maio, N.; Crooks, D.R.; Ricketts, C.J.; Yang, Y.; Wei, M.-H.; Fan, T.W.-M.; Lane, A.N.; Sourbier, C.; Singh, A.; et al. SDHB-deficient cancers: The role of mutations that impair iron sulfur cluster delivery. JNCI J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Park, Y.-K.; Hong, H.; Jang, B.-C. Transcriptional and translational regulation of COX-2 expression by cadmium in C6 glioma cells. Int. J. Mol. Med. 2012, 30, 960–966. [Google Scholar] [CrossRef]
- Arico, S.; Pattingre, S.; Bauvy, C.; Gane, P.; Barbat, A.; Codogno, P.; Ogier-Denis, E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J. Biol. Chem. 2002, 277, 27613–27621. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Menezes, S.V.; Kalinowski, D.S.; Sahni, S.; Jansson, P.J.; Kovacevic, Z.; Richardson, D.R. Identification of differential phosphorylation and sub-cellular localization of the metastasis suppressor, NDRG1. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2644–2663. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Park, K.C.; Kovacevic, Z.; Richardson, D.R. Two mechanisms involving the autophagic and proteasomal pathways process the metastasis suppressor protein, N-myc downstream regulated gene 1. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Happold, C.; Roth, P.; Wick, W.; Schmidt, N.; Florea, A.-M.; Silginer, M.; Reifenberger, G.; Weller, M. Distinct molecular mechanisms of acquired resistance to temozolomide in glioblastoma cells. J. Neurochem. 2012, 122, 444–455. [Google Scholar] [CrossRef]
- Yip, S.; Miao, J.; Cahill, D.P.; Iafrate, A.J.; Aldape, K.; Nutt, C.L.; Louis, D.N. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4622–4629. [Google Scholar] [CrossRef]
- Liu, L.; Markowitz, S.; Gerson, S.L. Mismatch repair mutations override alkyltransferase in conferring resistance to temozolomide but not to 1,3-bis(2-chloroethyl)nitrosourea. Cancer Res. 1996, 56, 5375–5379. [Google Scholar]
- Kunkel, T.A.; Erie, D.A. DNA mismatch repair. Annu. Rev. Biochem. 2005, 74, 681–710. [Google Scholar] [CrossRef]
- Montecucco, A.; Biamonti, G. Cellular response to etoposide treatment. Cancer Lett. 2007, 252, 9–18. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Kovacevic, Z.; Jansson, P.J.; Sahni, S.; Huang, M.L.-H.; Lane, D.J.R.; Lok, H.; Richardson, D.R. Exploiting cancer metal metabolism using anti-cancer metal-binding agents. Curr. Med. Chem. 2019, 26, 302–322. [Google Scholar] [CrossRef]
- Park, K.C.; Paluncic, J.; Kovacevic, Z.; Richardson, D.R. Pharmacological targeting and the diverse functions of the metastasis suppressor, NDRG1, in cancer. Free Radic. Biol. Med. 2020, 157, 154–175. [Google Scholar] [CrossRef]
- Dixon, K.M.; Lui, G.Y.L.; Kovacevic, Z.; Zhang, D.; Yao, M.; Chen, Z.; Dong, Q.; Assinder, S.J.; Richardson, D.R. Dp44mT targets the AKT, TGF-β and ERK pathways via the metastasis suppressor NDRG1 in normal prostate epithelial cells and prostate cancer cells. Br. J. Cancer 2013, 108, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Jansson, P.J.; Assinder, S.J.; Maleki, S.; Richardson, D.R.; Kovacevic, Z. Unique targeting of androgen-dependent and -independent AR signaling in prostate cancer to overcome androgen resistance. FASEB J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Zeng, M.-L.; Yu, D.; Ren, J.; Li, F.; Zheng, A.; Wang, Y.-P.; Chen, C.; Tao, Z.-Z. In vitro assessment of the role of DpC in the treatment of head and neck squamous cell carcinoma. Oncol. Lett. 2018, 15, 7999–8004. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.J.; Kalinowski, D.S.; Lane, D.J.R.; Kovacevic, Z.; Seebacher, N.A.; Fouani, L.; Sahni, S.; Merlot, A.M.; Richardson, D.R. The renaissance of polypharmacology in the development of anti-cancer therapeutics: Inhibition of the “Triad of Death” in cancer by Di-2-pyridylketone thiosemicarbazones. Pharmacol. Res. 2015, 100, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar] [PubMed]
- Rudner, J.; Elsaesser, S.J.; Müller, A.-C.; Belka, C.; Jendrossek, V. Differential effects of anti-apoptotic Bcl-2 family members Mcl-1, Bcl-2, and Bcl-xL on celecoxib-induced apoptosis. Biochem. Pharmacol. 2010, 79, 10–20. [Google Scholar] [CrossRef]
- Johnson, A.J.; Hsu, A.-L.; Lin, H.-P.; Song, X.; Chen, C.-S. The cyclo-oxygenase-2 inhibitor celecoxib perturbs intracellular calcium by inhibiting endoplasmic reticulum Ca2+-ATPases: A plausible link with its anti-tumour effect and cardiovascular risks. Biochem. J. 2002, 366, 831–837. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Sivagurunathan, S.; Mangs, H.; Chikhani, S.; Zhang, D.; Richardson, D.R. The metastasis suppressor, N-myc downstream regulated gene 1 (NDRG1), upregulates p21 via p53-independent mechanisms. Carcinogenesis 2011, 32, 732–740. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, D.; Bae, D.-H.; Sahni, S.; Jansson, P.; Zheng, Y.; Zhao, Q.; Yue, F.; Zheng, M.; Kovacevic, Z.; et al. Metastasis suppressor, NDRG1, mediates its activity through signaling pathways and molecular motors. Carcinogenesis 2013, 34, 1943–1954. [Google Scholar] [CrossRef]
- Ma, W.; Na, M.; Tang, C.; Wang, H.; Lin, Z. Overexpression of N-myc downstream-regulated gene 1 inhibits human glioma proliferation and invasion via phosphoinositide 3-kinase/AKT pathways. Mol. Med. Rep. 2015, 12, 1050–1058. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Gerson, S.L. Clinical relevance of MGMT in the treatment of cancer. J. Clin. Oncol. 2002, 20, 2388–2399. [Google Scholar] [CrossRef]
- Woo, P.Y.M.; Li, Y.; Chan, A.H.Y.; Ng, S.C.P.; Loong, H.H.F.; Chan, D.T.M.; Wong, G.K.C.; Poon, W.-S. A multifaceted review of temozolomide resistance mechanisms in glioblastoma beyond O-6-methylguanine-DNA methyltransferase. Glioma 2019, 2, 68. [Google Scholar] [CrossRef]
- Duguid, E.M.; Rice, P.A.; He, C. The structure of the human AGT protein bound to DNA and its implications for damage detection. J. Mol. Biol. 2005, 350, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Wickström, M.; Dyberg, C.; Milosevic, J.; Einvik, C.; Calero, R.; Sveinbjörnsson, B.; Sandén, E.; Darabi, A.; Siesjö, P.; Kool, M.; et al. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat. Commun. 2015, 6, 8904. [Google Scholar] [CrossRef]
- Alexander, B.M.; Pinnell, N.; Wen, P.Y.; D’Andrea, A. Targeting DNA repair and the cell cycle in glioblastoma. J. Neurooncol. 2012, 107, 463–477. [Google Scholar] [CrossRef]
- Rao, V.A.; Klein, S.R.; Agama, K.K.; Toyoda, E.; Adachi, N.; Pommier, Y.; Shacter, E.B. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res. 2009, 69, 948–957. [Google Scholar] [CrossRef]
- Yalowich, J.C.; Wu, X.; Zhang, R.; Kanagasabai, R.; Hornbaker, M.; Hasinoff, B.B. The anticancer thiosemicarbazones Dp44mT and triapine lack inhibitory effects as catalytic inhibitors or poisons of DNA topoisomerase IIα. Biochem. Pharmacol. 2012, 84, 52–58. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Emanuelli, A.; Borroni, A.P.; Apel-Sarid, L.; Shah, P.A.; Ayyathan, D.M.; Koganti, P.; Levy-Cohen, G.; Blank, M. Smurf2-mediated stabilization of DNA topoisomerase IIα controls genomic integrity. Cancer Res. 2017, 77, 4217–4227. [Google Scholar] [CrossRef]
- Krzyzankova, M.; Chovanova, S.; Chlapek, P.; Radsetoulal, M.; Neradil, J.; Zitterbart, K.; Sterba, J.; Veselska, R. LOX/COX inhibitors enhance the antineoplastic effects of all-trans retinoic acid in osteosarcoma cell lines. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 7617–7627. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | IC50 Values | ||||
|---|---|---|---|---|---|
| CX [µM] | TMZ [µM] | ETO [µM] | DpC [nM] | Dp44mT [nM] | |
| Saos-2 | 75.6 ± 7.1 | 116.5 ± 3.0 | 11.5 ± 0.7 | 8.8 ± 0.2 | 9.3 ± 0.6 |
| Daoy | 90.6 ± 6.7 | 212.9 ± 38.4 | 6.8 ± 2.0 | 8.5 ± 2.2 | 5.4 ± 0.8 |
| SH-SY5Y | 73.7 ± 9.1 | 112.4 ± 3.8 | 2.8 ± 0.3 | 3.7 ± 0.6 | 0.8 ± 0.1 |
| Cell Line | Combination Index (CI) ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CX | TMZ | ETO | |||||||
| +DpC | +Dp44mT | +DpC | +Dp44mT | +DpC | +Dp44mT | ||||
| Saos-2 | 0.29 ± 0.19 | 0.33 ± 0.15 | 0.48 ± 0.07 | 0.92 ± 0.37 | 1.55 ± 0.44 | 2.19 ± 0.70 | |||
| Daoy | 0.52 ± 0.03 | 0.39 ± 0.05 | 0.89 ± 0.11 | 1.59 ± 0.31 | 0.45 ± 0.12 | 0.64 ± 0.12 | |||
| SH-SY5Y | 1.56 ± 0.08 | 0.84 ± 0.11 | 1.62 ± 0.32 | 1.38 ± 0.25 | 1.66 ± 0.66 | 1.67 ± 0.25 | |||
| Categories of Interactions | |||||||||
| 0.10–0.30 | strong synergism | 0.91–1.10 | nearly additive | ||||||
| 0.31–0.70 | synergism | 1.11–1.20 | slight antagonism | ||||||
| 0.71–0.85 | moderate synergism | 1.21–1.45 | moderate antagonism | ||||||
| 0.86–0.90 | slight synergism | 1.46–3.30 | antagonism | ||||||
| Gene | Primer Sequence | Product Length (bp) |
|---|---|---|
| GAPDH | F: 5′-AGC CAC ATC GCT CAG ACA CC-3′ R: 5′-GTA CTC AGC GCC AGC ATC G-3′ | 302 |
| MGMT | F: 5′-CCGTTTGCGACTTGGTACTTG-3′ R: 5′-TGGTGAACGACTCTTGCTGG-3′ | 312 |
| PTGS2 | F: 5′-GATGATTGCCCGACTCCCTT-3′ R: 5′-TGAAAAGGCGCAGTTTACGC-3′ | 273 |
| TOP2A | F: 5′-ACCATTGCAGCCTGTAAATGA-3′ R: 5′-GGGCGGAGCAAAATATGTTCC-3′ | 129 |
| Primary Antibodies | |||||
| Antigen | Type/Host | Clone | Catalog No. | Manufacturer | Dilution |
| AKT (pan) | * Mono/Rb | C67E7 | 4691S | CST | 1:2000 |
| p-AKT (Ser473) | Mono/Rb | D9E | 4060S | CST | 1:2000 |
| COX-2 | Mono/Rb | D5H5 | 12282S | CST | 1:1000 |
| GAPDH | Mono/Rb | 14C10 | 2118S | CST | 1:10,000 |
| MGMT | Mono/Mo | - | 51234M | Bioss | 1:1000 |
| MLH1 | Mono/Mo | 4C9C7 | 3515S | CST | 1:1000 |
| MSH2 | Mono/Rb | D24B5 | 2017S | CST | 1:2500 |
| MSH6 | Mono/Rb | D60G2 | 5424S | CST | 1:2500 |
| NDRG1 | Mono/Rb | - | 9485 | CST | 1:2000 |
| TOP2α | Mono/Rb | D10G9 | 12286 | CST | 1:1000 |
| Secondary Antibodies | |||||
| Host | Specificity | Conjugate | Catalog No. | Manufacturer | Dilution |
| Goat | Anti-Rb IgG | HRP | 7074 | CST | 1:5000 |
| Horse | Anti-Mo IgG | HRP | 7076 | CST | 1:5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paukovcekova, S.; Skoda, J.; Neradil, J.; Mikulenkova, E.; Chlapek, P.; Sterba, J.; Richardson, D.R.; Veselska, R. Novel Thiosemicarbazones Sensitize Pediatric Solid Tumor Cell-Types to Conventional Chemotherapeutics through Multiple Molecular Mechanisms. Cancers 2020, 12, 3781. https://doi.org/10.3390/cancers12123781
Paukovcekova S, Skoda J, Neradil J, Mikulenkova E, Chlapek P, Sterba J, Richardson DR, Veselska R. Novel Thiosemicarbazones Sensitize Pediatric Solid Tumor Cell-Types to Conventional Chemotherapeutics through Multiple Molecular Mechanisms. Cancers. 2020; 12(12):3781. https://doi.org/10.3390/cancers12123781
Chicago/Turabian StylePaukovcekova, Silvia, Jan Skoda, Jakub Neradil, Erika Mikulenkova, Petr Chlapek, Jaroslav Sterba, Des R. Richardson, and Renata Veselska. 2020. "Novel Thiosemicarbazones Sensitize Pediatric Solid Tumor Cell-Types to Conventional Chemotherapeutics through Multiple Molecular Mechanisms" Cancers 12, no. 12: 3781. https://doi.org/10.3390/cancers12123781
APA StylePaukovcekova, S., Skoda, J., Neradil, J., Mikulenkova, E., Chlapek, P., Sterba, J., Richardson, D. R., & Veselska, R. (2020). Novel Thiosemicarbazones Sensitize Pediatric Solid Tumor Cell-Types to Conventional Chemotherapeutics through Multiple Molecular Mechanisms. Cancers, 12(12), 3781. https://doi.org/10.3390/cancers12123781






