Cancer Testis Antigens and Immunotherapy: Expression of PRAME Is Associated with Prognosis in Soft Tissue Sarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Cohort
2.2. Cancer-Testis Antigens Show Distinct Expression Patterns in Sarcoma Subtypes
2.3. Cancer-Testis Antigens PRAME and NY-ESO-1 Are Expressed More Frequently in Soft Tissue Sarcomas with Low Counts of Tumour-Infiltrating Lymphocytes
2.4. Expression of PRAME and NY-ESO-1 Is Associated with Grading in Opposing Ways
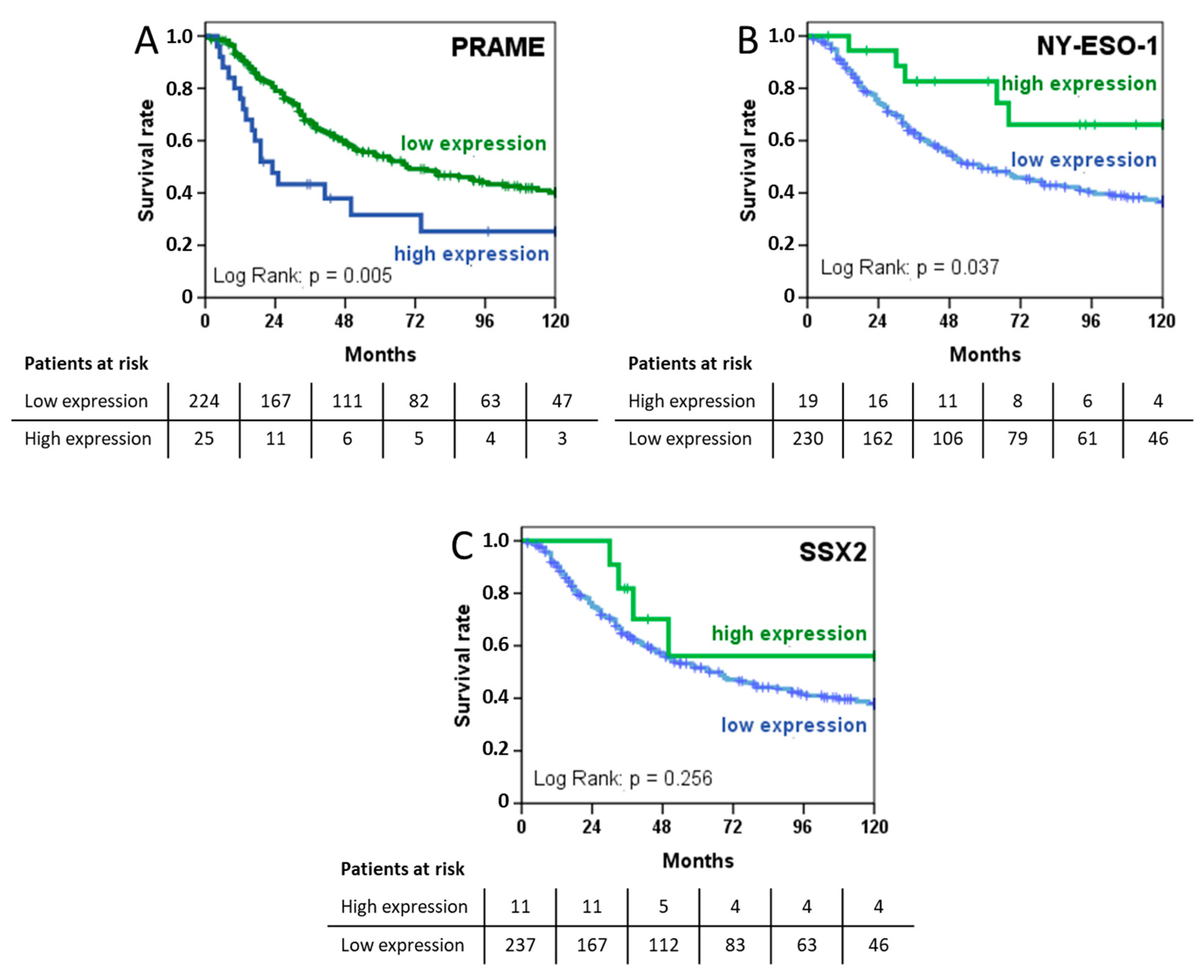
2.5. PRAME Expression Is Prognostic of Shorter Survival while NY-ESO-1 Is Associated with a More Favourable Prognosis
2.6. Expression of CTAs PRAME und SSX2 As Well As Radical Resections, Chemotherapy, and Metastatic Disease Influenced Overall Surivival in Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Histopathology and Tissue Microarray Construction Immunohistochemistry
4.3. Immunohistochemistry of Cancer Testis Antigens
4.4. Tumour-Infiltrating Lymphocytes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Expression | PRAME | NY-ESO-1 | SSX2 | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 0 | 224 | 90.0 | 214 | 85.9 | 237 | 95.6 |
| 1 | 6 | 2.4 | 8 | 3.2 | 2 | 0.8 |
| 2 | 8 | 3.2 | 8 | 3.2 | 4 | 1.6 |
| 3 | 11 | 4.4 | 19 | 7.6 | 5 | 2.0 |
| Total | 249 | 100.0 | 249 | 100.0 | 248 | 100.0 |
| Missing | 1 | |||||
| HR (95% CI) | p | ||
|---|---|---|---|
| Metastatic disease | M0 | 1 | <0.001 |
| M1 | 3.173 (1.889–5.330) | ||
| Surgical margins | R0/R1 | 1 | <0.001 |
| R2 or no resection | 1.618 (1.328–1.972) | ||
| PRAME | Low | 1 | 0.117 |
| High | 1.181 (0.959–1.453) | ||
| SSX2 | High | 1 | 0.229 |
| Low | 1.841 (0.681–4.982) | ||
| NY-ESO-1 | High | 1 | 0.096 |
| Low | 1.600 (0.921–2.782) | ||
| TILs count | Low | 1 | 0.635 |
| High | 1.207 (0.804–1.811) | ||
| Histological subgroup | 1.061 (1.007–1.119) | 0.028 | |
| Radiotherapy | Not done | 1 | 0.455 |
| Done | 1.166 (0.779–1.743) | ||
| Regional hyperthermia | Not done | 1 | 0.085 |
| Done | 1.446 (0.950–2.200) |
References
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Roland, C.L.; Keung, E.Z.-Y.; Lazar, A.J.; Torres, K.E.; Wang, W.-L.; Guadagnolo, A.; Bishop, A.J.; Lin, H.Y.; Hunt, K.; Feig, B.W.; et al. Preliminary results of a phase II study of neoadjuvant checkpoint blockade for surgically resectable undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated liposarcoma (DDLPS). J. Clin. Oncol. 2020, 38, 11505. [Google Scholar] [CrossRef]
- Simpson, A.J.; Caballero, O.L.; Jungbluth, A.; Chen, Y.T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Bolli, M.; Kocher, T.; Adamina, M.; Guller, U.; Dalquen, P.; Haas, P.; Mirlacher, M.; Gambazzi, F.; Harder, F.; Heberer, M.; et al. Tissue microarray evaluation of Melanoma antigen E (MAGE) tumor-associated antigen expression: Potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann. Surg. 2002, 236, 785–793. [Google Scholar] [CrossRef]
- Iura, K.; Kohashi, K.; Ishii, T.; Maekawa, A.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Matsumoto, Y.; Iwamoto, Y.; et al. MAGEA4 expression in bone and soft tissue tumors: Its utility as a target for immunotherapy and diagnostic marker combined with NY-ESO-1. Virchows Arch. Int. J. Pathol. 2017, 471, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Busam, K.J.; Iversen, K.; Kolb, D.; Coplan, K.; Spagnoli, G.C.; Ladanyi, M.; Old, L.J.; Jungbluth, A.A. MAGE antigen expression in monophasic and biphasic synovial sarcoma. Hum. Pathol. 2002, 33, 225–229. [Google Scholar] [CrossRef]
- Jungbluth, A.A.; Busam, K.J.; Kolb, D.; Iversen, K.; Coplan, K.; Chen, Y.T.; Spagnoli, G.C.; Old, L.J. Expression of MAGE-antigens in normal tissues and cancer. Int. J. Cancer 2000, 85, 460–465. [Google Scholar] [CrossRef]
- Iura, K.; Maekawa, A.; Kohashi, K.; Ishii, T.; Bekki, H.; Otsuka, H.; Yamada, Y.; Yamamoto, H.; Harimaya, K.; Iwamoto, Y.; et al. Cancer-testis antigen expression in synovial sarcoma: NY-ESO-1, PRAME, MAGEA4, and MAGEA1. Hum. Pathol. 2017, 61, 130–139. [Google Scholar] [CrossRef]
- Lai, J.P.; Robbins, P.F.; Raffeld, M.; Aung, P.P.; Tsokos, M.; Rosenberg, S.A.; Miettinen, M.M.; Lee, C.C. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: Significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod. Pathol. 2012, 25, 854–858. [Google Scholar] [CrossRef]
- Endo, M.; de Graaff, M.A.; Ingram, D.R.; Lim, S.; Lev, D.C.; Briaire-de Bruijn, I.H.; Somaiah, N.; Bovee, J.V.; Lazar, A.J.; Nielsen, T.O. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod. Pathol. 2015, 28, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Toland, A.E.; Scharschmidt, T.J.; Mayerson, J.L.; Guttridge, D.C.; Iwenofu, O.H. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod. Pathol. 2014, 27, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; Jungbluth, A.A.; Hoch, B.L.; Farrar, E.A.; Bleakley, M.; Schneider, D.J.; Loggers, E.T.; Rodler, E.; Eary, J.F.; Conrad, E.U., 3rd; et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer 2012, 118, 4564–4570. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Ewart Toland, A.; Scharschmidt, T.J.; Mayerson, J.L.; Kraybill, W.G.; Guttridge, D.C.; Iwenofu, O.H. The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas. Mod. Pathol. 2013, 26, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Iwenofu, O.H. NY-ESO-1 is a sensitive and specific immunohistochemical marker for myxoid and round cell liposarcomas among related mesenchymal myxoid neoplasms. Mod. Pathol. 2013, 26, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Shurell, E.; Vergara-Lluri, M.E.; Li, Y.; Crompton, J.G.; Singh, A.; Bernthal, N.; Wu, H.; Eilber, F.C.; Dry, S.M. Comprehensive adipocytic and neurogenic tissue microarray analysis of NY-ESO-1 expression—A promising immunotherapy target in malignant peripheral nerve sheath tumor and liposarcoma. Oncotarget 2016, 7, 72860–72867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, R.; Dean, D.C.; Thanindratarn, P.; Hornicek, F.J.; Guo, W.; Duan, Z. Cancer testis antigens in sarcoma: Expression, function and immunotherapeutic application. Cancer Lett. 2020, 479, 54–60. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kepp, O.; Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160. [Google Scholar] [CrossRef]
- Issels, R.; Bücklein, V.; Kampmann, E.; Knösel, T.; Nössner, E.; Subklewe, M.; Lindner, L. Dissecting the role of tumor-infiltrating lymphocytes (TIL) in patients with high-risk soft-tissue sarcoma (STS) receiving neo-adjuvant chemotherapy (NAC) with regional hyperthermia (RHT). Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Raj, S.; Miller, L.D.; Triozzi, P.L. Addressing the Adult Soft Tissue Sarcoma Microenvironment with Intratumoral Immunotherapy. Sarcoma 2018, 2018, 9305294. [Google Scholar] [CrossRef] [PubMed]
- Roszik, J.; Wang, W.L.; Livingston, J.A.; Roland, C.L.; Ravi, V.; Yee, C.; Hwu, P.; Futreal, A.; Lazar, A.J.; Patel, S.R.; et al. Overexpressed PRAME is a potential immunotherapy target in sarcoma subtypes. Clin. Sarcoma Res. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Garcia Lora, A.M.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Spel, L.; Boelens, J.J.; van der Steen, D.M.; Blokland, N.J.; van Noesel, M.M.; Molenaar, J.J.; Heemskerk, M.H.; Boes, M.; Nierkens, S. Natural killer cells facilitate PRAME-specific T-cell reactivity against neuroblastoma. Oncotarget 2015, 6, 35770–35781. [Google Scholar] [CrossRef]
- Al-Khadairi, G.; Decock, J. Cancer Testis Antigens and Immunotherapy: Where Do We Stand in the Targeting of PRAME? Cancers 2019, 11, 984. [Google Scholar] [CrossRef]
- Sharma, A.; Bode, B.; Wenger, R.H.; Lehmann, K.; Sartori, A.A.; Moch, H.; Knuth, A.; Von Boehmer, L.; Van Den Broek, M. γ-Radiation Promotes Immunological Recognition of Cancer Cells through Increased Expression of Cancer-Testis Antigens In Vitro and In Vivo. PLoS ONE 2011, 6, e28217. [Google Scholar] [CrossRef]
- Kakimoto, T.; Matsumine, A.; Kageyama, S.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Iino, T.; Ikeda, H.; Shiku, H.; Sudo, A. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol. Lett. 2019, 17, 3937–3943. [Google Scholar] [CrossRef]
- Jungbluth, A.A.; Antonescu, C.R.; Busam, K.J.; Iversen, K.; Kolb, D.; Coplan, K.; Chen, Y.T.; Stockert, E.; Ladanyi, M.; Old, L.J. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int. J. Cancer 2001, 94, 252–256. [Google Scholar] [CrossRef]
- Gure, A.O.; Chua, R.; Williamson, B.; Gonen, M.; Ferrera, C.A.; Gnjatic, S.; Ritter, G.; Simpson, A.J.; Chen, Y.T.; Old, L.J.; et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 8055–8062. [Google Scholar] [CrossRef]
- Odunsi, K.; Jungbluth, A.A.; Stockert, E.; Qian, F.; Gnjatic, S.; Tammela, J.; Intengan, M.; Beck, A.; Keitz, B.; Santiago, D.; et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003, 63, 6076–6083. [Google Scholar]
- Mischo, A.; Kubuschok, B.; Ertan, K.; Preuss, K.D.; Romeike, B.; Regitz, E.; Schormann, C.; de Bruijn, D.; Wadle, A.; Neumann, F.; et al. Prospective study on the expression of cancer testis genes and antibody responses in 100 consecutive patients with primary breast cancer. Int. J. Cancer 2006, 118, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Al-Khadairi, G.; Roelands, J.; Hendrickx, W.; Dermime, S.; Bedognetti, D.; Decock, J. NY-ESO-1 Based Immunotherapy of Cancer: Current Perspectives. Front. Immunol. 2018, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.F.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Sherry, R.M.; Dudley, M.E.; Wunderlich, J.R.; Nahvi, A.V.; Helman, L.J.; Mackall, C.L.; et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011, 29, 917–924. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Melchiori, L.; Merchant, M.S.; Bernstein, D.; Glod, J.; Kaplan, R.; Grupp, S.; Tap, W.D.; Chagin, K.; Binder, G.K.; et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018, 8, 944–957. [Google Scholar] [CrossRef]
- Chawla, S.P.; Van Tine, B.A.; Pollack, S.; Ganjoo, K.N.; Elias, A.D.; Riedel, R.F.; Attia, S.; Choy, E.; Okuno, S.H.; Agulnik, M.; et al. A phase II randomized study of CMB305 and atezolizumab versus atezolizumab in NY-ESO-1+ soft tissue sarcoma: Analysis of immunogenicity, tumor control, and patient survival. J. Clin. Oncol. 2019, 37, 11011. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Giavina-Bianchi, P.; Sotto, M.N.; Muzikansky, A.; Kalil, J.; Festa-Neto, C.; Duncan, L.M. Increased NY-ESO-1 expression and reduced infiltrating CD3+ T cells in cutaneous melanoma. J. Immunol. Res. 2015, 2015, 761378. [Google Scholar] [CrossRef]
- Orth, M.F.; Buecklein, V.L.; Kampmann, E.; Subklewe, M.; Noessner, E.; Cidre-Aranaz, F.; Romero-Perez, L.; Wehweck, F.S.; Lindner, L.; Issels, R.; et al. A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol. Immunother. CII 2020, 69, 1353–1362. [Google Scholar] [CrossRef]
- Ramachandran, I.; Lowther, D.E.; Dryer-Minnerly, R.; Wang, R.; Fayngerts, S.; Nunez, D.; Betts, G.; Bath, N.; Tipping, A.J.; Melchiori, L.; et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J. Immunother. Cancer 2019, 7, 276. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e928. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef]
- Knösel, T.; Emde, A.; Schluns, K.; Chen, Y.; Jurchott, K.; Krause, M.; Dietel, M.; Petersen, I. Immunoprofiles of 11 biomarkers using tissue microarrays identify prognostic subgroups in colorectal cancer. Neoplasia 2005, 7, 741–747. [Google Scholar] [CrossRef] [PubMed]



| n | % | ||
|---|---|---|---|
| Total | 249 | 100 | |
| Sex | Male | 124 | 50 |
| Female | 125 | 50 | |
| Histological subtype | UPS | 82 | 33 |
| Leiomyosarcoma | 50 | 20 | |
| Synovial sarcoma | 28 | 11 | |
| Dedifferentiated Liposarcoma | 47 | 19 | |
| Angiosarcoma | 9 | 4 | |
| MPNST | 13 | 5 | |
| Other | 20 | 8 | |
| Location | Extremities | 85 | 34 |
| Retroperitoneal | 54 | 22 | |
| Abdominal/visceral | 42 | 17 | |
| Trunk | 60 | 24 | |
| Other | 8 | 3 | |
| Grading | Intermediate (G2) | 118 | 47 |
| High (G3) | 131 | 53 | |
| Size | <50 mm | 20 | 8 |
| 50–79 mm | 61 | 24 | |
| 80–120 mm | 64 | 26 | |
| >120 mm | 76 | 31 | |
| Missing | 28 | 11 | |
| Metastatic disease | M0 | 227 | 91 |
| M1 | 22 | 9 | |
| Surgical margins | R0/R1 | 202 | 81 |
| R2 or no resection | 47 | 19 | |
| Radiotherapy | Done | 53 | 21 |
| Not done | 180 | 72 | |
| Unknown | 16 | 2 | |
| Regional hyperthermia | Done | 195 | 78 |
| Not done | 54 | 22 |
| Total | PRAME | NY-ESO-1 | SSX2 | TILs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Histologic Subtype | n | n | % | n | % | n | % | n | % |
| UPS | 82 a | 6 | 7% | 2 | 2% | 3 | 4% | 71 | 87% |
| Leiomyosarcoma | 50 | 3 | 6% | 1 | 2% | 0 | 0% | 33 | 66% |
| Synovial Sarcoma | 28 | 3 | 11% | 12 | 43% | 7 | 25% | 13 | 46% |
| DDLPS | 47 | 2 | 4% | 3 | 6% | 0 | 0% | 39 | 83% |
| Angiosarcoma | 9 | 3 | 33% | 1 | 11% | 0 | 0% | 8 | 89% |
| MPNST | 13 | 5 | 38% | 0 | 0% | 1 | 8% | 11 | 85% |
| Other | 20 | 3 | 15% | 0 | 0% | 0 | 0% | 18 | 90% |
| Total | 249 a | 25 | 10% | 19 | 8% | 11 | 4% | 193 | 78% |
| HR (95% CI) | p | ||
|---|---|---|---|
| Metastatic disease | M0 | 1 | <0.001 |
| M1 | 3.182 (1.875–5.401) | ||
| Surgical margins | R0/R1 | 1 | <0.001 |
| R2 or no resection | 2.531 (1.682–3.809) | ||
| PRAME | Low | 1 | <0.001 |
| High | 2.675 (1.548–4.622) | ||
| SSX2 | High | 1 | 0.039 |
| Antigen | Product No. | Supplier | Clone | Dilution | Pre-Treatment |
|---|---|---|---|---|---|
| NY-ESO-1 | SC-53869 | Santa Cruz | E978 | 1:100 | ER2 |
| PRAME | ab219650 | Abcam | EPR20330 | 1:1000 | ER2 |
| SSX2 | AMAb91141 | Atlas Antibodies | CL3202 | 1:3000 | ER2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertsmeier, M.; Altendorf-Hofmann, A.; Lindner, L.H.; Issels, R.D.; Kampmann, E.; Dürr, H.-R.; Schubert-Fritschle, G.; Angele, M.K.; Kirchner, T.; Jungbluth, A.A.; et al. Cancer Testis Antigens and Immunotherapy: Expression of PRAME Is Associated with Prognosis in Soft Tissue Sarcoma. Cancers 2020, 12, 3612. https://doi.org/10.3390/cancers12123612
Albertsmeier M, Altendorf-Hofmann A, Lindner LH, Issels RD, Kampmann E, Dürr H-R, Schubert-Fritschle G, Angele MK, Kirchner T, Jungbluth AA, et al. Cancer Testis Antigens and Immunotherapy: Expression of PRAME Is Associated with Prognosis in Soft Tissue Sarcoma. Cancers. 2020; 12(12):3612. https://doi.org/10.3390/cancers12123612
Chicago/Turabian StyleAlbertsmeier, Markus, Annelore Altendorf-Hofmann, Lars H. Lindner, Rolf D. Issels, Eric Kampmann, Hans-Roland Dürr, Gabriele Schubert-Fritschle, Martin K. Angele, Thomas Kirchner, Achim A. Jungbluth, and et al. 2020. "Cancer Testis Antigens and Immunotherapy: Expression of PRAME Is Associated with Prognosis in Soft Tissue Sarcoma" Cancers 12, no. 12: 3612. https://doi.org/10.3390/cancers12123612
APA StyleAlbertsmeier, M., Altendorf-Hofmann, A., Lindner, L. H., Issels, R. D., Kampmann, E., Dürr, H.-R., Schubert-Fritschle, G., Angele, M. K., Kirchner, T., Jungbluth, A. A., & Knösel, T. (2020). Cancer Testis Antigens and Immunotherapy: Expression of PRAME Is Associated with Prognosis in Soft Tissue Sarcoma. Cancers, 12(12), 3612. https://doi.org/10.3390/cancers12123612





