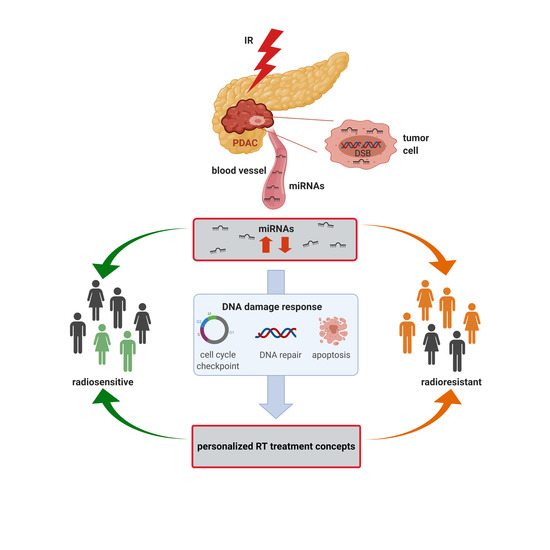
The Emerging Role of miRNAs for the Radiation Treatment of Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. miRNAs as Biomarker in Pancreatic Cancer
2.2. miRNA Response to Ionizing Radiation
2.3. miRNAs and Radioresistanc
2.4. miRNAs and Radioresistance in Pancreatic Cancer
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Wallace, M.B.; Stauffer, J.; Bolan, C.; Raimondo, M.; Woodward, T.A.; Gomez, V.; Ritter, A.W.; Asbun, H.; Mody, K. Survival of Patients with Oligometastatic Pancreatic Ductal Adenocarcinoma Treated with Combined Modality Treatment Including Surgical Resection: A Pilot Study. J. Pancreat. Cancer 2018, 4, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combs, S.E.; Habermehl, D.; Kessel, K.A.; Bergmann, F.; Werner, J.; Naumann, P.; Jäger, D.; Büchler, M.W.; Debus, J. Prognostic Impact of CA 19-9 on Outcome after Neoadjuvant Chemoradiation in Patients with Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2014, 21, 2801–2807. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Büschenfelde, C.M.Z.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [Green Version]
- Dobiasch, S.; Fietkau, R.; Goerig, N.L.; Combs, S.E. Essential role of radiation therapy for the treatment of pancreatic cancer. Strahlenther. Onkol. 2017, 194, 185–195. [Google Scholar] [CrossRef]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouché, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.F.; Van Laethem, J.-L.; Goldstein, D.D.; Glimelius, B.; Artru, P.P.; Borbath, I.; Bouché, O.; Shannon, J.J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled after 4 Months of Gemcitabine with or without Erlotinib. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine Alone Versus Gemcitabine Plus Radiotherapy in Patients With Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef]
- Brunner, M.; Wu, Z.; Krautz, C.; Pilarsky, C.; Grützmann, R.; Weber, G.F. Current Clinical Strategies of Pancreatic Cancer Treatment and Open Molecular Questions. Int. J. Mol. Sci. 2019, 20, 4543. [Google Scholar] [CrossRef] [Green Version]
- Moertel, C.G.; Frytak, S.; Hahn, R.G.; O’Connell, M.J.; Reitemeier, R.J.; Rubin, J.; Schutt, A.J.; Weiland, L.H.; Childs, D.S.; Holbrook, M.A.; et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. The gastrointestinal tumor study group. Cancer 1981, 48, 1705–1710. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hurt, C.N.; Bridgewater, J.; Falk, S.; Cummins, S.; Wasan, H.; Crosby, T.; Jephcott, C.; Roy, R.; Radhakrishna, G.; et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): A multicentre, randomised, phase 2 trial. Lancet Oncol. 2013, 14, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Suker, M.; Nuyttens, J.J.; Koerkamp, B.G.; Eskens, F.A.L.M.; Van Eijck, C.H. FOLFIRINOX and radiotherapy for locally advanced pancreatic cancer: A cohort study. J. Surg. Oncol. 2018, 118, 1021–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, B.R.; Stein, S.; Lloyd, S.; Rutter, C.E.; James, E.; Chang, B.W.; Lacy, J.; Johung, K.L. Chemoradiation after FOLFIRINOX for borderline resectable or locally advanced pancreatic cancer. J. Gastrointest. Oncol. 2018, 9, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy with FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.H.; Sahai, V.; Griffith, K.A.; Nathan, H.; Kaza, R.; Cuneo, K.C.; Shi, J.; Kim, E.; Sonnenday, C.J.; Cho, C.S.; et al. Phase 2 Trial of Neoadjuvant FOLFIRINOX and Intensity Modulated Radiation Therapy Concurrent With Fixed-Dose Rate-Gemcitabine in Patients With Borderline Resectable Pancreatic Cancer. Int. J. Radiat. Oncol. 2020, 106, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.H.G.; Ou, F.S.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chuong, M.; Schwartz, L.H.; et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017, 17, 505. [Google Scholar] [CrossRef]
- Ng, S.P.; Koay, E.J. Current and emerging radiotherapy strategies for pancreatic adenocarcinoma: Stereotactic, intensity modulated and particle radiotherapy. Ann. Pancreat. Cancer 2018, 1, 22. [Google Scholar] [CrossRef]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2019, 68, 159–171. [Google Scholar] [CrossRef]
- Wu, X.; Tang, W.; Marquez, R.T.; Li, K.; Highfill, C.A.; He, F.; Lian, J.; Lin, J.; Fuchs, J.R.; Ji, M.; et al. Overcoming chemo/radio-resistance of pancreatic cancer by inhibiting STAT3 signaling. Oncotarget 2016, 7, 11708–11723. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Xia, X.; Yang, C.; Shen, J.; Mai, J.; Kim, H.-C.; Kirui, D.; Kang, Y.; Fleming, J.B.; Koay, E.J.; et al. SMAD4Gene Mutation Renders Pancreatic Cancer Resistance to Radiotherapy through Promotion of Autophagy. Clin. Cancer Res. 2018, 24, 3176–3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Zhu, E.C.; Qu, Y.-L.; Wang, D.-Y.; Qu, W.-W.; Zhang, C.; Wu, T.; Gao, Z.-H. Serum level of co-expressed hub miRNAs as diagnostic and prognostic biomarkers for pancreatic ductal adenocarcinoma. J. Cancer 2018, 9, 3991–3999. [Google Scholar] [CrossRef] [PubMed]
- Quattrochi, B.; Gulvady, A.; Driscoll, D.R.; Sano, M.; Klimstra, D.S.; Turner, C.E.; Lewis, B.C. MicroRNAs of the mir-17~92 cluster regulate multiple aspects of pancreatic tumor development and progression. Oncotarget 2017, 8, 35902–35918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Royam, M.M.; Ramesh, N.; Shanker, R.; Sabarimurugan, S.; Kumarasamy, C.; Muthukaliannan, G.K.; Baxi, S.; Gupta, A.; Krishnan, S.; Jayaraj, R. miRNA Predictors of Pancreatic Cancer Chemotherapeutic Response: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 900. [Google Scholar] [CrossRef] [Green Version]
- Fesler, A.; Ju, J. Development of microRNA-based therapy for pancreatic cancer. J. Pancreatol. 2019, 2, 147–151. [Google Scholar] [CrossRef]
- Chhatriya, B.; Mukherjee, M.; Ray, S.K.; Sarkar, P.; Chatterjee, S.; Nath, D.; Das, K.; Goswami, S. Comparison of tumour and serum specific microRNA changes dissecting their role in pancreatic ductal adenocarcinoma: A meta-analysis. BMC Cancer 2019, 19, 1175. [Google Scholar] [CrossRef]
- Ouyang, H.; Gore, J.; Deitz, S.; Korc, M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-β actions. Oncogene 2013, 33, 4664–4674. [Google Scholar] [CrossRef] [Green Version]
- Qu, K.; Zhang, X.; Lin, T.; Liu, T.; Wang, Z.; Liu, S.; Zhou, L.; Wei, J.; Chang, H.; Li, K.; et al. Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: Evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci. Rep. 2017, 7, 1692. [Google Scholar] [CrossRef] [Green Version]
- Vila-Navarro, E.; Duran-Sanchon, S.; Vila-Casadesús, M.; Moreira, L.; Ginès, À.; Cuatrecasas, M.; Lozano, J.J.; Bujanda, L.; Castells, A.; Gironella, M. Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clin. Transl. Gastroenterol. 2019, 10, e00029. [Google Scholar] [CrossRef]
- Karasek, P.; Gablo, N.; Hlavsa, J.; Kiss, I.; Vychytilova-Faltejskova, P.; Hermanova, M.; Kala, Z.; Slaby, O.; Prochazka, V. Pre-operative Plasma miR-21-5p Is a Sensitive Biomarker and Independent Prognostic Factor in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Surgical Resection. Cancer Genom. Proteom. 2018, 15, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, T.; Komatsu, S.; Ichikawa, D.; Morimura, R.; Tsujiura, M.; Konishi, H.; Takeshita, H.; Nagata, H.; Arita, T.; Hirajima, S.; et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br. J. Cancer 2013, 108, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemberger, M.; Loewenstein, S.; Lubezky, N.; Nizri, E.; Pasmanik-Chor, M.; Barazovsky, E.; Klausner, J.M.; Lahat, G. MicroRNA profiling of pancreatic ductal adenocarcinoma (PDAC) reveals signature expression related to lymph node metastasis. Oncotarget 2019, 10, 2644–2656. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Wald, P.; Webb, A.; Sebastian, N.; Walston, S.; Robb, R.; Chen, W.; Vedaie, M.; Dillhoff, M.; Frankel, W.L.; et al. A microRNA-based signature predicts local-regional failure and overall survival after pancreatic cancer resection. Oncotarget 2020, 11, 913–923. [Google Scholar] [CrossRef]
- Schmiegel, W.-H.; Kreiker, C.; Eberl, W.; Arndt, R.; Classen, M.; Greten, H.; Jessen, K.; Kalthoff, H.; Soehendra, N.; Thiele, H.-G. Monoclonal antibody defines CA 19-9 in pancreatic juices and sera. Gut 1985, 26, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, J.; Du, Y.; Li, Z.; Ren, Y.; Gu, J.; Wang, X.; Gong, Y.; Wang, W.; Kong, X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int. J. Cancer 2012, 131, 683–691. [Google Scholar] [CrossRef]
- LaConti, J.J.; Shivapurkar, N.; Preet, A.; Mays, A.D.; Peran, I.; Kim, S.E.; Marshall, J.L.; Riegel, A.T.; Wellstein, A. Tissue and Serum microRNAs in the KrasG12D Transgenic Animal Model and in Patients with Pancreatic Cancer. PLoS ONE 2011, 6, e20687. [Google Scholar] [CrossRef] [Green Version]
- Mazza, T.; Copetti, M.; Capocefalo, D.; Fusilli, C.; Biagini, T.; Carella, M.; De Bonis, A.; Mastrodonato, N.; Piepoli, A.; Pazienza, V.; et al. MicroRNA co-expression networks exhibit increased complexity in pancreatic ductal compared to Vater’s papilla adenocarcinoma. Oncotarget 2017, 8, 105320–105339. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Lu, Y.; Li, X. miR-339-3p inhibits proliferation and metastasis of colorectal cancer. Oncol. Lett. 2015, 10, 2842–2848. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Gao, J.; Wang, D.; Lin, J.-H.; Liao, Z.; Ji, J.-T.; Du, T.-T.; Jiang, F.; Hu, L.-H.; Li, Z. Novel blood-based microRNA biomarker panel for early diagnosis of chronic pancreatitis. Sci. Rep. 2017, 7, 40019. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, X.; Pan, B.; He, B.; Chen, X.; Zeng, K.; Xu, M.; Pan, Y.; Sun, H.; Xu, T.; et al. Circulating miR-1290 and miR-320d as Novel Diagnostic Biomarkers of Human Colorectal Cancer. J. Cancer 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ding, X.; Wang, S.; Xu, L.; Yin, T.; Han, S.; Geng, J.; Sun, W. Downregulation of serum exosomal miR-320d predicts poor prognosis in hepatocellular carcinoma. J. Clin. Lab. Anal. 2020, 34, e23239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Zhan, M.; Xu, S.; Yang, R.; Chen, W.; Zhang, S.; Shi, Y.; Yongheng, S.; Mohan, M.; Liu, Q.; et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol. Cancer 2017, 16, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Cheng, J.; Yao, Y.; Lou, C.; Wang, L.; Huang, X.; Zhang, Y. Combination of Four Serum Exosomal MiRNAs as Novel Diagnostic Biomarkers for Early-Stage Gastric Cancer. Front. Genet. 2020, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, Z.; Wei, S.; Wang, W.; Chen, Z.; Zhang, L.; Chen, L.; Li, B.; Sun, G.; Xu, J.; et al. Overexpression of miR-584-5p inhibits proliferation and induces apoptosis by targeting WW domain-containing E3 ubiquitin protein ligase 1 in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 59. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Zhu, W.; Xu, J.; Guo, R.; Cheng, W.; Wang, F.; Qi, L.-W.; Chen, Y.; et al. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget 2017, 8, 6513–6525. [Google Scholar] [CrossRef]
- Ni, J.; Zheng, H.; Huang, Z.; Hong, Y.; Ou, Y.; Tao, Y.; Wang, M.; Wang, Z.; Yang, Y.; Zhou, W. MicroRNA-197-3p acts as a prognostic marker and inhibits cell invasion in hepatocellular carcinoma. Oncol. Lett. 2018, 17, 2317–2327. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, A.; Shiino, S.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Matsuzaki, J.; Ono, M.; Takeshita, F.; Niida, S.; Shimizu, C.; et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016, 107, 326–334. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget 2017, 8, 77028–77040. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Wu, Y.-N.; Xu, J. Serum miR-1290 and miR-1246 as Potential Diagnostic Biomarkers of Human Pancreatic Cancer. J. Cancer 2020, 11, 1325–1333. [Google Scholar] [CrossRef]
- Madhavan, B.; Yue, S.; Galli, U.; Rana, S.; Gross, W.; Müller, M.; Giese, N.A.; Kalthoff, H.; Becker, T.; Büchler, M.W.; et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int. J. Cancer 2015, 136, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Z.; Zhu, X.; Chen, L.; Ma, Y.; Wang, J.; Yang, X.-Z.; Liu, Z. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int. J. Clin. Oncol. 2019, 25, 89–99. [Google Scholar] [CrossRef]
- Takeshita, N.; Hoshino, I.; Mori, M.; Akutsu, Y.; Hanari, N.; Yoneyama, Y.; Ikeda, N.; Isozaki, Y.; Maruyama, T.; Akanuma, N.; et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br. J. Cancer 2013, 108, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Nagamitsu, Y.; Nishi, H.; Sasaki, T.; Takaesu, Y.; Terauchi, F.; Isaka, K. Profiling analysis of circulating microRNA expression in cervical cancer. Mol. Clin. Oncol. 2016, 5, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czochor, J.R.; Glazer, P.M. microRNAs in Cancer Cell Response to Ionizing Radiation. Antioxid. Redox Signal. 2014, 21, 293–312. [Google Scholar] [CrossRef]
- Chaudhry, M.A. Radiation-induced microRNA: Discovery, functional analysis, and cancer radiotherapy. J. Cell. Biochem. 2014, 115, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Liu, Y.; Zhang, H.; Di, C.; Sun, C. microRNA Expression and Biogenesis in Cellular Response to Ionizing Radiation. DNA Cell Biol. 2014, 33, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wan, G.; Berger, F.G.; He, X.; Lu, X. The ATM Kinase Induces MicroRNA Biogenesis in the DNA Damage Response. Mol. Cell 2011, 41, 371–383. [Google Scholar] [CrossRef] [Green Version]
- He, L.; He, X.; Lim, L.P.; De Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.F.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nat. Cell Biol. 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.D.; Savage, J.E.; Cao, L.; Soule, B.P.; Ly, D.; DeGraff, W.; Harris, C.C.; Mitchell, J.B.; Simone, N.L. Cellular Stress Induced Alterations in MicroRNA let-7a and let-7b Expression Are Dependent on p53. PLoS ONE 2011, 6, e24429. [Google Scholar] [CrossRef]
- Wei, F.; Liu, Y.; Guo, Y.; Xiang, A.; Wang, G.-Y.; Xue, X.; Lu, Z. miR-99b-targeted mTOR induction contributes to irradiation resistance in pancreatic cancer. Mol. Cancer 2013, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.-J.; Chen, Y.-Y.; Dai, J.-J.; Gu, D.-N.; Mei, Z.; Liu, F.-R.; Huang, Q.; Tian, L. Dying tumor cell-derived exosomal miR-194-5p potentiates survival and repopulation of tumor repopulating cells upon radiotherapy in pancreatic cancer. Mol. Cancer 2020, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Małachowska, B.; Tomasik, B.; Stawiski, K.; Kulkarni, S.; Guha, C.; Chowdhury, D.; Fendler, W. Circulating microRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. 2020, 106, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Farsinejad, S.; Rahaie, M.; Alizadeh, A.M.; Mir-Derikvand, M.; Gheisary, Z.; Nosrati, H.; Khalighfard, S. Expression of the circulating and the tissue microRNAs after surgery, chemotherapy, and radiotherapy in mice mammary tumor. Tumor Biol. 2016, 37, 14225–14234. [Google Scholar] [CrossRef]
- Long, Z.-W.; Wu, J.-H.; Hong, C.; Wang, Y.-N.; Zhou, Y. MiR-374b Promotes Proliferation and Inhibits Apoptosis of Human GIST Cells by Inhibiting PTEN through Activation of the PI3K/Akt Pathway. Mol. Cells 2018, 41, 532–544. [Google Scholar]
- Sun, W.; Lan, J.; Chen, L.; Qiu, J.; Luo, Z.; Li, M.; Wang, J.; Zhao, J.; Zhang, T.; Long, X.; et al. A mutation in porcine pre-miR-15b alters the biogenesis of MiR-15b\16-1 cluster and strand selection of MiR-15b. PLoS ONE 2017, 12, e0178045. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-L.; Zhang, J.; Wu, X.-Z.; Yan, T.; Lv, W. miR-15b promotes epithelial-mesenchymal transition by inhibiting SMURF2 in pancreatic cancer. Int. J. Oncol. 2015, 47, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhou, C.; Luo, M.; Shi, X.; Li, Y.; Sun, Z.; Zhou, F.; Chen, Z.; He, J. MiR-652-3p is upregulated in non-small cell lung cancer and promotes proliferation and metastasis by directly targeting Lgl1. Oncotarget 2016, 7, 16703–16715. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Luan, J.; Ding, Y. miR-144-3p Targets FosB Proto-oncogene, AP-1 Transcription Factor Subunit (FOSB) to Suppress Proliferation, Migration, and Invasion of PANC-1 Pancreatic Cancer Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 683–690. [Google Scholar] [CrossRef]
- Cioffi, M.; Trabulo, S.M.; Vallespinos, M.; Raj, D.; Kheir, T.B.; Lin, M.-L.; Begum, J.; Baker, A.-M.; Amgheib, A.; Saif, J.; et al. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget 2017, 8, 21609–21625. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, I.; Kinoshita, T.; Hanazawa, T.; Kikkawa, N.; Chiyomaru, T.; Enokida, H.; Yamamoto, N.; Goto, Y.; Nishikawa, R.; Nakagawa, M.; et al. Identification of tumour suppressive microRNA-451a in hypopharyngeal squamous cell carcinoma based on microRNA expression signature. Br. J. Cancer 2014, 111, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-L.; Bai, Z.-H.; Wang, X.-B.; Bai, L.; Miao, F.; Pei, H.-H. miR-186 and 326 Predict the Prognosis of Pancreatic Ductal Adenocarcinoma and Affect the Proliferation and Migration of Cancer Cells. PLoS ONE 2015, 10, e0118814. [Google Scholar] [CrossRef]
- Yu, J.; Ohuchida, K.; Mizumoto, K.; Fujita, H.; Nakata, K.; Tanaka, M. MicroRNAmiR-17-5pis overexpressed in pancreatic cancer, associated with a poor prognosis, and involved in cancer cell proliferation and invasion. Cancer Biol. Ther. 2010, 10, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Gu, J.; Li, Y.; Peng, C.; Shi, M.; Wang, X.; Wei, G.; Ge, O.; Wang, D.; Zhang, B.; et al. MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett. 2018, 412, 59–68. [Google Scholar] [CrossRef]
- Yan, H.-J.; Liu, W.-S.; Sun, W.-H.; Wu, J.; Ji, M.; Wang, Q.; Zheng, X.; Jiang, J.; Wu, C. miR-17-5p Inhibitor Enhances Chemosensitivity to Gemcitabine Via Upregulating Bim Expression in Pancreatic Cancer Cells. Dig. Dis. Sci. 2012, 57, 3160–3167. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, S.; Zhou, Y.; Liu, C.; Hu, X.-G.; Shao, C. MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer. Biochem. Biophys. Res. Commun. 2011, 406, 552–557. [Google Scholar] [CrossRef]
- Siragam, V.; Rutnam, Z.J.; Yang, W.; Fang, L.; Luo, L.; Yang, X.; Li, M.; Deng, Z.; Qian, J.; Peng, C.; et al. MicroRNA miR-98 inhibits tumor angiogenesis and invasion by targeting activin receptor-like kinase-4 and matrix metalloproteinase-11. Oncotarget 2012, 3, 1370–1385. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yuan, C.; Lv, K.; Xie, S.; Fu, P.; Liu, X.; Chen, Y.; Qin, C.; Deng, W.; Hu, W. Lin28 Mediates Radiation Resistance of Breast Cancer Cells via Regulation of Caspase, H2A.X and Let-7 Signaling. PLoS ONE 2013, 8, e67373. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liu, X.; Chen, Q.; Liu, T.; Lu, C.; Yu, J.; Miao, Y.; Wei, J. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J. Exp. Clin. Cancer Res. 2018, 37, 130. [Google Scholar] [CrossRef] [Green Version]
- Morimura, R.; Komatsu, S.; Ichikawa, D.; Takeshita, H.; Tsujiura, M.; Nagata, H.; Konishi, H.; Shiozaki, A.; Ikoma, H.; Okamoto, K.; et al. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br. J. Cancer 2011, 105, 1733–1740. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Pu, Y.; Qian, L.; Zang, C.; Tao, Z.; Gao, J. MiR-20a-5p promotes radio-resistance by targeting NPAS2 in nasopharyngeal cancer cells. Oncotarget 2017, 8, 105873–105881. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zheng, L.; Ding, Y.; Li, Q.; Wang, R.; Liu, T.; Sun, Q.; Yang, H.; Peng, S.; Wang, W.; et al. MiR-20a Induces Cell Radioresistance by Activating the PTEN/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2015, 92, 1132–1140. [Google Scholar] [CrossRef]
- Huang, J.-W.; Wang, Y.; Dhillon, K.K.; Calses, P.; Villegas, E.; Mitchell, P.S.; Tewari, M.; Kemp, C.J.; Taniguchi, T. Systematic Screen Identifies miRNAs That Target RAD51 and RAD51D to Enhance Chemosensitivity. Mol. Cancer Res. 2013, 11, 1564–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Ji, N.; Wei, W.; Sun, W.; Gong, X.; Wang, X. MiR-142 modulates human pancreatic cancer proliferation and invasion by targeting hypoxia-inducible factor 1 (HIF-1α) in the tumor microenvironments. Biol. Open 2017, 6, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; He, M.; Chen, L.; Chen, C.; Zheng, J.; Cai, Z. The Loss of miR-26a-Mediated Post-Transcriptional Regulation of Cyclin E2 in Pancreatic Cancer Cell Proliferation and Decreased Patient Survival. PLoS ONE 2013, 8, e76450. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Gong, X.; Zhang, G.; Huang, G.; Lu, Y.; Li, Y.-X. MicroRNA-140 regulates cell growth and invasion in pancreatic duct adenocarcinoma by targeting iASPP. Acta Biochim. Biophys. Sin. (Shanghai) 2016, 48, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Lan, F.; Yue, X.; Ren, G.; Li, H.; Ping, L.; Wang, Y.; Xia, T. miR-15a/16 Enhances Radiation Sensitivity of Non-Small Cell Lung Cancer Cells by Targeting the TLR1/NF-κB Signaling Pathway. Int. J. Radiat. Oncol. 2015, 91, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.R.; Frampton, A.E.; Jacob, J.; Pellegrino, L.; Krell, J.; Giamas, G.; Tsim, N.; Vlavianos, P.; Cohen, P.; Ahmad, R.; et al. MicroRNAs Targeting Oncogenes Are Down-Regulated in Pancreatic Malignant Transformation from Benign Tumors. PLoS ONE 2012, 7, e32068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xiang, H.; Ge, W.; Wang, H.; Wang, T.; Xiong, M. Expression and functional perspectives of miR-184 in pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 12313–12318. [Google Scholar]
- Zhao, L.; Bode, A.M.; Cao, Y.; Dong, Z. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis 2012, 33, 2220–2227. [Google Scholar] [CrossRef]
- Lal, A.; Pan, Y.; Navarro, F.; Dykxhoorn, D.M.; Moreau, L.; Meire, E.; Bentwich, Z.; Lieberman, J.; Chowdhury, D. miR-24–mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat. Struct. Mol. Biol. 2009, 16, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Luo, J.; Liu, Z.; Zhou, R.; Luo, H. MicroRNA-138 Regulates DNA Damage Response in Small Cell Lung Cancer Cells by Directly Targeting H2AX. Cancer Investig. 2015, 33, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zou, F.; Zhang, X.; Li, H.; Dulak, A.; Tomko, R.J.; Lazo, J.S.; Wang, Z.; Zhang, L.; Yu, J.; et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009, 69, 8157–8165. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Ng, W.L.; Zhang, X.; Wang, P.; Zhang, Z.; Mo, Y.-Y.; Mao, H.; Hao, C.; Olson, J.J.; Curran, W.J.; et al. Targeting DNA-PKcs and ATM with miR-101 Sensitizes Tumors to Radiation. PLoS ONE 2010, 5, e11397. [Google Scholar] [CrossRef]
- Hu, H.; Du, L.; Nagabayashi, G.; Seeger, R.C.; Gatti, R.A. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc. Natl. Acad. Sci. USA 2010, 107, 1506–1511. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, S.; Ogusu, R.; Wada, Y.; Matsuyama, S.; Mori, T. PTEN, A Target of Microrna-374b, Contributes to the Radiosensitivity of Canine Oral Melanoma Cells. Int. J. Mol. Sci. 2019, 20, 4631. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Su, T.; Ye, J.; Yang, C.; Zhang, S.; Xie, C. The miR-15 Family Enhances the Radiosensitivity of Breast Cancer Cells by Targeting G2Checkpoints. Radiat. Res. 2015, 183, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Peng, L.; Hua, S.; Li, X.; Ma, L.; Jie, J.; Chen, D.; Wang, Y.; Li, D. miR-144-5p Enhances the Radiosensitivity of Non-Small-Cell Lung Cancer Cells via Targeting ATF2. BioMed Res. Int. 2018, 2018, 5109497. [Google Scholar] [CrossRef] [Green Version]
- Lan, F.; Yu, H.; Hu, M.; Xia, T.; Yue, X. miR-144-3p exerts anti-tumor effects in glioblastoma by targeting c-Met. J. Neurochem. 2015, 135, 274–286. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Y.; Hou, J.; Zhai, C.; Song, Y.; Zhang, Z.; Qiu, L.; Jia, X. MicroRNA-144 affects radiotherapy sensitivity by promoting proliferation, migration and invasion of breast cancer cells. Oncol. Rep. 2015, 34, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, M.; Ding, C.; Wang, X.; Wang, R.; Wu, X.; Fan, R. Hypoxia-responsive miR-124 and miR-144 reduce hypoxia-induced autophagy and enhance radiosensitivity of prostate cancer cells via suppressing PIM 1. Cancer Med. 2016, 5, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, J.; Zhang, Q.; Liu, B.; Cheng, Y.; Zhang, Y.; Sun, Y.; Ge, H.; Liu, Y. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J. Exp. Clin. Cancer Res. 2020, 39, 65. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, S.; Zhou, H.; Guo, L. Direct Downregulation of B-Cell Translocation Gene 3 by microRNA-93 Is Required for Desensitizing Esophageal Cancer to Radiotherapy. Dig. Dis. Sci. 2017, 62, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, R.; Rana, S.; Kelley, K.; Espinosa-Diez, C.; Hudson, C.; Lanciault, C.; Thomas, C.R., Jr.; Liana Tsikitis, V.; Anand, S. microRNA-451a regulates colorectal cancer proliferation in response to radiation. BMC Cancer 2018, 18, 517. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Han, Y.; Yan, X.; Zhong, D.; Yang, G.; Lei, J.; Li, X.; Wang, X. Upregulation of microrna-451 increases the sensitivity of A 549 cells to radiotherapy through enhancement of apoptosis. Thorac. Cancer 2015, 7, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, D.-Q.; Huang, J.-Y.; Zhang, K.; Feng, B.; Pan, B.-Z.; Chen, J.; De, W.; Chen, L.-B. Acquisition of radioresistance in docetaxel-resistant human lung adenocarcinoma cells is linked with dysregulation of miR-451/c-Myc-survivin/rad-51 signaling. Oncotarget 2014, 5, 6113–6129. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Sun, Q.; Liu, T.; Chen, J.; Du, S.; Ren, C.; Liao, G.; Yuan, Y. MiR-451 increases radiosensitivity of nasopharyngeal carcinoma cells by targeting ras-related protein 14 (RAB14). Tumor Biol. 2014, 35, 12593–12599. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W. FOXD1, negatively regulated by miR-186, promotes the proliferation, metastasis and radioresistance of nasopharyngeal carcinoma cells. Cancer Biomark. 2020, 28, 511–521. [Google Scholar] [CrossRef]
- Lynam-Lennon, N.; Heavey, S.; Sommerville, G.; Bibby, B.A.; Ffrench, B.; Quinn, J.; Gasch, C.; O’Leary, J.J.; Gallagher, M.F.; Reynolds, J.V.; et al. MicroRNA-17 is downregulated in esophageal adenocarcinoma cancer stem-like cells and promotes a radioresistant phenotype. Oncotarget 2016, 8, 11400–11413. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Wu, A.T.; Liu, S.-H. MicroRNA-17-5p regulated apoptosis-related protein expression and radiosensitivity in oral squamous cell carcinoma caused by betel nut chewing. Oncotarget 2016, 7, 51482–51493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Cui, S.; Zhang, R.; Shi, Y.; Luo, L. MiR-421 inhibits the malignant phenotype in glioma by directly targeting MEF2D. Am. J. Cancer Res. 2017, 7, 857–868. [Google Scholar] [PubMed]
- Mansour, W.Y.; Bogdanova, N.V.; Kasten-Pisula, U.; Rieckmann, T.; Köcher, S.; Borgmann, K.; Baumann, M.; Krause, M.; Petersen, C.; Hu, H.; et al. Aberrant overexpression of miR-421 downregulates ATM and leads to a pronounced DSB repair defect and clinical hypersensitivity in SKX squamous cell carcinoma. Radiother. Oncol. 2013, 106, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-Y.; Chen, Q.-J.; Wei, Y.; Wang, Y.-L.; Wang, Z.-W.; Xu, K.; He, Y.; Ma, H. Upregulation of microRNA-98 increases radiosensitivity in esophageal squamous cell carcinoma. J. Radiat. Res. 2016, 57, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Shi, X.-B.; Nori, D.; Chao, C.K.; Chen, A.M.; Valicenti, R.; White, R.D. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate 2011, 71, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Troschel, F.M.; Böhly, N.; Borrmann, K.; Braun, T.; Schwickert, A.; Kiesel, L.; Eich, H.T.; Götte, M.; Greve, B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumor Biol. 2018, 40. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Liu, L.; Lei, Y.; Hu, Y. MiRNA-142-3p increases radiosensitivity in human umbilical cord blood mononuclear cells by inhibiting the expression of CD133. Sci. Rep. 2018, 8, 5674. [Google Scholar] [CrossRef]
- Jin, Q.; Li, X.J.; Cao, P.G. MicroRNA-26b Enhances the Radiosensitivity of Hepatocellular Carcinoma Cells by Targeting EphA2. Tohoku J. Exp. Med. 2016, 238, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Duru, N.; Gernapudi, R.; Zhang, Y.; Yao, Y.; Lo, P.-K.; Wolfson, B.; Zhou, Q. NRF2/miR-140 signaling confers radioprotection to human lung fibroblasts. Cancer Lett. 2015, 369, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Mao, A.; Tang, J.; Zhang, Q.; Yan, J.; Wang, Y.; Di, C.; Gan, L.; Sun, C.; Zhang, H. microRNA-16-5p enhances radiosensitivity through modulating Cyclin D1/E1–pRb–E2F1 pathway in prostate cancer cells. J. Cell. Physiol. 2019, 234, 13182–13190. [Google Scholar] [CrossRef]
- Samadi, P.; Afshar, S.; Amini, R.; Najafi, R.; Mahdavinezhad, A.; Pashaki, A.S.; Gholami, M.H.; Saidijam, M. Let-7e enhances the radiosensitivity of colorectal cancer cells by directly targeting insulin-like growth factor 1 receptor. J. Cell. Physiol. 2019, 234, 10718–10725. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Swoboda, J.; Fendler, W.; Lerch, M.M.; Sendler, M.; Moskwa, P. MiR-502 is the first reported miRNA simultaneously targeting two components of the classical non-homologous end joining (C-NHEJ) in pancreatic cell lines. Heliyon 2020, 6, e03187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhang, J.; Zhang, L.; Zhu, Z.; Fan, J.; Chen, L.; Zhuang, L.; Luo, J.; Chen, H.; Liu, L.; et al. MicroRNA 23b Regulates Autophagy Associated With Radioresistance of Pancreatic Cancer Cells. Gastroenterology 2013, 145, 1133–1143.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Lin, S.; Ba, M.-C.; Cui, S. MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy. Oncol. Rep. 2015, 34, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Tomihara, H.; Yamada, D.; Eguchi, H.; Iwagami, Y.; Noda, T.; Asaoka, T.; Wada, H.; Kawamoto, K.; Gotoh, K.; Takeda, Y.; et al. MicroRNA-181b-5p, ETS1, and the c-Met pathway exacerbate the prognosis of pancreatic ductal adenocarcinoma after radiation therapy. Cancer Sci. 2017, 108, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; DeSano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 Inhibits Human Pancreatic Cancer Tumor-Initiating Cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef]
- Fang, C.; Dai, C.-Y.; Mei, Z.; Jiang, M.-J.; Gu, D.-N.; Huang, Q.; Tian, L. microRNA-193a stimulates pancreatic cancer cell repopulation and metastasis through modulating TGF-β2/TGF-βRIII signalings. J. Exp. Clin. Cancer Res. 2018, 37, 25. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Taeb, S.; Jahangiri, S.; Korpela, E.; Cadonic, I.; Yu, N.; Krylov, S.N.; Fokas, E.; Boutros, P.C.; Liu, S.K. miR-620 promotes tumor radioresistance by targeting 15-hydroxyprostaglandin dehydrogenase (HPGD). Oncotarget 2015, 6, 22439–22451. [Google Scholar] [CrossRef]
- Oh, J.-S.; Kim, J.-J.; Byun, J.-Y.; Kim, I.A. Lin28-let7 Modulates Radiosensitivity of Human Cancer Cells with Activation of K-Ras. Int. J. Radiat. Oncol. 2010, 76, 5–8. [Google Scholar] [CrossRef]
- Baek, S.-J.; Azuma, R.; Hayashi, K.; Ishii, H.; Sato, K.; Nishida, N.; Koseki, J.; Kawamoto, K.; Konno, M.; Satoh, T.; et al. MicroRNA miR-374, a potential radiosensitizer for carbon ion beam radiotherapy. Oncol. Rep. 2016, 36, 2946–2950. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 Broadly Influences Gene Expression and Promotes Apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Hao, X.; Meng, Y.; Zhang, M.; DeSano, J.; Fan, D.; Xu, L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer 2008, 8, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Matsui, W.; Khaki, L.; Stearns, D.; Chun, J.; Li, Y.-M.; Eberhart, C.G. Notch Pathway Inhibition Depletes Stem-like Cells and Blocks Engraftment in Embryonal Brain Tumors. Cancer Res. 2006, 66, 7445–7452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raufi, A.G.; Manji, G.A.; Chabot, J.A.; Bates, S.E. Neoadjuvant Treatment for Pancreatic Cancer. Semin. Oncol. 2019, 46, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Goodman, K.A. Radiation therapy for pancreatic adenocarcinoma, a treatment option that must be considered in the management of a devastating malignancy. Radiat. Oncol. 2019, 14, 114. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Fesler, A.; Hwang, G.-R.; Ju, J. microRNA based prognostic biomarkers in pancreatic Cancer. Biomark. Res. 2018, 6, 18. [Google Scholar] [CrossRef]
- Moertl, S.; Mutschelknaus, L.; Heider, T.; Atkinson, M.J. MicroRNAs as novel elements in personalized radiotherapy. Transl. Cancer Res. 2016, 5, S1262–S1269. [Google Scholar] [CrossRef]
- Li, X.; Gao, P.; Wang, Y.; Wang, X. Blood-Derived microRNAs for Pancreatic Cancer Diagnosis: A Narrative Review and Meta-Analysis. Front. Physiol. 2018, 9, 685. [Google Scholar] [CrossRef]
- Buscail, E.; Maulat, C.; Muscari, F.; Chiche, L.; Cordelier, P.; Dabernat, S.; Alix-Panabières, C.; Buscail, L. Liquid Biopsy Approach for Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 852. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, D.; Haberberger, A.; Kirchner, B.; Spornraft, M.; Riedmaier, I.; Schelling, G.; Pfaffl, M.W. Toward reliable biomarker signatures in the age of liquid biopsies—How to standardize the small RNA-Seq workflow. Nucleic Acids Res. 2016, 44, 5995–6018. [Google Scholar] [CrossRef]
- Cacheux, J.; Bancaud, A.; Leïchlé, T.; Cordelier, P. Technological Challenges and Future Issues for the Detection of Circulating MicroRNAs in Patients with Cancer. Front. Chem. 2019, 7, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpela, E.; Vesprini, D.; Liu, S.K. MicroRNA in radiotherapy: miRage or miRador? Br. J. Cancer 2015, 112, 777–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
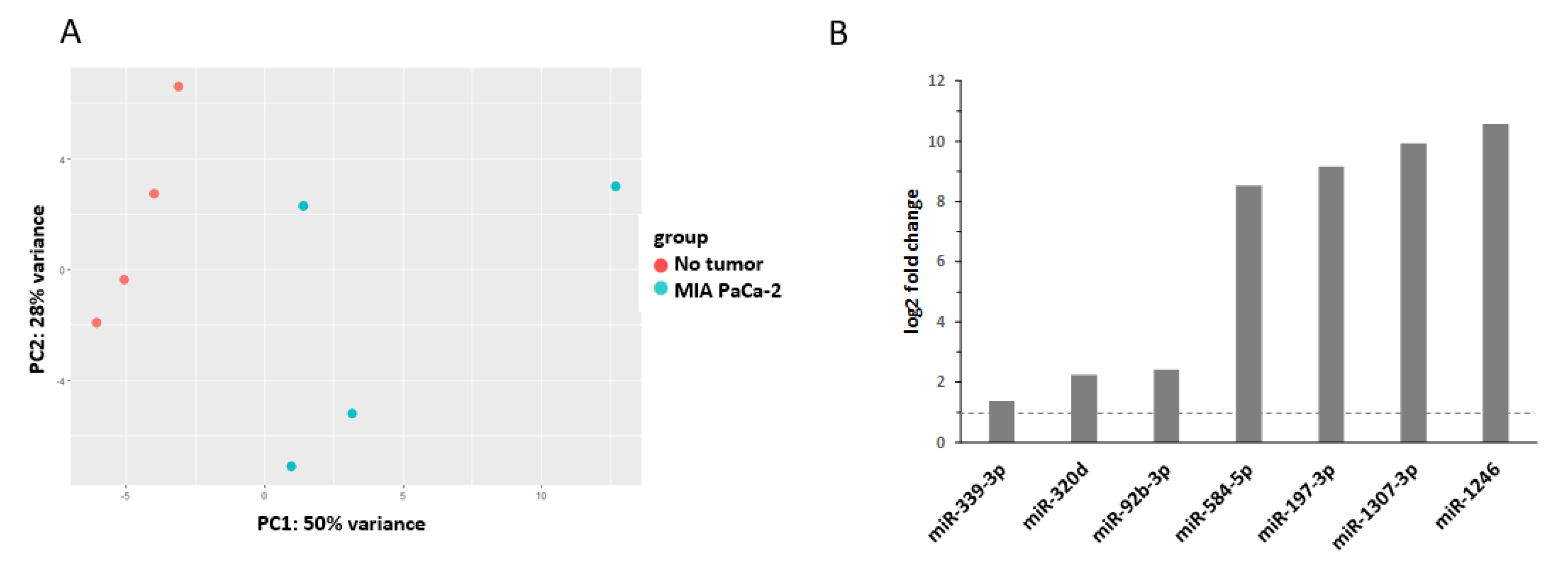

| miRNA | (Tumor) Entity | Expression | Source | Reference |
|---|---|---|---|---|
| miR-339-3p | Vater’s papilla adenocarcinoma | downregulated | tissue | [38] |
| colorectal cancer | downregulated | tissue | [39] | |
| miR-320d | chronic pancreatitis | upregulated | tissue | [40] |
| colorectal cancer | downregulated | plasma and tissue | [41] | |
| hepatocellular carcinoma (HCC) | downregulated | serum exosomes | [42] | |
| miR-92b-3p | pancreatic ductal adenocarcinoma (PDAC) | downregulated | tissue | [43] |
| gastric cancer | upregulated | serum exosomes | [44] | |
| miR-584-5p | gastric cancer | downregulated | tissue | [45] |
| lung cancer | upregulated | plasma | [46] | |
| miR-197-3p | HCC | downregulated | tissue | [47] |
| miR-1307-3p | breast cancer | upregulated | serum | [48] |
| miR-1246 | breast cancer | upregulated | serum | [48] |
| PDAC | upregulated | plasma exosomes | [49] | |
| PDAC | upregulated | serum | [50] | |
| PDAC | upregulated | serum exosomes | [51] | |
| gastric cancer | upregulated | serum exosomes | [52] | |
| esophageal squamous cell carcinoma (SCC) | upregulated | serum | [53] | |
| cervical cancer | upregulated | serum | [54] |
| miRNA from Figure 2 | miRNA in Reference | Radio-Resistance | Entity (In Vitro/In Vivo) * | Targets # | Proteins/ Pathways § | Ref. |
|---|---|---|---|---|---|---|
| miR-374b-5p | miR-374b-5p | increased | Canine oral melanoma (in vitro) | PTEN 1 | [97] | |
| miR-15b-3p | miR-15b | decreased | Breast cancer (in vitro) | Chk1, Wee1 1 | [98] | |
| miR-144-5p/ miR-144-3p | miR-144-5p | decreased | NSCLC (in vitro/in vivo) | ATF2 1 | [99] | |
| miR-144-3p | decreased | Glioblastoma (in vitro) | c-MET 1 | Phosphorylation of STAT3, ERK1/2, AKT, mTOR (all down) 4 | [100] | |
| miR-144 | increased | Breast cancer (in vitro) | PTEN (down) 4 AKT, Snail, N-cadherin, Vimentin (all up) 4 | [101] | ||
| miR-144 | decreased | Prostate cancer (in vitro) | PIM1 1 | [102] | ||
| miR-93-5p | miR-93-5p | increased | Colorectal cancer(in vitro) | FOXA1 1 | TGFB3 (up) 4 | [103] |
| miR-93 | increased | Esophageal squamous carcinoma (in vitro) | BTG3 1 | [104] | ||
| miR-451a | miR-451a | decreased | Mouse colorectal cancer (in vitro) | CAB39, EMSY, MEX3C, EREG 2 | [105] | |
| miR-451 | decreased | NSCLC (in vitro) | PTEN (up) 4 | [106] | ||
| miR-451 | decreased | Lung adenocarcinoma (in vitro) | c-MYC 1 | Survivin, rad-51 (both down) 4 | [107] | |
| miR-451 | decreased | Nasopharyngeal carcinoma (in vitro) | RAB14 1 | [108] | ||
| miR-186-5p | miR-186 | decreased | Nasopharyngeal carcinoma (in vitro) | FOXD1 1 | [109] | |
| miR-17-5p | miR-17-5p | decreased | Esophageal adenocarcinoma (in vitro) | PRKACB, C6orf120 3 | PRKACB, C6orf120 (both down) 5 | [110] |
| miR-17-5p | increased | Oral squamous cell carcinoma (in vitro/in vivo) | p21, p-p53, TNF RI, FADD (all down) 4 cIAP1, HIF-1α, TRAIL R1 (all up) 4 | [111] | ||
| miR-421 | miR-421 | decreased | Glioma (in vitro) | MEF2D 1 | [112] | |
| miR-421 | decreased | Cervix carcinoma, NSCLC and SCCHN (in vitro) | ATM 1 | [113] | ||
| miR-98-5p | miR-98 | decreased | Esophageal squamous cell carcinoma (in vitro) | BCL2 1 | [114] | |
| miR-20a-5p | miR-20a-5p | increased | Nasopharyngeal cancer (in vitro) | NPAS2 1 | Notch pathway (down) 6 | [82] |
| miR-106b-5p | miR-106b | increased | Prostate cancer (in vitro) | p21 (down) 4 | [115] | |
| miR-142-3p | miR-142-3p | decreased | Breast cancer (in vitro) | β-catenin (down) 4 | [116] | |
| miR-142-3p | decreased | Umbilical cord blood mononuclear cells (in vitro) | CD133 1 | [117] | ||
| miR-26b-5p | miR-26b-5p | decreased | Hepatocellular carcinoma (in vitro) | EphA2 1 | [118] | |
| miR-140-5p | miR-140 | increased | Lung fibroblasts (in vitro/in vivo) | [119] | ||
| miR-16-5p | miR-16-5p | decreased | Prostate cancer (in vitro) | Cyclin D1, Cyclin E1 1 | pRb, E2F1 (both down) 4 | [120] |
| let 7i-5p let 7d-5p | let-7 family (let-7e) | decreased | Colorectal cancer (in vitro) | IGF-1R (down) 4 | [121] |
| miRNA | Radioresistance | Cell Lines | Targets | Intervention | Ref. |
|---|---|---|---|---|---|
| miR-502 | reduced | Mia PaCa-2, PaTuT, PaTu02 | Ku70, XLF | miR-502 overexpression | [122] |
| miR-23b | reduced | Panc-1, BxPc3 | ATG12 | miR-23b mimic/inhibitor | [123] |
| miR-216a | reduced | Panc-1, BxPc3 | beclin-1 | miR-216a mimic | [124] |
| miR-99b | reduced | Panc-1, BxPc3, Capan-2 | mTOR | miR-99b precursor/inhibitor | [61] |
| miR-181b | reduced | Panc-1, MIA PaCa-2 | ETS (c-Met) | miR-181b precursor | [125] |
| miR-34 | reduced | BxPc3, MIA PaCa-2 | Bcl-2, Notch1-2 | miR-34 mimic | [126] |
| miR-193a | increased | Panc-1, SW1990, AsPc-1 | TGF-β2/TGF-βRIII, E2F6 | miR-193a antagonist | [127] |
| miR-620 | increased | MIA PaCa-2 | HPGD | miR-620 mimic | [128] |
| Let-7a | reduced | AsPc-1 | K-Ras | Lin28 siRNA (repressor of let-7a) | [129] |
| miR-374 | unchanged/ reduced | Panc-1, MIA PaCa-2 | - | miR-374 overexpression | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.; Schilling, D.; Dobiasch, S.; Raulefs, S.; Santiago Franco, M.; Buschmann, D.; Pfaffl, M.W.; Schmid, T.E.; Combs, S.E. The Emerging Role of miRNAs for the Radiation Treatment of Pancreatic Cancer. Cancers 2020, 12, 3703. https://doi.org/10.3390/cancers12123703
Nguyen L, Schilling D, Dobiasch S, Raulefs S, Santiago Franco M, Buschmann D, Pfaffl MW, Schmid TE, Combs SE. The Emerging Role of miRNAs for the Radiation Treatment of Pancreatic Cancer. Cancers. 2020; 12(12):3703. https://doi.org/10.3390/cancers12123703
Chicago/Turabian StyleNguyen, Lily, Daniela Schilling, Sophie Dobiasch, Susanne Raulefs, Marina Santiago Franco, Dominik Buschmann, Michael W. Pfaffl, Thomas E. Schmid, and Stephanie E. Combs. 2020. "The Emerging Role of miRNAs for the Radiation Treatment of Pancreatic Cancer" Cancers 12, no. 12: 3703. https://doi.org/10.3390/cancers12123703