Newcastle Disease Virus at the Forefront of Cancer Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
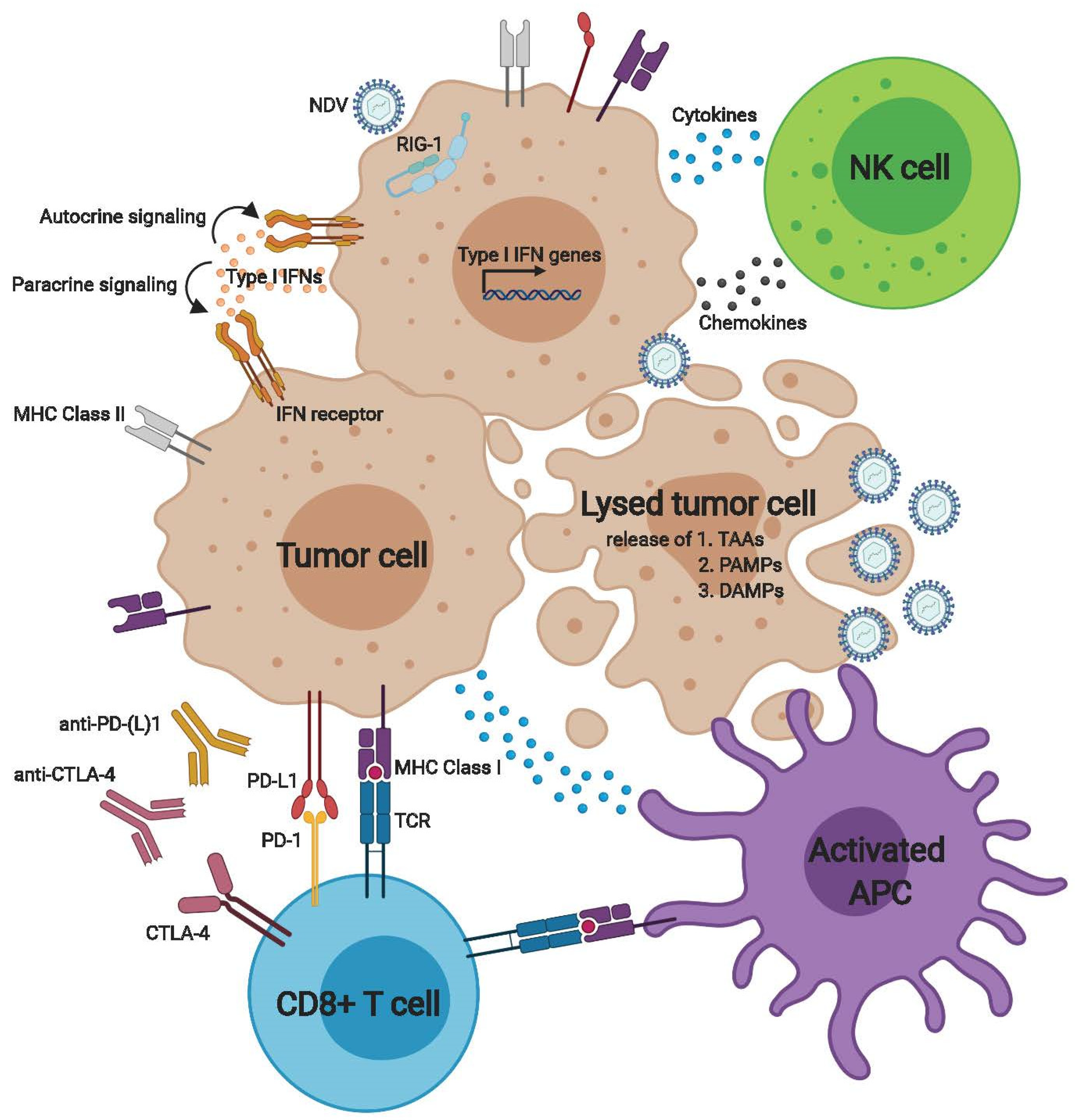
2. Activation of the Innate Anti-Tumor Immune Response by NDV
3. Activation of the Adaptive Antitumor Immune Response by NDV
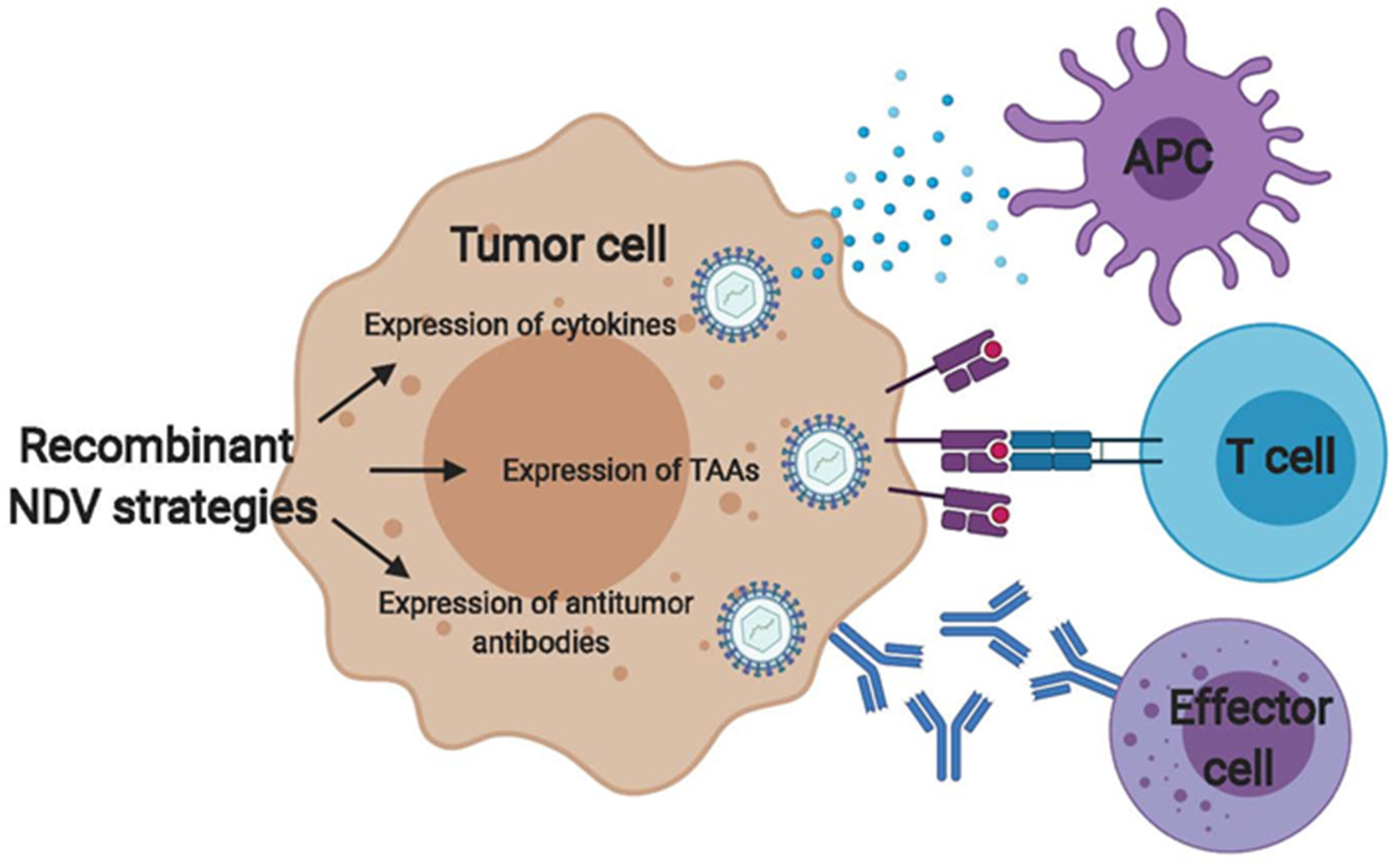
4. Engineering NDV to Modulate Innate and Adaptive Immune Responses
5. Clinical Experience with NDV
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.D.; Love, R.; Tesar, W. Propagation of Newcastle Disease Virus in Ehrlich Ascites Cells In Vitro and In vivo. Exp. Biol. Med. 1955, 90, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Sinkovics, J. Studies on the biological characteristics of the newcastle disease virus (NDV) adapted to the brain of newborne mice. Arch. Virol. 1957, 7, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Cassel, W.A.; Garrett, R.E. Newcastle disease virus as an antineoplastic agent. Cancer 1965, 18, 863–868. [Google Scholar] [CrossRef]
- Cassel, W.A.; Garrett, R.E. Tumor immunity after viral oncolysis. J. Bacteriol. 1966, 92, 792. [Google Scholar] [CrossRef] [Green Version]
- Wheelock, E.F.; Dingle, J.H. Observations on the Repeated Administration of Viruses to a Patient with Acute Leukemia. A Preliminary Report. N. Engl. J. Med. 1964, 271, 645–651. [Google Scholar] [CrossRef]
- Lamb, R.; Parks, G. Paramyxoviridae: The Viruses and Their Replication. Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1449–1496. [Google Scholar]
- Sinkovics, J.G.; Horvath, J.C. Newcastle disease virus (NDV): Brief history of its oncolytic strains. J. Clin. Virol. 2000, 16, 1–15. [Google Scholar] [CrossRef]
- Leighton, F.A.; Heckert, R.A. Newcastle Disease and Related Avian Paramyxoviruses. Infect. Dis. Wild Birds 2008, 1, 1–16. [Google Scholar]
- Dortmans, J.C.F.M.; Koch, G.; Rottier, P.J.M.; Peeters, B. Virulence of newcastle disease virus: What is known so far? Vet. Res. 2011, 42, 122. [Google Scholar] [CrossRef] [Green Version]
- Connaris, H.; Takimoto, T.; Russell, R.; Crennell, S.; Moustafa, I.; Portner, A.; Taylor, G.L. Probing the Sialic Acid Binding Site of the Hemagglutinin-Neuraminidase of Newcastle Disease Virus: Identification of Key Amino Acids Involved in Cell Binding, Catalysis, and Fusion. J. Virol. 2002, 76, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Komada, H.; Kusagawa, S.; Tsurudome, M.; Matsumura, H.; Kawano, M.; Ohta, H.; Nishio, M. Fusion regulation proteins on the cell surface: Isolation and characterization of monoclonal antibodies which enhance giant polykaryocyte formation in Newcastle disease virus-infected cell lines of human origin. J. Virol. 1992, 66, 5999–6007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.A.; Jardetzky, T.S. Structural basis of viral invasion: Lessons from paramyxovirus F. Curr. Opin. Struct. Biol. 2007, 17, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leeuw, O.S.; Hartog, L.; Koch, G.; Peeters, B.P.H. Effect of fusion protein cleavage site mutations on virulence of Newcastle disease virus: Non-virulent cleavage site mutants revert to virulence after one passage in chicken brain. J. Gen. Virol. 2003, 84, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Huang, Z.; Elankumaran, S.; Rockemann, D.D.; Samal, S.K. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 2004, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; King, D.J.; Bennett, J.D. Characterization of Newcastle disease virus isolates by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotype prediction and molecular epidemiological analysis. J. Clin. Microbiol. 1995, 33, 2624–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Samal, S.K. Newcastle Disease Virus as a Vaccine Vector for Development of Human and Veterinary Vaccines. Viruses 2016, 8, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginting, T.E.; Suryatenggara, J.; Christian, S.; Mathew, G. Proinflammatory response induced by Newcastle disease virus in tumor and normal cells. Oncolytic Virother. 2017, 6, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, S.; Takimoto, T.; Scroggs, R.A.; Portner, A. Differentially Regulated Interferon Response Determines the Outcome of Newcastle Disease Virus Infection in Normal and Tumor Cell Lines. J. Virol. 2006, 80, 5145–5155. [Google Scholar] [CrossRef] [Green Version]
- Fiola, C.; Peeters, B.; Fournier, P.; Arnold, A.; Bucur, M.; Schirrmacher, V. Tumor selective replication of Newcastle disease virus: Association with defects of tumor cells in antiviral defence. Int. J. Cancer 2006, 119, 328–338. [Google Scholar] [CrossRef]
- Zamarin, D.; Palese, P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012, 7, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Matveeva, O.V.; Chumakov, P.M. Defects in interferon pathways as potential biomarkers of sensitivity to oncolytic viruses. Rev. Med. Virol. 2018, 28, e2008. [Google Scholar] [CrossRef] [PubMed]
- Wilden, H.; Fournier, P.; Zawatzky, R.; Schirrmacher, V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle Disease Virus. Int. J. Oncol. 2009, 34, 971–982. [Google Scholar] [PubMed] [Green Version]
- Liu, T.; Zhang, Y.; Cao, Y.; Jiang, S.; Sun, R.; Yin, J.; Gao, Z.; Ren, G.; Wang, Z.; Yu, Q.; et al. Optimization of oncolytic effect of Newcastle disease virus Clone30 by selecting sensitive tumor host and constructing more oncolytic viruses. Gene Ther. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Palese, P.; Zamarin, D. Oncolytic Specificity of Newcastle Disease Virus Is Mediated by Selectivity for Apoptosis-Resistant Cells. J. Virol. 2011, 85, 6015–6023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadrado-Castano, S.; Ayllon, J.; Mansour, M.; De La Iglesia-Vicente, J.; Jordan, S.; Tripathi, S.; García-Sastre, A.; Villar, E. Enhancement of the proapoptotic properties of newcastle disease virus promotes tumor remission in syngeneic murine cancer models. Mol. Cancer Ther. 2015, 14, 1247–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, I.; Yaacov, B.; Shiloach, T.; Eliahoo, E.; Kadouri, L.; Lotem, M.; Perlman, R.; Zakay-Rones, Z.; Panet, A.; Ben-Yehuda, D. The Oncolytic Activity of Newcastle Disease Virus NDV-HUJ on Chemoresistant Primary Melanoma Cells Is Dependent on the Proapoptotic Activity of the Inhibitor of Apoptosis Protein Livin. J. Virol. 2009, 84, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.-H.; Sun, Y.-J.; Zhang, F.-Q.; Zhang, X.-R.; Qiu, X.-S.; Yu, L.-P.; Wu, Y.; Ding, C. Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci. Rep. 2016, 6, 24721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, T.; Jiang, K.; Wei, L.; Barr, M.P.; Xu, Q.; Zhang, G.; Ding, C.; Meng, S.; Piao, H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am. J. Cancer Res. 2018, 8, 1514–1527. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.-X.; Mao, X.; Fang, H.; Wang, H.; Li, Y.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget 2017, 8, 43201–43217. [Google Scholar] [CrossRef]
- Zeng, J.; Fournier, P.; Schirrmacher, V. Induction of Interferon-α and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Human Blood Mononuclear Cells by Hemagglutinin-Neuraminidase but Not F Protein of Newcastle Disease Virus. Virology 2002, 297, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghrici, M.; El Zowalaty, M.E.; Omar, A.R.; Ideris, A. Induction of apoptosis in MCF-7 cells by the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus Malaysian strain AF2240. Oncol. Rep. 2013, 30, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Elankumaran, S.; Rockemann, D.; Samal, S.K. Newcastle Disease Virus Exerts Oncolysis by both Intrinsic and Extrinsic Caspase-Dependent Pathways of Cell Death. J. Virol. 2006, 80, 7522–7534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Fournier, P.; Schirrmacher, V. High cell surface expression of Newcastle disease virus proteins via replicon vectors demonstrates syncytia forming activity of F and fusion promotion activity of HN molecules. Int. J. Oncol. 2004, 25, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral Actions of Interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [Green Version]
- Washburn, B.; Schirrmacher, V. Human tumor cell infection by Newcastle Disease Virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int. J. Oncol. 2002, 21, 85–93. [Google Scholar] [CrossRef]
- Vitale, G.; van Eijck, C.H.; Koetsvelt, P.M.; Erdman, J.; Speel, E.J.M.; Mooji, D.M.; Colao, A.; Lombardi, G.; Croze, E.; Lamberts, S.W.J.; et al. Type I interferons in the treatment of pancreatic cancer: Mechanisms of action and role of related receptors. Ann. Surg. 2007, 246, 259–268. [Google Scholar] [CrossRef]
- Ten, R.M.; Blank, V.; Le Bail, O.; Kourilsky, P.; Israël, A. Two factors, IRF1 and KBF1/NF-kappa B, cooperate during induction of MHC class I gene expression by interferon alpha beta or Newcastle disease virus. Comptes Rendus Académie Sci. Ser. III 1993, 316, 496–501. [Google Scholar]
- Ni, J.; Galani, I.E.; Cerwenka, A.; Schirrmacher, V.; Fournier, P. Antitumor vaccination by Newcastle Disease Virus Hemagglutinin–Neuraminidase plasmid DNA application: Changes in tumor microenvironment and activation of innate anti-tumor immunity. Vaccine 2011, 29, 1185–1193. [Google Scholar] [CrossRef]
- Zamarin, D.; Wolchok, J.D. Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer. Mol. Ther. Oncolytics 2014, 1, 14004. [Google Scholar] [CrossRef]
- Jarahian, M.; Watzl, C.; Fournier, P.; Arnold, A.; Djandji, D.; Zahedi, S.; Cerwenka, A.; Paschen, A.; Schirrmacher, V.; Momburg, F. Activation of Natural Killer Cells by Newcastle Disease Virus Hemagglutinin-Neuraminidase. J. Virol. 2009, 83, 8108–8121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.-R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nat. Cell Biol. 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Kumar, S.R.; Allen, A.; Yong, W.; Nimmanapalli, R.; Samal, S.K.; Elankumaran, S. Cell-Type-Specific Innate Immune Response to Oncolytic Newcastle Disease Virus. Viral Immunol. 2012, 25, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Elankumaran, S.; Chavan, V.; Qiao, D.; Shobana, R.; Moorkanat, G.; Biswas, M.; Samal, S.K. Type I Interferon-Sensitive Recombinant Newcastle Disease Virus for Oncolytic Virotherapy. J. Virol. 2010, 84, 3835–3844. [Google Scholar] [CrossRef] [Green Version]
- Buijs, P.R.A.; Van Eijck, C.H.J.; Hofland, L.J.; Fouchier, R.A.M.; Hoogen, B.V.D. Different responses of human pancreatic adenocarcinoma cell lines to oncolytic Newcastle disease virus infection. Cancer Gene Ther. 2014, 21, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Oseledchyk, A.; Ricca, J.M.; Gigoux, M.; Ko, B.; Redelman-Sidi, G.; Walther, T.; Liu, C.; Iyer, G.; Merghoub, T.; Wolchok, J.D.; et al. Lysis-independent potentiation of immune checkpoint blockade by oncolytic virus. Oncotarget 2018, 9, 28702–28716. [Google Scholar] [CrossRef] [Green Version]
- Zamarin, D.; Martínez-Sobrido, L.; Kelly, K.; Mansour, M.; Sheng, G.; Vigil, A.; García-Sastre, A.; Palese, P.; Fong, Y. Enhancement of Oncolytic Properties of Recombinant Newcastle Disease Virus Through Antagonism of Cellular Innate Immune Responses. Mol. Ther. 2009, 17, 697–706. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, T.; Knittler, M.R.; Grund, C.; Roemer-Oberdoerfer, A.; Kapp, J.-F.; Lerch, M.M.; Mettenleiter, T.C.; Mayerle, J.; Blohm, U. Newcastle disease virus mediates pancreatic tumor rejection via NK cell activation and prevents cancer relapse by prompting adaptive immunity. Int. J. Cancer 2017, 141, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Ricca, J.M.; Oseledchyk, A.; Walther, T.; Liu, C.; Mangarin, L.; Merghoub, T.; Wolchok, J.D.; Zamarin, D. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol. Ther. 2018, 26, 1008–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamarin, D.; Ricca, J.M.; Sadekova, S.; Oseledchyk, A.; Yu, Y.; Blumenschein, W.M.; Wong, J.; Gigoux, M.; Merghoub, T.; Wolchok, J.D. PD-L1 in tumor microenvironment mediates resistance to oncolytic immunotherapy. J. Clin. Investig. 2018, 128, 1413–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirrmacher, V.; Bai, L.; Umansky, V.; Yu, L.; Xing, Y.; Qian, Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int. J. Oncol. 2000, 16, 363–373. [Google Scholar] [CrossRef]
- Shortman, K.; Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 2010, 234, 18–31. [Google Scholar] [CrossRef]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.S.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Schreiber, R.D.; et al. Batf3 Deficiency Reveals a Critical Role for CD8 + Dendritic Cells in Cytotoxic T Cell Immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Van Gool, S.; Stuecker, W. Breaking Therapy Resistance: An Update on Oncolytic Newcastle Disease Virus for Improvements of Cancer Therapy. Biomedicines 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Zamarin, D.; Holmgaard, R.B.; Ricca, J.; Plitt, T.; Palese, P.; Sharma, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 2017, 8, 14340. [Google Scholar] [CrossRef]
- Da Silva, E.S.; Flores, R.A.; Ribas, A.S.; Taschetto, A.P.D.; Faria, M.S.; Lima, L.B.; Metzger, M.; Donato, J.; Paschoalini, M.A. Injections of the of the α 1 -adrenoceptor antagonist prazosin into the median raphe nucleus increase food intake and Fos expression in orexin neurons of free-feeding rats. Behav. Brain Res. 2017, 324, 87–95. [Google Scholar] [CrossRef]
- Porter, C.E.; Shaw, A.R.; Jung, Y.; Yip, T.; Castro, P.D.; Sandulache, V.C.; Sikora, A.; Gottschalk, S.; Ittman, M.M.; Brenner, M.K.; et al. Oncolytic Adenovirus Armed with BiTE, Cytokine, and Checkpoint Inhibitor Enables CAR T Cells to Control the Growth of Heterogeneous Tumors. Mol. Ther. 2020, 28, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020, 12, eaax7992. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Møller, A.-S.W.; Jaderberg, M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. OncoImmunology 2019, 8, 1532763. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, Y.; Wang, H.; Ma, C.; Feist, M.; Ju, S.; Guo, Z.S.; Bartlett, D.L. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017, 8, 14754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilett, E.; Kottke, T.; Thompson, J.; Rajani, K.; Zaidi, S.; Evgin, L.; Coffey, M.; Ralph, C.; Diaz, R.; Pandha, H.; et al. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther. 2017, 24, 21–30. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, P.-Y.; Hutzen, B.; Sprague, L.; Swain, H.M.; Love, J.K.; Stanek, J.R.; Boon, L.; Conner, J.; Cripe, T.P. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hardcastle, J.; Mills, L.; Malo, C.S.; Jin, F.; Kurokawa, C.; Geekiyanage, H.; Schroeder, M.; Sarkaria, J.; Johnson, A.J.; Galanis, E. Immunovirotherapy with measles virus strains in combination with anti–PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro-Oncology 2016, 19, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Woller, N.; Gürlevik, E.; Fleischmann-Mundt, B.; Schumacher, A.; Knocke, S.; Kloos, A.M.; Saborowski, M.; Geffers, R.; Manns, M.P.; Wirth, T.C.; et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol. Ther. 2015, 23, 1630–1640. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Patnaik, M.M.; Ruiz, A.; Russell, S.J.; Peng, K.-W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood 2016, 127, 1449–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajani, K.; Parrish, C.; Kottke, T.; Thompson, J.; Zaidi, S.; Ilett, L.; Shim, K.G.; Diaz, R.-M.; Pandha, H.; Harrington, K.; et al. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol. Ther. 2016, 24, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Pesonen, S. Replication-Competent Viruses as Cancer Immunotherapeutics: Emerging Clinical Data. Hum. Gene Ther. 2015, 26, 538–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.H.; Lemoine, N.R.; Wang, Y. Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles. Viruses 2010, 2, 78–106. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.S.; Lemoine, N.R.; Wang, Y. Systemic Delivery of Oncolytic Viruses: Hopes and Hurdles. Adv. Virol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Bridle, B.W.; Stephenson, K.B.; Boudreau, J.E.; Koshy, S.; Kazdhan, N.; Pullenayegum, E.; Brunellière, J.; Bramson, J.L.; Lichty, B.D.; Wan, Y. Potentiating Cancer Immunotherapy Using an Oncolytic Virus. Mol. Ther. 2010, 18, 1430–1439. [Google Scholar] [CrossRef]
- Hotte, S.J.; Lorence, R.M.; Hirte, H.; Polawski, S.R.; Bamat, M.K.; O’Neil, J.D.; Roberts, M.S.; Groene, W.S.; Major, P.P. An Optimized Clinical Regimen for the Oncolytic Virus PV701. Clin. Cancer Res. 2007, 13, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Lorence, R.M.; Roberts, M.S.; Neil, J.D.O.; Groene, W.S.; Miller, J.A.; Mueller, S.N.; Bamat, M.K. Phase 1 Clinical Experience Using Intravenous Administration of PV701, an Oncolytic Newcastle Disease Virus. Curr. Cancer Drug Targets 2007, 7, 157–167. [Google Scholar] [CrossRef]
- Vigil, A.; Park, M.-S.; Martinez, O.; Chua, M.A.; Xiao, S.; Cros, J.; Martínez-Sobrido, L.; Woo, S.L.; García-Sastre, A. Use of Reverse Genetics to Enhance the Oncolytic Properties of Newcastle Disease Virus. Cancer Res. 2007, 67, 8285–8292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.; Shergold, A.; Elder, M.J.; Whitworth, J.; Cheng, X.; Jin, H.; Wilkinson, R.W.; Harper, J.; Carroll, D.K. Oncolytic Newcastle disease virus activation of the innate immune response and priming of antitumor adaptive responses in vitro. Cancer Immunol. Immunother. 2020, 69, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yi, C.; Yang, X.; Xu, J.; Sun, Q.; Liu, Y.; Zhao, L. Tumor Cells Modified with Newcastle Disease Virus Expressing IL-24 as a Cancer Vaccine. Mol. Ther. Oncolytics 2019, 14, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Quezada, S.A.; Sepulveda, M.A.; Sharma, P.; Allison, J.P. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 2014, 211, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, G.; McCroskery, S.; Palese, P. Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines. J. Virol. 2019, 94. [Google Scholar] [CrossRef]
- Vigil, A.; Martinez, O.; Chua, M.A.; García-Sastre, A. Recombinant Newcastle Disease Virus as a Vaccine Vector for Cancer Therapy. Mol. Ther. 2008, 16, 1883–1890. [Google Scholar] [CrossRef]
- Cassel, W.A.; Murray, D.R.; Torbin, A.H.; Olkowski, Z.L.; Moore, M.E. Viral oncolysate in the management of malignant melanoma. I. Preparation of the oncolysate and measurement of immunologic responses. Cancer 1977, 40, 672–679. [Google Scholar] [CrossRef]
- Murray, D.R.; Cassel, W.A.; Torbin, A.H.; Olkowski, Z.L.; Moore, M.E. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer 1977, 40, 680–686. [Google Scholar] [CrossRef]
- Cassel, W.A.; Murray, D.R. Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat. Immun. Cell Growth Regul. 1988, 7, 351–352. [Google Scholar]
- Lehner, B.; Schlag, P.; Liebrich, W.; Schirrmacher, V. Postoperative active specific immunization in curatively resected colorectal cancer patients with a virus-modified autologous tumor cell vaccine. Cancer Immunol. Immunother. 1990, 32, 173–178. [Google Scholar] [CrossRef]
- Liebrich, W.; Schlag, P.; Manasterski, M.; Lehner, B.; Stohr, M.; Möller, P.; Schirrmacher, V. In vitro and clinical characterisation of a newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur. J. Cancer Clin. Oncol. 1991, 27, 703–710. [Google Scholar] [CrossRef]
- Cassel, W.A.; Murray, D.R. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 1992, 9, 169–171. [Google Scholar] [PubMed]
- Schlag, P.; Manasterski, M.; Gerneth, T.; Hohenberger, P.; Dueck, M.; Herfarth, C.; Liebrich, W.; Schirrmacher, V. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol. Immunother. 1992, 35, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V.; Schlag, P.; Liebrich, W.; Patel, B.T.; Stoeck, M. Specific Immunotherapy of Colorectal Carcinoma with Newcastle-Disease Virus-Modified Autologous Tumor Cells Prepared from Resected Liver Metastasis. Ann. N. Y. Acad. Sci. 1993, 690, 364–366. [Google Scholar] [CrossRef]
- Kirchner, H.; Anton, P.; Atzpodien, J. Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines. World J. Urol. 1995, 13, 171–173. [Google Scholar] [CrossRef]
- Pomer, S.; Schirrmacher, V.; Thiele, R.; Lohrke, H.; Brkovic, D.; Staehler, G. Tumor Response and 4 year survival-data of patients with advanced renal-cell carcinoma treated with autologous tumor vaccine AND subcutaneous R-IL-2 and Ifn-Alpha(2B). Int. J. Oncol. 1995, 6, 947–954. [Google Scholar] [CrossRef]
- Ockert, D.; Schirrmacher, V.; Beck, N.; Stoelben, E.; Ahlert, T.; Flechtenmacher, J.; Hagmüller, E.; Buchcik, R.; Nagel, M.; Saeger, H.D. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin. Cancer Res. 1996, 2, 21–28. [Google Scholar]
- Ahlert, T.; Sauerbrei, W.; Bastert, G.; Ruhland, S.; Bartik, B.; Simiantonaki, N.; Schumacher, J.; Häcker, B.; Schirrmacher, V.; Schumacher, M. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J. Clin. Oncol. 1997, 15, 1354–1366. [Google Scholar] [CrossRef]
- Zorn, U.; Duensing, S.; Langkopf, F.; Anastassiou, G.; Kirchner, H.; Hadam, M.; Knüver-Hopf, J.; Atzpodien, J. Active Specific Immunotherapy of Renal Cell Carcinoma: Cellular and Humoral Immune Responses. Cancer Biother. Radiopharm. 1997, 12, 157–165. [Google Scholar] [CrossRef]
- Batliwalla, F.M.; Bateman, B.A.; Serrano, D.; Murray, D.; MacPhail, S.; Maino, V.C.; Ansel, J.C.; Gregersen, P.K.; Armstrong, C.A. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol. Med. 1998, 4, 783–794. [Google Scholar] [CrossRef]
- Csatary, L.K.; Moss, R.W.; Beuth, J.; Töröcsik, B.; Szeberenyi, J.; Bakacs, T. Beneficial treatment of patients with advanced cancer using a Newcastle disease virus vaccine (MTH-68/H). Anticancer. Res. 1999, 19, 635–638. [Google Scholar] [PubMed]
- Liang, W.; Wang, H.; Sun, T.-M.; Yao, W.-Q.; Chen, L.; Jin, Y.; Li, C.-L.; Meng, F.-J. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J. Gastroenterol. 2003, 9, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Voit, C.A.; Kron, M.; Schwürzer-Voit, M.; Sterry, W. Intradermal injection of Newcastle disease virus-modified autologous melanoma cell lysate and interleukin-2 for adjuvant treatment of melanoma patients with resectable stage III disease. J. Dtsch. Dermatol. Ges. 2003, 1, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.-H.; Bonsanto, M.M.; Beckhove, P.; Brysch, M.; Geletneky, K.; Ahmadi, R.; Schuele-Freyer, R.; Kremer, P.; Ranaie, G.; Matejic, D.; et al. Antitumor Vaccination of Patients With Glioblastoma Multiforme: A Pilot Study to Assess Feasibility, Safety, and Clinical Benefit. J. Clin. Oncol. 2004, 22, 4272–4281. [Google Scholar] [CrossRef]
- Schulze, T.; Kemmner, W.; Weitz, J.; Wernecke, K.-D.; Schirrmacher, V.; Schlag, P.M. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: Results of a prospective randomized trial. Cancer Immunol. Immunother. 2009, 58, 61–69. [Google Scholar] [CrossRef]
- Cassel, W.A.; Murray, D.R.; Phillips, H.S. A phase II study on the postsurgical management of stage II malignant melanoma with a Newcastle disease virus oncolysate. Cancer 1983, 52, 856–860. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Fournier, P. Newcastle Disease Virus: A Promising Vector for Viral Therapy, Immune Therapy, and Gene Therapy of Cancer. Methods Mol. Biol. 2009, 542, 565–605. [Google Scholar] [CrossRef]
- Bohle, W.; Schlag, P.; Liebrich, W.; Hohenberger, P.; Manasterski, M.; Möller, P.; Schirrmacher, V. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine. First clinical results with tumor-cell vaccines modified with live but avirulent newcastle disease virus. Cancer 1990, 66, 1517–1523. [Google Scholar] [CrossRef]
- Csatary, L.K. Use of Newcastle Disease Virus Vaccine (MTH-68/H) in a Patient With High-grade Glioblastoma. JAMA 1999, 281, 1588–1589. [Google Scholar] [CrossRef]
- Csatary, L.; Gosztonyi, G.; Szeberenyi, J.; Fabian, Z.; Liszka, V.; Bodey, B.; Csatary, C. MTH-68/H Oncolytic Viral Treatment in Human High-Grade Gliomas. J. Neuro-Oncol. 2004, 67, 83–93. [Google Scholar] [CrossRef]
- Pecora, A.L.; Rizvi, N.; Cohen, G.I.; Meropol, N.J.; Sterman, D.; Marshall, J.L.; Goldberg, S.; Gross, P.; O’Neil, J.D.; Groene, W.S.; et al. Phase I Trial of Intravenous Administration of PV701, an Oncolytic Virus, in Patients with Advanced Solid Cancers. J. Clin. Oncol. 2002, 20, 2251–2266. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Bell, J.C.; Atkins, H.L.; Roach, J.; Bamat, M.K.; O’Neil, J.D.; Roberts, M.S.; Groene, W.S.; Lorence, R.M. A Phase 1 Clinical Study of Intravenous Administration of PV701, an Oncolytic Virus, Using Two-Step Desensitization. Clin. Cancer Res. 2006, 12, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burman, B.; Pesci, G.; Zamarin, D. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers 2020, 12, 3552. https://doi.org/10.3390/cancers12123552
Burman B, Pesci G, Zamarin D. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers. 2020; 12(12):3552. https://doi.org/10.3390/cancers12123552
Chicago/Turabian StyleBurman, Bharat, Giulio Pesci, and Dmitriy Zamarin. 2020. "Newcastle Disease Virus at the Forefront of Cancer Immunotherapy" Cancers 12, no. 12: 3552. https://doi.org/10.3390/cancers12123552
APA StyleBurman, B., Pesci, G., & Zamarin, D. (2020). Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers, 12(12), 3552. https://doi.org/10.3390/cancers12123552




