Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Second Primary Malignancies
2.3. Association of SPMs with the Therapy of Myeloid Neoplasms
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Study Population
4.3. Ethics
4.4. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, J.; Nassiri, M. Acute Promyelocytic Leukemia: A Review and Discussion of Variant Translocations. Arch. Pathol. Lab. Med. 2015, 139, 1308–1313. [Google Scholar] [CrossRef] [Green Version]
- Mannan, A.; Muhsen, I.N.; Barragán, E.; Sanz, M.A.; Mohty, M.; Hashmi, S.K.; Aljurf, M. Genotypic and Phenotypic Characteristics of Acute Promyelocytic Leukemia Translocation Variants. Hematol. Stem Cell Ther. 2020. [Google Scholar] [CrossRef]
- Hussain, L.; Maimaitiyiming, Y.; Islam, K.; Naranmandura, H. Acute promyelocytic leukemia and variant fusion proteins: PLZF-RARα fusion protein at a glance. Semin. Oncol. 2019, 46, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C.C.; Tavakkoli, M.; Tallman, M.S. Acute promyelocytic leukemia: Where did we start, where are we now, and the future. Blood Cancer J. 2015, 5, e304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.; Yim, R.; Lee, H.K.K.; Mak, V.; Lin, S.-Y.; Kho, B.; Yip, S.-F.; Lau, J.S.M.; Li, W.; Ip, H.-W.; et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: A 15-year prospective study. Cancer 2018, 124, 2316–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicconi, L.; Lo-Coco, F. Current management of newly diagnosed acute promyelocytic leukemia. Ann. Oncol. 2016, 27, 1474–1481. [Google Scholar] [CrossRef]
- Tallman, M.S.; Altman, J.K. How I treat acute promyelocytic leukemia. Blood 2009, 114, 5126–5135. [Google Scholar] [CrossRef]
- Rego, E.M.; Jácomo, R.H. Epidemiology and treatment of acute promyelocytic leukemia in latin america. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011049. [Google Scholar] [CrossRef] [Green Version]
- Kamath, G.R.; Tremblay, D.; Coltoff, A.; Caro, J.; Lancman, G.; Bhalla, S.; Najfeld, V.; Mascarenhas, J.; Taioli, E. Comparing the epidemiology, clinical characteristics and prognostic factors of acute myeloid leukemia with and without acute promyelocytic leukemia. Carcinogenesis 2019, 40, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Hillestad, L.K. Acute Promyelocytc Leukemia. Acta Med. Scand. 2009, 159, 189–194. [Google Scholar] [CrossRef]
- Thomas, X. Acute Promyelocytic Leukemia: A History over 60 Years—From the Most Malignant to the most Curable Form of Acute Leukemia. Oncol. Ther. 2019, 7, 33–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.E.; Ye, Y.C.; Chen, S.R.; Chai, J.R.; Lu, J.X.; Zhoa, L.; Gu, L.J.; Wang, Z.Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-Y.; Chen, Z. Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 2008, 111, 2505–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengfelder, E.; Hofmann, W.-K.; Nowak, D.A. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 2012, 26, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Eghtedar, A.; Rodriguez, I.; Kantarjian, H.; O’Brien, S.; Daver, N.; Garcia-Manero, G.; Ferrajoli, A.; Kadia, T.; Pierce, S.; Cortes, J.; et al. Incidence of secondary neoplasms in patients with acute promyelocytic leukemia treated with all-transretinoic acid plus chemotherapy or with all-transretinoic acid plus arsenic trioxide. Leuk. Lymphoma 2015, 56, 1342–1345. [Google Scholar] [CrossRef] [Green Version]
- Shetty, A.V.; Ravandi, F.; Alapati, N.; Borthakur, G.; Garcia-Manero, G.; Kadia, T.M.; Wierda, W.; Estrov, Z.; Pierce, S.; O’Brien, S.; et al. Survivorship in APL- Outcomes of Acute Promyelocytic Leukemia (APL) Patients (pts) after Maintaining Complete Remission (CR) for at Least 3 Years. Blood 2014, 124, 954. [Google Scholar] [CrossRef]
- WHO/IARC. International rules for multiple primary cancers. Asian Pac. J. Cancer Prev. 2005, 6, 104–106. [Google Scholar]
- Ng, A.K.; Kenney, L.B.; Gilbert, E.S.; Travis, L.B. Secondary Malignancies Across the Age Spectrum. Semin. Radiat. Oncol. 2010, 20, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.; Pathak, R.; Aryal, M.R.; Karmacharya, P.; Bhatt, V.R. Second primary malignancy in acute promyelocytic leukemia: A Surveillance, Epidemiology and End Results database study. Futur. Oncol. 2017, 13, 1455–1457. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Bird, S.T.; Avagyan, A.; Li, Y.; Akhtar, S.; Liao, J.; Wernecke, M.; Deisseroth, A.B.; Chuk, M.; MaCurdy, T.E.; et al. Second Cancers in Adults with Acute Promyelocytic Leukemia (APL) Treated with or without Arsenic Trioxide (ATO): A SEER-Medicare Analysis. Blood 2019, 134, 3497. [Google Scholar] [CrossRef]
- Pagano, L.; Gimema, F.; Pulsoni, A.; Tosti, M.E.; Caramatti, C.; Cerri, R.; Falcucci, P.; Fazi, P.; Fianchi, L.; Martino, B.; et al. Second malignancy after treatment of adult acute myeloid leukemia: Cohort study on adult patients enrolled in the GIMEMA trials. Leukemia 2004, 18, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Onel, K.; Curtis, R.E.; Hungate, E.A.; Armstrong, G.T. The Rising Incidence of Second Cancers: Patterns of Occurrence and Identification of Risk Factors for Children and Adults. Am. Soc. Clin. Oncol. Educ. Book 2014, e57–e67. [Google Scholar] [CrossRef] [PubMed]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.J.; Ritchie, E.K.; Consedine, N.; Magai, C.; Neugut, A.I. Incidence rates of the major leukemia subtypes among U.S. Hispanics, Blacks, and non-Hispanic Whites. Leuk. Lymphoma 2006, 47, 2365–2370. [Google Scholar] [CrossRef]
- Sierra, M.; Alonso, A.; Odero, M.D.; González, M.B.; Lahortiga, I.; Pérez, J.J.; García, J.L.; Gutiérrez, N.C.; Calasanz, M.J.; Miguel, J.F.S.; et al. Geographic differences in the incidence of cytogenetic abnormalities of acute myelogenous leukemia (AML) in Spain. Leuk. Res. 2006, 30, 943–948. [Google Scholar] [CrossRef]
- Walsh, K.M.; De Smith, A.J.; Chokkalingam, A.P.; Metayer, C.; Roberts, W.; Barcellos, L.F.; Wiemels, J.L.; Buffler, P.A. GATA3 risk alleles are associated with ancestral components in Hispanic children with ALL. Blood 2013, 122, 3385–3387. [Google Scholar] [CrossRef] [Green Version]
- Isoherranen, N.; Zhong, G. Biochemical and physiological importance of the CYP26 retinoic acid hydroxylases. Pharmacol. Ther. 2019, 204, 107400. [Google Scholar] [CrossRef]
- Carratù, M.R.; Marasco, C.; Mangialardi, G.; Vacca, A. Retinoids: Novel immunomodulators and tumour-suppressive agents? Br. J. Pharmacol. 2012, 167, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Devalaraja, S.; To, T.K.J.; Folkert, I.W.; Natesan, R.; Alam, Z.; Li, M.; Tada, Y.; Budagyan, K.; Dang, M.T.; Zhai, L.; et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell 2020, 180, 1098–1114.e16. [Google Scholar] [CrossRef] [PubMed]



| Variables | APL | Non-APL | Non-APL AML | ||
|---|---|---|---|---|---|
| Total number of records | 4520 | 6,695,986 | 51,501 | ||
| First cancer and primary by WHO rules | 4019 | 5,677,898 | 38,495 | ||
| First primary malignancy only | 3774 | 4,898,363 | 35,292 | ||
| Mean age at diagnosis in years (SD) | 45.4 (19.3) | 63.1 (15.7) | 61.1 (21.9) | ||
| Sex | |||||
| Female | 1.855 (49.2) | 2,395,182 (48.9) | 16,145 (45.7) | ||
| Male | 1.919 (50.8) | 2,503,181 (51.1) | 19,147 (54.3) | ||
| Ethnicity | |||||
| Hispanic (all races) | 908 (24.1) | 511,927 (10.5) | 4475 (12.7) | ||
| Non-Hispanic (white) | 766 (20.3) | 3,451,992 (70.5) | 24,540 (69.5) | ||
| Non-Hispanic (other) | 2100 (55.6) | 934,444 (19.0) | 6277 (17.8) | ||
| Median follow-up time in years (range) | 4.8 (0.1–16.9) | 3.1 (0.1–16.9) | 0.5 (0.1–16.9) | ||
| Second primary malignancy | 116 | 392,016 | 755 | ||
| Mean age at diagnosis of the first malignancy in years (SD) | 53.9 (15.7) | 66.3 (11.8) | 61.2 (16.6) | ||
| Mean age at diagnosis of the SPM in years (SD) | 59.0 (14.5) | 70.1 (11.7) | 63.8 (15.7) | ||
| Sex | |||||
| Female | 56 (48.3) | 155,494 (39.7) | 311 (40.1) | ||
| Male | 60 (51.7) | 236,522 (60.3) | 464 (59.9) | ||
| Ethnicity | |||||
| Hispanic (all races) | 14 (12.1) | 27,787 (7.1) | 68 (8.8) | ||
| Non-Hispanic (white) | 78 (67.2) | 304,360 (77.7) | 604 (77.9) | ||
| * Non-Hispanic (other) | 24 (20.7) | 59,869 (15.2) | 103 (13.3) | ||
| Median latency time in years (range) | 4 (0.1–14) | 3 (0–16) | 1 (0–16) | ||
| Median follow-up time in years (range) | 7.8 (0.2–16.9) | 6.3 (0.1–16.9) | 2.9 (0.1–16.7) | ||
| Baseline Characteristics | APL/SPM | APL/only | p * | Non-APL/SPM | p * | Non-APL AML/SPM | p# | |
|---|---|---|---|---|---|---|---|---|
| Frequency of SPM | 2.9% | --- | --- | 6.9% | <0.001 | 2.0% | 0.193 | |
| Age | ||||||||
| <40 years | 18 (15.5) | 1486 (39.4) | <0.001 | 7667 (2.0) | <0.001 | 70 (9.0) | <0.001 | |
| ≥40 years | 98 (84.5) | 2288 (60.6) | 384,349 (98.0) | 705 (91.0) | ||||
| Sex | ||||||||
| Female | 56 (48.3) | 1.855 (49.2) | 0.942 | 155,494 (39.7) | 0.058 | 311 (40.1) | 0.096 | |
| Male | 60 (51.7) | 1.919 (50.8) | 236,522 (60.3) | 464 (59.9) | ||||
| Ethnicity | ||||||||
| Hispanic | 14 (12.1) | 908 (24.1) | 0.001 | 27,787 (7.1) | <0.001 | 68 (8.8) | <0.001 | |
| Non-Hispanic (white) | 78 (67.2) | 2100 (55.6) | 304,360 (77.7) | 604 (77.9) | ||||
| * Non-Hispanic (other) | 24 (20.7) | 766 (20.3) | 59,869 (15.2) | 103 (13.3) | ||||
| Median follow-up time in years (range) | 7.8 (0.2–16.9) | 4.8 (0.1–16.9) | <0.001 | 6.3 (0.1–16.9) | 0.001 | 2.9 (0.1–16.7) | <0.001 | |
| Risk Factors | APL/only n (%) | APL/SPM n (%) | HR (CI95%) | p# | |
|---|---|---|---|---|---|
| Age | |||||
| <40 years | 18 (15.5) | 1486 (39.4) | --- | ||
| ≥40 years | 98 (84.5) | 2288 (60.6) | 5.1 (3.1–8.4) | <0.001 | |
| Race/ethnicity | |||||
| Hispanic (all races) | 14 (12.1) | 908 (24.1) | --- | 0.020 | |
| Non-Hispanic white | 78 (67.2) | 2100 (55.6) | 2.3 (1.3–4.0) | 0.005 | |
| * Non-Hispanic (other) | 24 (20.7) | 766 (20.3) | 2.0 (1.0–3.8) | 0.041 | |
| Second Primary Malignancy | APL/SPM n (%) | Non-APL/SPM n (%) | RR (CI95%) | p * |
|---|---|---|---|---|
| Overall | 116 | 392,016 | ||
| Liver | 7 (6.0) | 5719 (1.5) | 4.6 (2.1–9.9) | <0.001 |
| Salivary gland | 4 (3.4) | 1542 (0.4) | 9.8 (3.6–26.5) | <0.001 |
| Soft tissue | 4 (3.4) | 3241 (0.8) | 4. 7 (1.7–12.6) | 0.003 |
| In men | 60 | 236,522 | ||
| Liver | 5 (8.3) | 4325 (1.8) | 7.6 (2.9–19.6) | <0.001 |
| Salivary gland | 2 (3.3) | 937 (0.4) | 14.0 (3.3–58.6) | <0.001 |
| Soft tissue, including heart | 2 (3.3) | 1911 (0.8) | 6.9 (1.6–28.7) | 0.008 |
| Prostate | 22 (36.7) | 39,068 (16.5) | 3.7 (2.1–6.4) | <0.001 |
| In women | 56 | 155,494 | ||
| Liver | 2 (3.6) | 1394 (0.9) | 4.4 (1.1–17.9) | 0.041 |
| Salivary gland | 2 (3.6) | 605 (0.4) | 10.0 (2.4–41.2) | 0.001 |
| Soft tissue | 2 (3.6) | 1330 (0.9) | 4.571 (1.1–18.8) | 0.035 |
| Female breast | 12 (21.4) | 24,270 (15.6) | 2. 5 (0.9–6.3) | 0.057 |
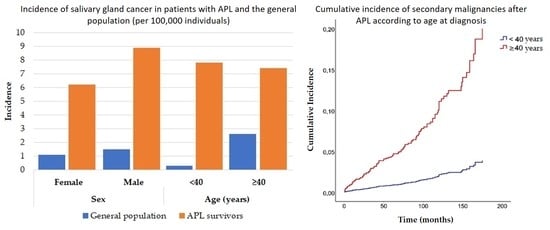
| Cancer | Incidence/100.000 (2000–2016) | |||
|---|---|---|---|---|
| All Registries SEER 18 * | All APL * | p | ||
| Liver | ||||
| Sex | Female | 3.8 | 6.2 | 0.016 |
| Male | 10.9 | 14.9 | 0.013 | |
| Age | <40 | 0.3 | 0.0 | 0.317 |
| ≥40 | 16.1 | 17.3 | 0.511 | |
| Salivary Gland | ||||
| Sex | Female | 1.1 | 6.2 | <0.001 |
| Male | 1.5 | 8.9 | <0.001 | |
| Age | <40 | 0.3 | 7.8 | <0.001 |
| ≥40 | 2.6 | 7.4 | <0.001 | |
| Soft Tissue | ||||
| Sex | Female | 2.9 | 9.2 | <0.001 |
| Male | 3.7 | 5.9 | 0.025 | |
| Age | <40 | 1.4 | 7.8 | <0.001 |
| ≥40 | 5.7 | 7.4 | 0.137 | |
| Cancer Sites in SPM | Observed | Expected | SIR | CI95% | Excess Risk | |
|---|---|---|---|---|---|---|
| APL/SPM | ||||||
| Salivary gland | 4 | 0.31 | 12.89 | (3.51–33.01) | 2.02 | |
| Liver | 6 | 2.07 | 2.90 | (1.07–6.32) | 2.16 | |
| Soft tissue | 4 | 0.77 | 5.18 | (1.41–13.25) | 1.77 | |
| Non-APL/SPM | ||||||
| Salivary gland | 1775 | 1191.23 | 1.49 | (1.42–1.56) | 0.2 | |
| Liver | 6254 | 6710.05 | 0.93 | (0.91–0.96) | −0.16 | |
| Soft tissue | 4025 | 2440.49 | 1.65 | (1.6–1.7) | 0.56 | |
| Second Primary Malignancy Site | APL/SPM (n = 116) n (%) | Non-APL AML (n = 775) n (%) | RR (CI95%) | p * |
|---|---|---|---|---|
| Overall | ||||
| Liver | 7 (6.0) | 4 (0.5) | 5.437 (2.528–11.697) | <0.001 |
| Salivary gland | 4 (3.4) | 3 (0.4) | 4.883 (1.798–13.262) | 0.002 |
| Soft tissue | 4 (3.4) | 6 (0.8) | 3.418 (1.258–9.283) | 0.016 |
| In men | 60 | 464 | ||
| Liver | 5 (8.3) | 4 (0.9) | 7.682 (2.974–19.845) | <0.001 |
| Salivary gland | 2 (3.3) | 2 (0.4) | 6.914 (1.650–28.974) | 0.008 |
| Soft tissue | 2 (3.3) | 3 (0.6) | 5.531 (1.320–23.180) | 0.019 |
| Prostate | 22 (36.7) | 83 (17.9) | 2.897 (1.665–5.042) | <0.001 |
| In women | 56 | 311 | ||
| Liver | 2 (3.6) | 0 (0.0) | 7.140 (1.737–29.343) | 0.006 |
| Salivary gland | 2 (3.6) | 1 (0.32) | 4.760 (1.158–19.562) | 0.030 |
| Soft tissue | 2 (3.6) | 3 (1.0) | 2.856 (0.695–11.737) | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzi, L.; Lee-Jones, L.; Mostofa, M.A.; de Andrade, D.P.; Ribeiro, R.C.; Figueiredo, B.C. Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study. Cancers 2020, 12, 3610. https://doi.org/10.3390/cancers12123610
Lenzi L, Lee-Jones L, Mostofa MA, de Andrade DP, Ribeiro RC, Figueiredo BC. Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study. Cancers. 2020; 12(12):3610. https://doi.org/10.3390/cancers12123610
Chicago/Turabian StyleLenzi, Luana, Lisa Lee-Jones, Maruf A. Mostofa, Diancarlos P. de Andrade, Raul C. Ribeiro, and Bonald C. Figueiredo. 2020. "Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study" Cancers 12, no. 12: 3610. https://doi.org/10.3390/cancers12123610
APA StyleLenzi, L., Lee-Jones, L., Mostofa, M. A., de Andrade, D. P., Ribeiro, R. C., & Figueiredo, B. C. (2020). Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study. Cancers, 12(12), 3610. https://doi.org/10.3390/cancers12123610