Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer
Abstract
:Simple Summary
Abstract
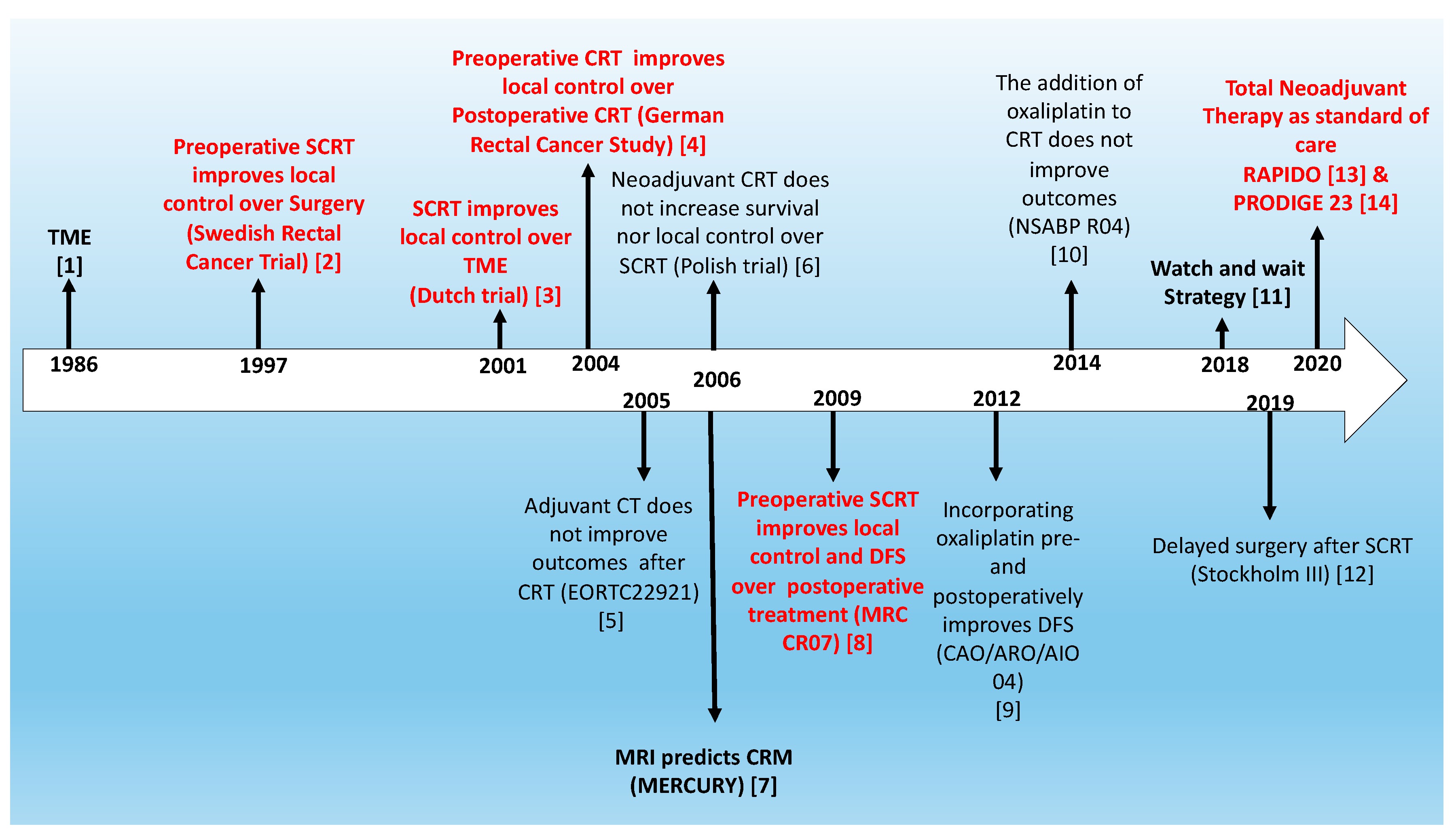
1. Introduction
2. Total Neoadjuvant Treatment: A Strategy to Provide All Treatment Modalities
3. Total Neoadjuvant Treatment as a New Standard of Care for LARC
4. Is There a Role for Neoadjuvant CT in Organ Preservation Strategies?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heald, R.J.; Ryall, R.D.H. Recurrence and Survival After Total Mesorectal Excision for Rectal Cancer. Lancet 1986, 327, 1479–1482. [Google Scholar] [CrossRef]
- Swedish Rectal Cancer Trial. Improved Survival with Preoperative Radiotherapy in Resectable Rectal Cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.M.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.T.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.J.M.; et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for Resectable Rectal Cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosset, J.-F.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Briffaux, A.; Collette, L. Enhanced Tumorocidal Effect of Chemotherapy With Preoperative Radiotherapy for Rectal Cancer: Preliminary Results—EORTC 22921. J. Clin. Oncol. 2005, 23, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006, 93, 1215–1223. [Google Scholar] [CrossRef]
- MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006, 333, 779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and Oxaliplatin in the Preoperative Multimodality Treatment of Rectal Cancer: Surgical End Points From National Surgical Adjuvant Breast and Bowel Project Trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Hospers, G.; Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.; Putter, H.; Meershoek—Klein Kranenbarg, E.; Roodvoets, A.G.; Nagtegaal, I.D.; Beets-Tan, R.G.; et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J. Clin. Oncol. 2020, 38, 4006. [Google Scholar] [CrossRef]
- Conroy, T.; Lamfichekh, N.; Etienne, P.-L.; Rio, E.; FRANCOIS, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2020, 38, 4007. [Google Scholar] [CrossRef]
- Taylor, F.G.M.; Quirke, P.; Heald, R.J.; Moran, B.J.; Blomqvist, L.; Swift, I.R.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-Year follow-up results of the MERCURY Study. J. Clin. Oncol. 2014, 32, 34–43. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Guren, M.G.; Kørner, H.; Pfeffer, F.; Myklebust, T.A.; Eriksen, M.T.; Edna, T.H.; Larsen, S.G.; Knudsen, K.O.; Nesbakken, A.; Wasmuth, H.H.; et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol. 2015, 54, 1714–1722. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [Green Version]
- Pellino, G.; Alós, R.; Biondo, S.; Codina-Cazador, A.; Enríquez-Navascues, J.M.; Espín-Basany, E.; Roig-Vila, J.V.; Cervantes, A.; García-Granero, E.; Carceller, R.A.; et al. Trends and outcome of neoadjuvant treatment for rectal cancer: A retrospective analysis and critical assessment of a 10-year prospective national registry on behalf of the Spanish Rectal Cancer Project. Eur. J. Surg. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Roselló, S.; Papaccio, F.; Roda, D.; Tarazona, N.; Cervantes, A. The role of chemotherapy in localized and locally advanced rectal cancer: A systematic revision. Cancer Treat. Rev. 2018, 63, 156–171. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.-H.; Kim, K.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.-Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Marijnen, C.; Beets, G.; Bujko, K.; De Bari, B.; Cervantes, A.; Chiloiro, G.; Coco, C.; Gambacorta, M.A.; Glynne-Jones, R.; et al. The 2017 Assisi Think Tank Meeting on rectal cancer: A positioning paper. Radiother. Oncol. 2020, 142, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.J.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cáncer de recto 3 study. J. Clin. Oncol. 2010, 28, 859–865. [Google Scholar] [CrossRef]
- Fernández-Martos, C.; Garcia-Albeniz, X.; Pericay, C.; Maurel, J.; Aparicio, J.; Montagut, C.; Safont, M.J.; Salud, A.; Vera, R.; Massuti, B.; et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial. Ann. Oncol. 2015, 26, 1722–1728. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Marijnen, C.A.M.; van Etten, B.; Dijkstra, E.A.; Hilling, D.E.; Kranenbarg, E.M.K.; Putter, H.; Roodvoets, A.G.H.; Bahadoer, R.R.; Fokstuen, T.; et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer—Results of the international randomized RAPIDO-trial. Radiother. Oncol. 2020, 147, 75–83. [Google Scholar] [CrossRef]
- Borg, C.; Rullier, E.; Marchal, F.; Etienne, P.-L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; et al. LBA21 Neoadjuvant mFOLFIRINOX and preoperative chemoradiation (CRT) versus preoperative CRT in patients with T3-4 rectal cancer: Surgical and quality of life results of PRODIGE 23 phase III trial. Ann. Oncol. 2020, 31, S1152. [Google Scholar] [CrossRef]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef]
- Zaborowski, A.; Stakelum, A.; Winter, D.C. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. BJS 2019, 106, 979–987. [Google Scholar] [CrossRef]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ArO/AIO-12. J. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Sclafani, F.; Brown, G.; Cunningham, D.; Wotherspoon, A.; Tait, D.; Peckitt, C.; Evans, J.; Yu, S.; Sena Teixeira Mendes, L.; Tabernero, J.; et al. PAN-EX: A pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann. Oncol. 2016, 27, 1557–1565. [Google Scholar] [CrossRef]
- Bujko, K.; Wyrwicz, L.; Rutkowski, A.; Malinowska, M.; Pietrzak, L.; Kryński, J.; Michalski, W.; Oledzki, J.; Kuśnierz, J.; Zajac, L.; et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann. Oncol. 2016, 27, 834–842. [Google Scholar] [CrossRef]
- Ciseł, B.; Pietrzak, L.; Michalski, W.; Wyrwicz, L.; Rutkowski, A.; Kosakowska, E.; Cencelewicz, A.; Spałek, M.; Polkowski, W.; Jankiewicz, M.; et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019, 30, 1298–1303. [Google Scholar] [CrossRef]
- Erlandsson, J.; Lörinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer—Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Radu, C.; Berglund, A.; Pahlman, L.; Glimelius, B. Short-course preoperative radiotherapy with delayed surgery in rectal cancer—A retrospective study. Radiother. Oncol. 2008, 87, 343–349. [Google Scholar] [CrossRef]
- Nilsson, P.J.; van Etten, B.; Hospers, G.A.; Påhlman, L.; van de Velde, C.J.; Beets-Tan, R.G.; Blomqvist, L.; Beukema, J.C.; Kapiteijn, E.; Marijnen, C.A.; et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer 2013, 13, 279. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, T.H.; Tamas, K.; Beukema, J.C.; Beets, G.L.; Gelderblom, A.J.; de Jong, K.P.; Nagtegaal, I.D.; Rutten, H.J.; van de Velde, C.J.; Wiggers, T.; et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann. Oncol. 2013, 24, 1762–1769. [Google Scholar] [CrossRef]
- Bisschop, C.; van Dijk, T.H.; Beukema, J.C.; Jansen, R.L.H.; Gelderblom, H.; de Jong, K.P.; Rutten, H.J.T.; van de Velde, C.J.H.; Wiggers, T.; Havenga, K.; et al. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann. Surg. Oncol. 2017, 24, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef]
- Beard, B.W.; Rettig, R.L.; Ryoo, J.J.; Parker, R.A.; McLemore, E.C.; Attaluri, V. Watch-and-Wait Compared to Operation for Patients with Complete Response to Neoadjuvant Therapy for Rectal Cancer. J. Am. Coll. Surg. 2020. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Chow, O.S.; Smith, D.D.; Marcet, J.E.; Cataldo, P.A.; Varma, M.G.; Kumar, A.S.; Oommen, S.; Coutsoftides, T.; Hunt, S.R.; et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Patil, S.; Kim, J.K.; Yuval, J.B.; Thompson, H.; Verheij, F.; Lee, M.; Saltz, L.B. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J. Clin. Oncol. 2020, 38, 4008. [Google Scholar] [CrossRef]



| Patient Characteristics | RAPIDO (TNT vs. CRT) | PRODIGE 23 (TNT vs. CRT) |
|---|---|---|
| Median age | 61 yrs vs. 61 yrs | 61 yrs vs. 62 yrs |
| Patients enrolled | 462 vs. 450 | 231 vs. 230 |
| cT4 (%) | 30.4% vs. 31.8% | 17.8% vs. 15.6% |
| cN2 (%) | 68% vs. 68% | Not stated |
| EMVI+ (%) | 32% vs. 28% | Not stated |
| MRF involved | 62% vs. 60% | 26% vs. 27.7% |
| Outcomes | RAPIDO | PRODIGE 23 |
|---|---|---|
| (TNT vs. CRT) | (TNT vs. CRT) | |
| Median FU | 4.6 yrs | 3.8 yrs |
| Primary endpoint | 3-yrs DrTF | 3-yrs DFS |
| 23.7% vs. 30.4% (HR 0.75 [95% CI 0.60–0.96]; p = 0.019) | 75.7% vs. 68.5% (HR 0.69 95% [CI 0.49–0.97]; p = 0.034) | |
| 3-year MFS | 80% vs. 73.2% | 78.8% vs. 71.7% |
| pCR rate | 28.4% vs. 14.3% | 27.5% vs. 11.7% |
| Local relapse | 8.7% vs. 5.4% | 4.8% vs. 7% |
| 3-year OS | 89.1% vs. 88.8% | 90.8% vs. 87.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaccio, F.; Roselló, S.; Huerta, M.; Gambardella, V.; Tarazona, N.; Fleitas, T.; Roda, D.; Cervantes, A. Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Cancers 2020, 12, 3611. https://doi.org/10.3390/cancers12123611
Papaccio F, Roselló S, Huerta M, Gambardella V, Tarazona N, Fleitas T, Roda D, Cervantes A. Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Cancers. 2020; 12(12):3611. https://doi.org/10.3390/cancers12123611
Chicago/Turabian StylePapaccio, Federica, Susana Roselló, Marisol Huerta, Valentina Gambardella, Noelia Tarazona, Tania Fleitas, Desamparados Roda, and Andres Cervantes. 2020. "Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer" Cancers 12, no. 12: 3611. https://doi.org/10.3390/cancers12123611







