Psychological Burden in Meningioma Patients under a Wait-and-Watch Strategy and after Complete Resection Is High—Results of a Prospective Single Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Psychological Burden as Measured by the HADS and DT
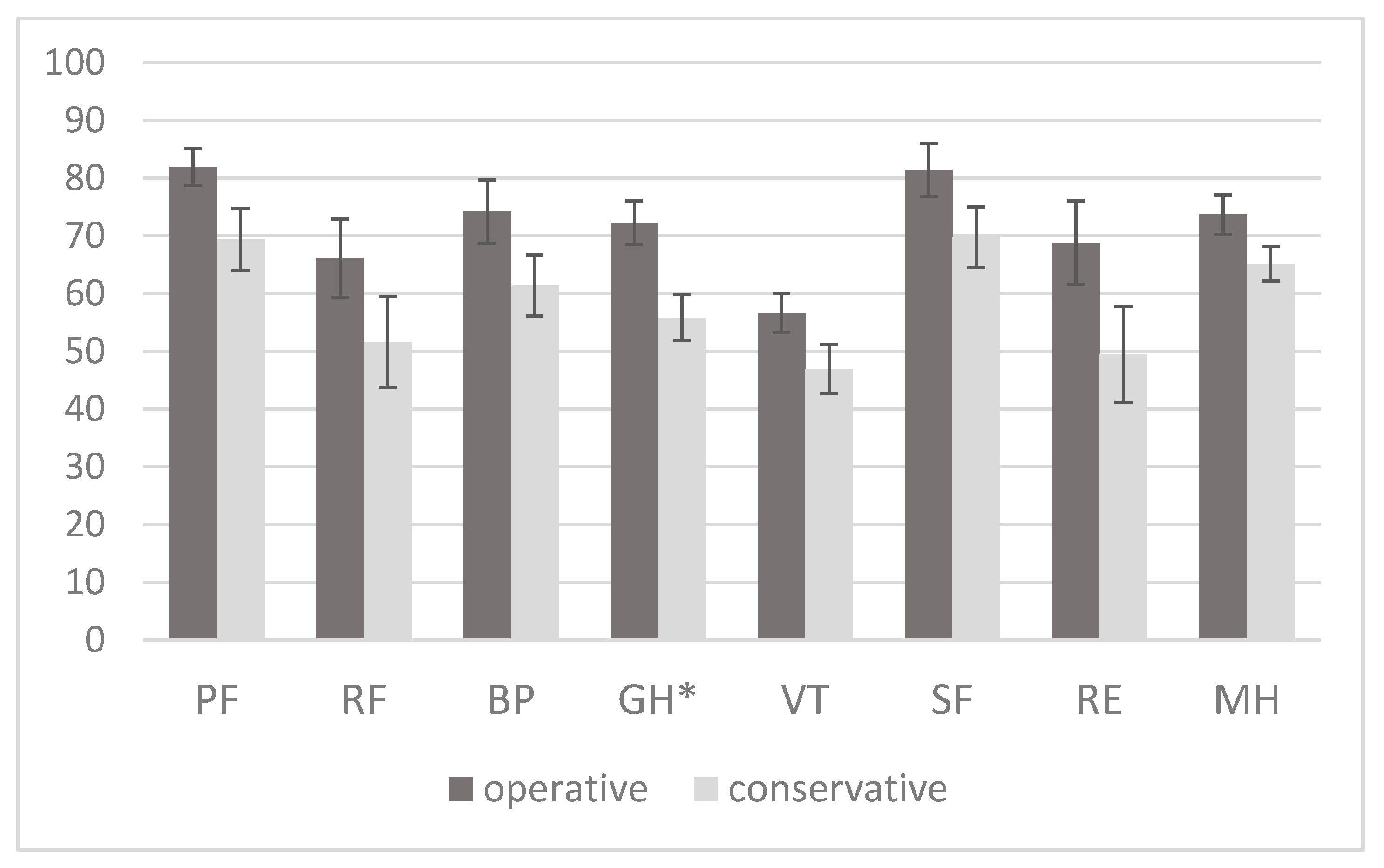
2.3. HRQoL as Measured by the SF-36
2.4. Fatigue as Measured by the BFI
2.5. Factors Associated with Higher Psychological Burden
3. Discussion
3.1. Depression and Anxiety in Meningioma Patients
3.2. Patients’ Quality of Life
3.3. Fatigue
3.4. Watch-and-Wait vs. Operative Strategy
3.5. Limitations of the Study
4. Materials and Methods
4.1. Study Design and Patients
4.2. Investigations
4.3. Applied Questionnaires
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, 1–100. [Google Scholar] [CrossRef]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and etiology of meningioma. J. Neurooncol. 2010, 99, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Vernooij, M.W.; Ikram, M.A.; Tanghe, H.L.; Vincent, A.J.P.E.; Hofman, A.; Krestin, G.P.; Niessen, W.J.; Breteler, M.M.B.; Van Der Lugt, A. Incidental findings on brain MRI in the general population. N. Engl. J. Med. 2007, 357, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Butts, A.M.; Weigand, S.; Brown, P.D.; Petersen, R.C.; Jack, C.R.; Machulda, M.M.; Cerhan, J.H. Neurocognition in individuals with incidentally-identified meningioma. J. Neurooncol. 2017, 134, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Roser, F.; Michel, J.; Med, C.; Jacobs, C.; Samii, M. The natural history of incidental meningiomas. Neurosurgery 2003, 53, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hickmann, A.K.; Nadji-Ohl, M.; Haug, M.; Hopf, N.J.; Ganslandt, O.; Giese, A.; Renovanz, M. Suicidal ideation, depression, and health-related quality of life in patients with benign and malignant brain tumors: A prospective observational study in 83 patients. Acta Neurochir. 2016, 158, 1669–1682. [Google Scholar] [CrossRef]
- Bunevicius, A.; Tamasauskas, S.; Deltuva, V.; Tamasauskas, A.; Radziunas, A.; Bunevicius, R. Predictors of health-related quality of life in neurosurgical brain tumor patients: Focus on patient-centered perspective. Acta Neurochir. 2014, 156, 367–374. [Google Scholar] [CrossRef]
- Zamanipoor Najafabadi, A.H.; Peeters, M.C.M.; Dirven, L.; Lobatto, D.J.; Groen, J.L.; Broekman, M.L.D.; Peerdeman, S.M.; Peul, W.C.; Taphoorn, M.J.B.; Van Furth, W.R. Impaired health-related quality of life in meningioma patients—A systematic review. Neuro-Oncology 2017, 19, 897–907. [Google Scholar] [CrossRef]
- Zamanipoor Najafabadi, A.H.; Peeters, M.C.M.; Lobatto, D.J.; Broekman, M.L.D.; Smith, T.R.; Biermasz, N.R.; Peerdeman, S.M.; Peul, W.C.; Taphoorn, M.J.B.; van Furth, W.R.; et al. Health-related quality of life of cranial WHO grade I meningioma patients: Are current questionnaires relevant? Acta Neurochir. 2017, 159, 2149–2159. [Google Scholar] [CrossRef] [Green Version]
- van Nieuwenhuizen, D.; Slot, K.M.; Klein, M.; Verbaan, D.; Aliaga, E.S.; Heimans, J.J.; Vandertop, W.P.; Peerdeman, S.M.; Reijneveld, J.C. The association between preoperative edema and postoperative cognitive functioning and health-related quality of life in WHO grade I meningioma patients. Acta Neurochir. 2019, 161, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Bunevicius, A.; Deltuva, V.P.; Tamasauskas, A. Association of pre-operative depressive and anxiety symptoms with five-year survival of glioma and meningioma patients: A prospective cohort study. Oncotarget 2017, 8, 57543–57551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A.; Shiban, Y.; Lange, N.; Joerger, A.K.; Hoffmann, U.; Meyer, B.; Shiban, E. The relevant psychological burden of having a benign brain tumor: A prospective study of patients undergoing surgical treatment of cranial meningiomas. J. Neurosurg. 2019, 131, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Goebel, S.; Mehdorn, H.M. Development of anxiety and depression in patients with benign intracranial meningiomas: A prospective long-term study. Support. Care Cancer 2013, 21, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vossen, S.; Schepers, V.P.M.; Van Der Sprenkel, J.W.B.; Visser-Meily, J.M.A.; Post, M.W.M. Cognitive and emotional problems in patients after cerebral meningioma surgery. J. Rehabil. Med. 2014, 46, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Goebel, S.; Mehdorn, H.M. A missing piece? Neuropsychiatric functioning in untreated patients with tumors within the cerebellopontine angle. J. Neurooncol. 2018, 140, 145–153. [Google Scholar] [CrossRef]
- Hinz, A.; Mitchell, A.J.; Dégi, C.L.; Mehnert-Theuerkauf, A. Normative values for the distress thermometer (DT) and the emotion thermometers (ET), derived from a German general population sample. Qual. Life Res. 2014, 28, 277–282. [Google Scholar] [CrossRef]
- Benz, L.S.; Wrensch, M.R.; Schildkraut, J.M.; Bondy, M.L.; Warren, J.L.; Wiemels, J.L.; Claus, E.B. Quality of life after surgery for intracranial meningioma. Cancer 2018, 124, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Yamashiro, S.; Nishi, T.; Koga, K.; Kaji, M.; Goto, T.; Muta, D.; Fujioka, S.; Kuratsu, J. Self-assesed quality of life in patients who underwent surgery for asymptomatic meningiomas. No Shinkei Geka 2007, 35, 1149–1155. [Google Scholar]
- van Nieuwenhuizen, D.; Klein, M.; Stalpers, L.J.A.; Leenstra, S.; Heimans, J.J.; Reijneveld, J.C. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J. Neurooncol. 2007, 84, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwenhuizen, D.; Ambachtsheer, N.; Heimans, J.J.; Reijneveld, J.C.; Peerdeman, S.M.; Klein, M. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J. Neurooncol. 2013, 113, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Price, B.; Shehab, A.; Au, K.; Cusimano, M.D.; Jenkinson, M.D.; Jungk, C.; Mansouri, A.; Santarius, T.; Suppiah, S.; et al. Life after surgical resection of a meningioma: A prospective cross-sectional study evaluating health-related quality of life. Neuro-Oncology 2019, 21 (Suppl. S1), i32–i43. [Google Scholar] [CrossRef] [Green Version]
- Nassiri, F.; Suppiah, S.; Wang, J.Z.; Badhiwala, J.H.; Juraschka, K.; Meng, Y.; Nejad, R.; Au, K.; Willmarth, N.E.; Cusimano, M.; et al. How to live with a meningioma: Experiences, symptoms, and challenges reported by patients. Neuro-Oncol. Adv. 2020, 2, vdaa086. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, S.D.; Gehring, K.; Gehring, K.; Rutten, G.J.M.; Kop, W.J.; Sitskoorn, M.M. Prevalence and correlates of fatigue in patients with meningioma before and after surgery. Neuro-Oncol. Pract. 2020, 7, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Portenoy, R.K.; Itri, L.M. Cancer-Related Fatigue: Guidelines for Evaluation and Management. Oncologist 1999, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.M.; Paul, L.; Chappell, F.M.; Macleod, M.; Whiteley, W.N.; Salman, R.A.S.; Wardlaw, J.M.; Sudlow, C.L.M. Potentially serious incidental findings on brain and body magnetic resonance imaging of apparently asymptomatic adults: Systematic review and meta-analysis. BMJ 2018, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunevicius, A. Personality traits, patient-centered health status and prognosis of brain tumor patients. J. Neurooncol. 2018, 137, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Renovanz, M.; Hickmann, A.K.; Nadji-Ohl, M.; Keric, N.; Weimann, E.; Wirtz, C.R.; Singer, S.; Ringel, F.; Coburger, J. Health-related quality of life and distress in elderly vs. younger patients with high-grade glioma—results of a multicenter study. Support. Care Cancer 2020, 28, 5165–5175. [Google Scholar] [CrossRef] [Green Version]
- Wirsching, H.-G.; Morel, C.; Roth, P.; Weller, M. Socioeconomic burden and quality of life in meningioma patients. Qual. Life Res. 2020, 29, 1801–1808. [Google Scholar] [CrossRef]
- Nayak, L.; DeAngelis, L.M.; Brandes, A.A.; Peereboom, D.M.; Galanis, E.; Lin, N.U.; Soffietti, R.; Macdonald, D.R.; Chamberlain, M.; Perry, J.; et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: A tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology. Neuro-Oncology 2017, 19, 625–635. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (Sf-36): I. conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Bullinger, M. German translation and psychometric testing of the SF-36 Health Survey: Preliminary results from the IQOLA project. Soc. Sci. Med. 1995, 41, 1359–1366. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.; Buss, U.; Snaith, R.P. HADS-D Hospital Anxiety and Depression Scale–Deutsche Version; Huber: Bern, Switzerland, 1995. [Google Scholar]
- Goebel, S.; Mehdorn, H.M. Measurement of psychological distress in patients with intracranial tumours: The NCCN distress thermometer. J. Neurooncol. 2011, 104, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, L.; Sabatowski, R.; Elsner, F.; Everts, J.; Mendoza, T.; Cleeland, C. Validation of the German version of the brief fatigue inventory. J. Pain Symptom Manag. 2003, 25, 449–458. [Google Scholar] [CrossRef]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]

| Postoperative | Wait-and-Watch | Total | |||
|---|---|---|---|---|---|
| N | 31 | 31 | 62 | ||
| Age (SD) | 57 (13) | 66 (12) | 61 (13) | p = 0.008 | |
| Female, % | 25 (81%) | 26 (84%) | 51 (82%) | ||
| Family situation, % | |||||
| living with a partner | 22 (71%) | 18 (62%) | 40 (67%) | ||
| living alone | 9 (29%) | 11 (38%) | 20 (33%) | ||
| Employment, % | p < 0.001 | ||||
| Full | 12 (39) | 9 (31%) | 21 (35%) | ||
| part-time | 2 (6%) | 0 (0%) | 2 (3%) | ||
| Unemployed | 6 (19%) | 0 (0%) | 6 (10%) | ||
| Retired | 11 (35%) | 20 (69%) | 31 (52%) | ||
| ECOG, % | |||||
| 0 | 24 (77%) | 26 (84%) | 50 (81%) | ||
| 1 | 7 (23%) | 2 (6%) | 9 (15%) | ||
| 2 | 0 (0%) | 3 (10%) | 3 (5%) | ||
| NANO scale, mean (SD) | 0.5 (1.0) | 0.4 (0.8) | 0.4 (0.9) | ||
| Psychiatric disorder | 1 (3%) | 2 (6%) | 3 (5%) | ||
| Tumor localisation, % | |||||
| Convexity | 10 (36%) | 11 (37%) | 21 (36%) | ||
| Falx | 4 (14%) | 5 (17%) | 9 (15%) | ||
| Anterior fossa | 4 (14%) | 2 (7%) | 6 (10%) | ||
| Middle fossa | 2 (7%) | 5 (7%) | 7(12%) | ||
| Posterior fossa | 5 (18%) | 4 (13%) | 9 (15%) | ||
| Sella/sinus cavernosus | 3 (11%) | 2 (7%) | 5 (9%) | ||
| WHO histological grade | |||||
| Grade I | 27 (87%) | ||||
| Grade II | 4 (13%) | ||||
| Time after diagnosis, months (SD) | 39 (47) | ||||
| Time after operation, months (SD) | 32 (44) | ||||
| Tumor size, mm | 30 (18) | 18 (10) | p = 0.004 | ||
| Tumor growth | 2 (6%) | 6 (19%) |
| Mean (SD) | Proportion with Significant Fatigue (≥7) | |||
|---|---|---|---|---|
| Subscales | Postoperative | Wait-and-Watch | Postoperative | Wait-and-Watch |
| Fatigue right now | 3.5 (2.2) | 3.8 (2.3) | 12.9% | 9.7% |
| Usual fatigue | 3.7 (1.9) | 4.2 (2.3) | 6.5% | 19.4% |
| Worst fatigue | 6.2 (2.9) | 5.6 (2.5) | 58.1% | 45.2% |
| Activity | 3.4 (3.4) | 3.4 (2.5) | 12.9% | 12.9% |
| Mood | 2.8 (1.9) | 3.4 (2.3) | 6.5% | 12.9% |
| Walking | 2.2 (1.8) | 2.8 (1.9) | 6.5% | 12.9% |
| Working | 2.6 (2.0) | 3.4 (2.3) | 3.2% | 6.5% |
| Relation to others | 2.2 (2.0) | 2.7 (2.3) | 6.5% | 12.9% |
| Enjoyment of life | 2.2 (2.0) * | 3.5 (2.3) * | 6.5% | 16.1% |
| Mean score | 3.4 (1.8) | 3.3 (1.5) | 6.5% | 3.4% |
| OR (95%CI) | |||
|---|---|---|---|
| Variable | DT ≥ 6 | HADS-A ≥ 11 | HADS-D ≥ 11 |
| Gender | 1.24 (0.30–5.18) | 1.44 (0.37–5.53) | 0.91 (0.21–3.96) |
| Age ≥ 65 years | 1.49 (0.52–4.28) | 1.23 (0.44–3.38) | 0.50 (0.15–1.66) |
| Family status | 0.41 (0.12–1.36) | 0.82 (0.27–2.42) | 0.54 (0.17–1.76) |
| Employment | 0.81 (0.55–1.19) | 0.96 (0.66–1.39) | 0.48 (0.14–1.71) |
| Education | - | 0.36 (0.03–4.25) | 1.40 (0.12–16.58) |
| ECOG 1 vs.0 | 1.22 (0.29–5.13) | 1.02 (0.24–4.25) | 0.35 (0.81–1.54) |
| NANO Score | 0.99 (0.23–1.97) | 1.13 (0.64–1.98) | 0.34 (0.16–0.70) * |
| Significant fatigue | 2.50 (0.86–7.31) | 0.60 (0.22–1.65) | 1.53 (0.49–4.81) |
| Wait-and-watch vs. operative treatment | 1.32 (0.47–3.72) | 0.88 (0.32–2.40) | 4.26 (1.19–15.25) * |
| Tumor location | 0.68 (0.23–1.97) | 0.62 (0.22–1.80) | 0.96 (0.30–3.14) |
| Tumor size | 0.99 (0.96–1.03) | 0.98 (0.97–1.03) | 0.99 (0.96–1.03) |
| Time since diagnosis/operation | 1.00 (0.99–1.01) | 0.99 (0.99–1.01) | 0.99 (0.98–1.01) |
| Tumor progress | 1.14 (0.23–5.63) | 0.75 (0.16–3.46) | 2.69 (0.31–23.78) |
| OR (95%CI) | |
|---|---|
| Variable | HADS-D ≥ 11 |
| NANO Score | 0.27 (0.11–0.65) * |
| Wait-and-watch vs. operative treatment | 5.83 (1.12–28.85) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalasauskas, D.; Keric, N.; Abu Ajaj, S.; von Cube, L.; Ringel, F.; Renovanz, M. Psychological Burden in Meningioma Patients under a Wait-and-Watch Strategy and after Complete Resection Is High—Results of a Prospective Single Center Study. Cancers 2020, 12, 3503. https://doi.org/10.3390/cancers12123503
Kalasauskas D, Keric N, Abu Ajaj S, von Cube L, Ringel F, Renovanz M. Psychological Burden in Meningioma Patients under a Wait-and-Watch Strategy and after Complete Resection Is High—Results of a Prospective Single Center Study. Cancers. 2020; 12(12):3503. https://doi.org/10.3390/cancers12123503
Chicago/Turabian StyleKalasauskas, Darius, Naureen Keric, Salman Abu Ajaj, Leoni von Cube, Florian Ringel, and Mirjam Renovanz. 2020. "Psychological Burden in Meningioma Patients under a Wait-and-Watch Strategy and after Complete Resection Is High—Results of a Prospective Single Center Study" Cancers 12, no. 12: 3503. https://doi.org/10.3390/cancers12123503





