Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer
Abstract
:1. Introduction
2. Signaling Pathways and Oncogene Involvement in Breast Cancer
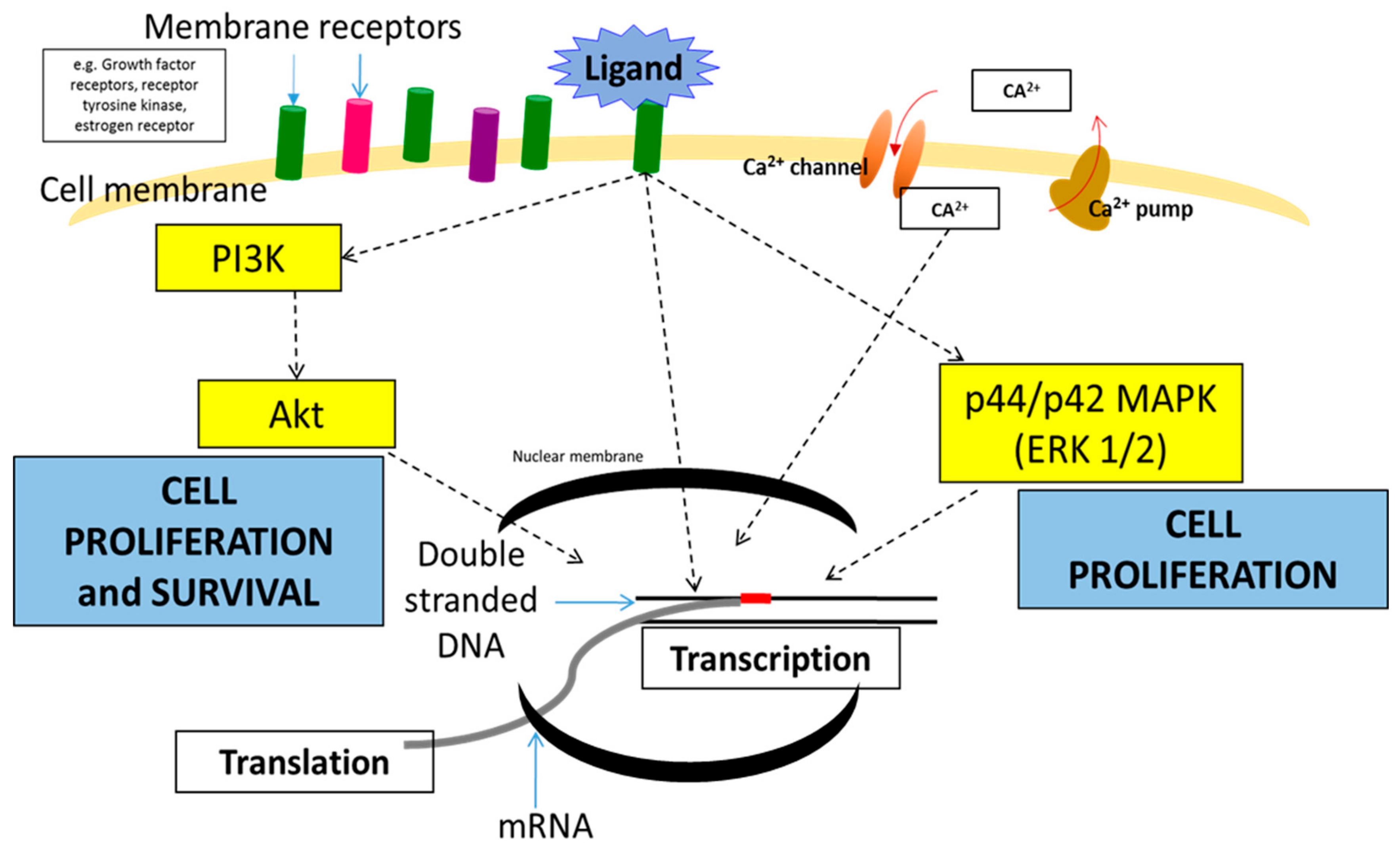
2.1. Mitogen-Activated Protein Kinase (MAPK) Pathway
2.2. PI3K/AKT Pathway
2.3. Calcium Signaling Pathway
2.4. Notch Signaling Pathway
2.5. Hedgehog Signaling Pathway
2.6. JAK/STAT Signaling Pathway
2.7. Anti-Apoptotic Signaling Pathway
3. Growth Factor Receptors and Breast Cancer
3.1. Epidermal Growth Factor Receptor (EGFR)
3.2. Insulin-Like Growth Factor 1 Receptor (IGF1R)
3.3. Transforming Growth Factor-Beta Receptor (TGF-βR)
3.4. Vascular Endothelial Growth Factor Receptor (VEGFR)
3.5. Human Epidermal Growth Factor Receptor 2 (HER2/ERBB2)
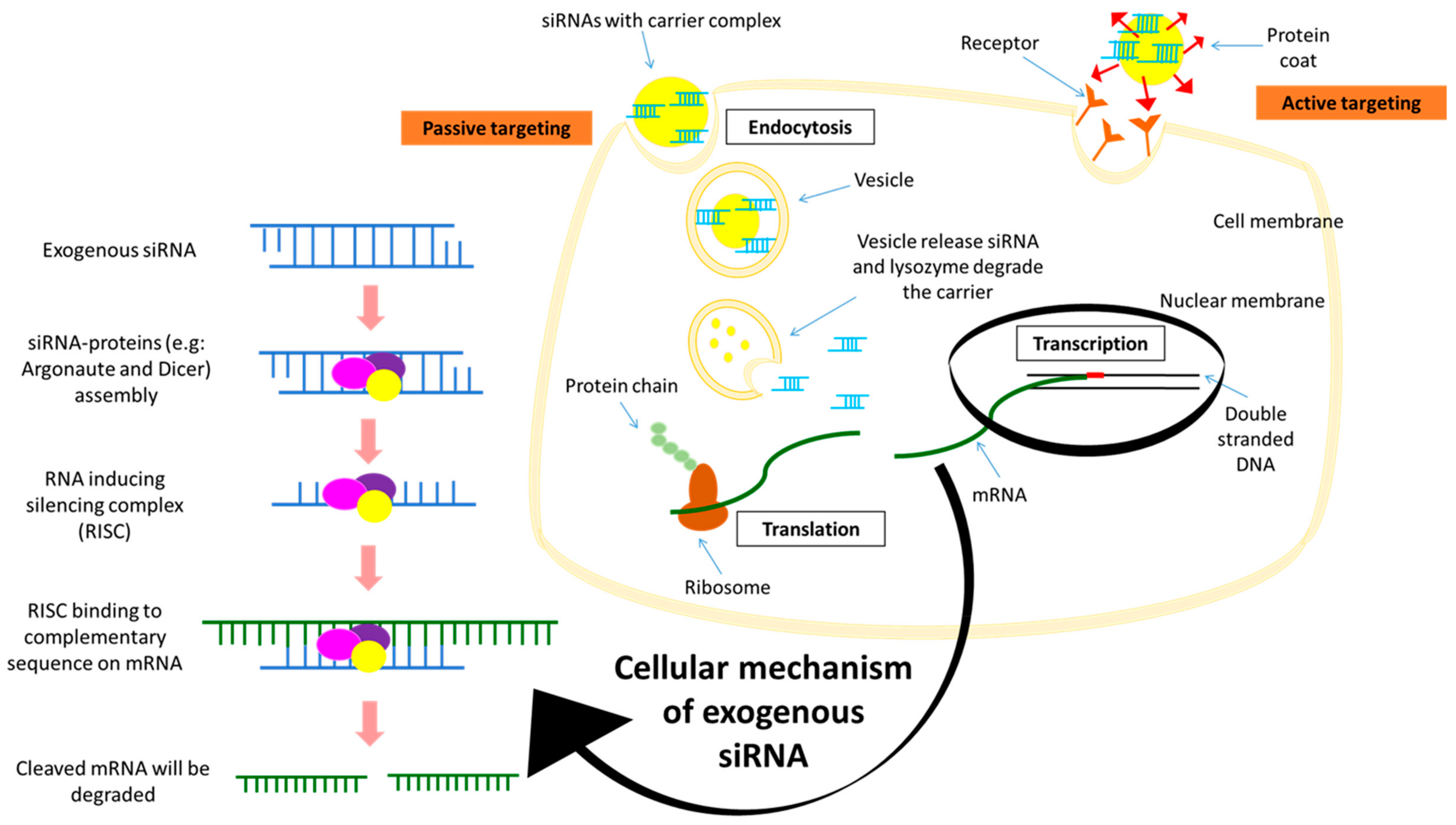
4. siRNA Silencing Technique
4.1. Advantages of siRNA Delivery
4.2. Limitations of siRNA Delivery
5. Delivery Systems of Potential Therapeutic siRNAs
6. Current Targets for Nanoparticles-Facilitated siRNA Silencing
7. Clinical Trials of nano-siRNA for Cancer Therapy
8. Conclusions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Ministry of Health; National Cancer Registry Department; National Cancer Institute. Malaysian National Cancer Registry Report (2007–2011); Ministry of Health Malaysia: Putrajaya, Malaysia, 2016.
- Globocan. Breast Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2018. 2018. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 5 April 2019).
- Van Pham, P. Introduction to Breast Cancer. In Breast Cancer Stem Cells & Therapy Resistance; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–4. [Google Scholar]
- Carlson, N.; King, J. Overview of breast cancer treatment and reconstruction for primary care providers. J. Midwifery Women’s Health 2012, 57, 558–568. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef]
- Chan, M.; Chang, M.C.; González, R.; Lategan, B.; del Barco, E.; Vera-Badillo, F.; Quesada, P.; Goldstein, R.; Cruz, I.; Ocana, A.; et al. Outcomes of estrogen receptor negative and progesterone receptor positive breast cancer. PLoS ONE 2015, 10, e0132449. [Google Scholar] [CrossRef]
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Fodstad, O.; Jiang, W.G. Metastasis of breast cancer: An introduction. In Metastasis of Breast Cancer; Mansel, R.E., Fodstad, O., Jiang, W.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–5. [Google Scholar]
- Tiash, S.; Chowdhury, E.H. Growth factor receptors: Promising drug targets in cancer. J. Cancer Metastasis Treat. 2015, 1, 190–200. [Google Scholar]
- Lokate, M.; Peeters, P.H.; Peelen, L.M.; Haars, G.; Veldhuis, W.B.; van Gils, C.H. Mammographic density and breast cancer risk: The role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011, 13, R103. [Google Scholar] [CrossRef] [PubMed]
- Urlep, Z.; Rozman, D. The interplay between circadian system, cholesterol synthesis, and steroidogenesis affects various aspects of female reproduction. Front Endocrinol. 2013, 4, 111. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Colditz, G.A. Estrogens in the breast tissue: A systematic review. Cancer Causes Control CCC 2011, 22, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Radiotherapy in the management of early breast cancer. J. Med. Radiat. Sci. 2013, 60, 40–46. [Google Scholar] [CrossRef]
- Harbeck, N. HER2-positive breast cancer: Neoadjuvant and adjuvant therapy. In Handbook of HER2-Targeted Agents in Breast Cancer; Springer International Publishing: Cham, Switzerland, 2016; pp. 29–49. [Google Scholar]
- Figueroa-Magalhães, M.C.; Jelovac, D.; Connolly, R.; Wolff, A.C. Treatment of HER2-positive breast cancer. Breast (Edinb. Scotl.) 2014, 23, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Gonzalez-Angulo, A.M.; Pusztai, L. Definition of triple-negative breast cancer and relationship to basal-like molecular subtype. In DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 1–6. [Google Scholar]
- Wang, M.; Zhu, J.; Lubman, D.; Gao, C. Aberrant glycosylation and cancer biomarker discovery: A promising and thorny journey. Clin. Chem. Lab. Med. 2018, 57, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Oon, C. How glycosylation aids tumor angiogenesis: An updated review. Biomed. Pharmacother. 2018, 103, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Lang, J.; Hung, M.; Sengupta, K.; Banerjee, S.; Baksi, K.; Banerjee, D. Unfolded Protein Response Is Required innu/nuMice Microvasculature for Treating Breast Tumor with Tunicamycin. J. Biol. Chem. 2011, 286, 29127–29138. [Google Scholar] [CrossRef] [PubMed]
- Kwa, M.; Adams, S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef]
- Thamarajah, L. Complementary and Alternative Therapies for Breast Cancer Worldwide. Lett. Health Biol. Sci. 2018, 4, 27–32. [Google Scholar]
- Barrie, R.C.; Gary, D. Complementary and Alternative Therapies for Cancer. Oncologist 2003, 9, 80–89. [Google Scholar]
- Azim, H.A., Jr.; de Azambuja, E.; Colozza, M.; Bines, J.; Piccart, M.J. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann. Oncol. 2011, 22, 1939–1947. [Google Scholar] [CrossRef]
- Nooren, I.M.A.; Thornton, J.M. CHAPTER 4—Molecular Sociology A2—Bradshaw, Ralph A. In Handbook of Cell Signaling; Dennis, E.A., Ed.; Academic Press: Burlington, NJ, USA, 2003; pp. 21–26. [Google Scholar]
- Chial, H. Proto-oncogenes to Oncogenes to Cancer. Nat. Educ. 2008, 1, 33. [Google Scholar]
- Basu, A.K. DNA damage, mutagenesis and cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Schaeffer, H.J.; Weber, M.J. Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol. Cell. Biol. 1999, 19, 2435–2444. [Google Scholar] [CrossRef]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. MMBR 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Sivaraman, V.S.; Wang, H.; Nuovo, G.J.; Malbon, C.C. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J. Clin. Investig. 1997, 99, 1478–1483. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, H.; Liu, G.; Kreike, B.; Chen, W.; Sethi, S.; Miller, F.R.; Wu, G. p38gamma mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer. Neoplasia 2011, 13, 472–482. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Baselga, J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist 2011, 16 (Suppl. 1), 12–19. [Google Scholar] [CrossRef]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Wickenden, J.A.; Watson, C.J. Key signaling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: A play in 3 Akts. Breast Cancer Res. 2010, 12, 202. [Google Scholar] [CrossRef]
- Bose, S.; Chandran, S.; Mirocha, J.M.; Bose, N. The Akt pathway in human breast cancer: A tissue-array-based analysis. Mod. Pathol. 2006, 19, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Krishnamurthy, S.; Paranandi, R.; Basu, A. PKCepsilon induces Bcl-2 by activating CREB. Int. J. Oncol. 2010, 36, 883–888. [Google Scholar]
- Clapham, D.E. Calcium signaling. Cell 1995, 80, 259–268. [Google Scholar] [CrossRef]
- Roderick, H.L.; Cook, S.J. Ca2+ signaling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Di, J.; Huang, H.; Qu, D.; Tang, J.; Cao, W.; Lu, Z.; Cheng, Q.; Yang, J.; Bai, J.; Zhang, Y.; et al. Rap2B promotes proliferation, migration, and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci. Rep. 2015, 5, 12363. [Google Scholar] [CrossRef]
- Reczek, C.; Chandel, N. ROS Promotes Cancer Cell Survival through Calcium Signaling. Cancer Cell 2018, 33, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Simoes, B.M.; Clarke, R.B.; Brennan, K. A role for Notch signaling in breast cancer and endocrine resistance. Stem Cells Int. 2016, 2016, 2498764. [Google Scholar] [CrossRef]
- Ling, H.; Sylvestre, J.R.; Jolicoeur, P. Notch1-induced mammary tumor development is cyclin D1-dependent and correlates with expansion of pre-malignant multipotent duct-limited progenitors. Oncogene 2010, 29, 4543–4554. [Google Scholar] [CrossRef]
- Cohen, B.; Shimizu, M.; Izrailit, J.; Ng, N.F.; Buchman, Y.; Pan, J.G.; Dering, J.; Reedijk, M. Cyclin D1 is a direct target of JAG1-mediated Notch signaling in breast cancer. Breast Cancer Res. Treat. 2010, 123, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Jaks, V.; Fiaschi, M.; Toftgård, R. Hedgehog signaling in breast cancer. Carcinogenesis 2009, 30, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Mott, L.; Su, K.; Pack, D. Evaluation of FOXC1 as a therapeutic target for basal-like breast cancer. Cancer Gene Ther. 2018, 25, 84–91. [Google Scholar] [CrossRef]
- Han, B.; Qu, Y.; Jin, Y.; Yu, Y.; Deng, N.; Wawrowsky, K.; Zhang, X.; Li, N.; Bose, S.; Wang, Q.; et al. FOXC1 activates Smoothened-independent Hedgehog signaling in basal-like breast cancer. Cell Rep. 2015, 13, 1046–1058. [Google Scholar] [CrossRef]
- Bai, C.B.; Stephen, D.; Joyner, A.L. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell 2004, 6, 103–115. [Google Scholar] [CrossRef]
- Kasper, M.; Regl, G.; Frischauf, A.M.; Aberger, F. GLI transcription factors: Mediators of oncogenic Hedgehog signaling. Eur. J. Cancer 2006, 42, 437–445. [Google Scholar] [CrossRef]
- Ten Haaf, A.; Bektas, N.; von Serenyi, S.; Losen, I.; Arweiler, E.C.; Hartmann, A.; Knüchel, R.; Dahl, E. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer 2009, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, A.; Isebaert, S.; Haustermans, K. Targeting the Hedgehog signaling pathway in cancer: Beyond Smoothened. Oncotarget 2015, 6, 13899–13913. [Google Scholar] [CrossRef]
- Croker, B.A.; Kiu, H.; Nicholson, S.E. SOCS regulation of the JAK/STAT signaling pathway. Semin. Cell Dev. Biol. 2008, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signaling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Dolled-Filhart, M.; Camp, R.L.; Kowalski, D.P.; Smith, B.L.; Rimm, D.L. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin. Cancer Res. 2003, 9, 594–600. [Google Scholar] [PubMed]
- Alvarez, J.V.; Febbo, P.G.; Ramaswamy, S.; Loda, M.; Richardson, A.; Frank, D.A. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res. 2005, 65, 5054–5062. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Joos, S.; Zornig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 2004, 1644, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Amstrong, R.C.; Augeri, D.J.; Belli, B.A. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Klasa, R.J.; Gillum, A.M.; Klem, R.E.; Frankel, S.R. Oblimersen Bcl-2 antisense: Facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002, 12, 193–213. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.W. Regulation of apoptosis by HER2 in breast cancer. J. Carcinog. Mutagen. 2013, 2013 (Suppl. 7), 003. [Google Scholar]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL-2 family: Key mediators of the apoptotic response to targeted anti-cancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef]
- Voudouri, K.; Berdiaki, A.; Tzardi, M.; Tzanakakis, G.N.; Nikitovic, D. Insulin-like growth factor and epidermal growth factor signaling in breast cancer cell growth: Focus on endocrine resistant disease. Anal. Cell. Pathol. (Amst.) 2015, 2015, 975495. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L.; Nicholson, S.; Sainsbury, R.; Wright, C.; Farndon, J. Epidermal growth factor receptor and other oncogenes as prognostic markers. J. Natl. Cancer Inst. Monogr. 1992, 11, 181–187. [Google Scholar]
- Price, J.T.; Tiganis, T.; Agarwal, A.; Djakiew, D.; Thompson, E.W. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999, 59, 5475–5478. [Google Scholar]
- Witsch, E.; Sela, M.; Yarden, Y. Roles for growth factors in cancer progression. Physiology 2010, 25, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, C.; Liu, J.; Liu, J.; Li, C.; Xu, C.; Niu, Y. The importance of EGFR as a biomarker in molecular apocrine breast cancer. Hum. Pathol. 2018, 77, 1–10. [Google Scholar] [CrossRef]
- Ullrich, A.; Gray, A.; Tam, A.W.; Yang-Feng, T.; Tsubokawa, M.; Collins, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E.; et al. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Cheifetz, S.; Andres, J.L.; Massague, J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J. Biol. Chem. 1988, 263, 16984–16991. [Google Scholar] [PubMed]
- Busch, S.; Acar, A.; Magnusson, Y.; Gregersson, P.; Ryden, L.; Landberg, G. TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene 2015, 34, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hooper, A.T.; Zhong, Z.; Witte, L.; Bohlen, P.; Rafii, S.; Hicklin, D.J. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int. J. Cancer 2006, 119, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.; Shi, W.; Yang, L.; Zhang, Q.; Cui, J.; Fanf, Y.; Li, Y.; Ren, G.; Yang, S.; et al. The novel VEGF receptor 2 inhibitor YLL545 inhibits angiogenesis and growth in breast cancer. Oncotarget 2016, 7, 41067–41080. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, J.; Wang, Z.; Liu, J.; Ning, Z.; Hu, M. Isomangiferin, a novel potent vascular endothelial growth factor receptor 2 kinase inhibitor, suppresses breast cancer growth, metastasis and angiogenesis. J. Breast Cancer 2018, 21, 11–20. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [PubMed]
- Rubin, I.; Yarden, Y. The Basic Biology of HER2. Ann. Oncol. 2001, 12 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Kamaruzman, N.I.; Tiash, S.; Ashaie, M.; Chowdhury, E.H. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Red Brewer, M. EpCAM: Another surface-to-nucleus missile. Cancer Cell 2009, 15, 165–166. [Google Scholar] [CrossRef]
- Imrich, S.; Hachmeister, M.; Gires, O. EpCAM and its potential role in tumor-initiating cells. Cell Adhes. Migr. 2012, 6, 30–38. [Google Scholar] [CrossRef]
- Munz, M.; Baeuerle, P.A.; Gires, O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009, 69, 5627–5629. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Gao, J.; Sun, Y.; Liu, T.; Yan, Q.; Yang, X. The role of epithelial cell adhesion molecule N-glycosylation on apoptosis in breast cancer cells. Tumor Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Peiris, D.; Spector, A.; Lomax-Browne, H.; Azimi, T.; Ramesh, B.; Loizidou, M.; Welch, H.; Dwek, M. Cellular glycosylation affects Herceptin binding and sensitivity of breast cancer cells to doxorubicin and growth factors. Sci. Rep. 2017, 7, 43006. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Tagliabue, E.; Campiglio, M.; Pupa, S.M. Role of HER2 gene overexpression in breast carcinoma. J. Cell. Physiol. 2000, 182, 150–162. [Google Scholar] [CrossRef]
- Daly, R.J.; Binder, M.D.; Sutherland, R.L. Overexpression of the Grb2 gene in human breast cancer cell lines. Oncogene 1994, 9, 2723–2727. [Google Scholar]
- Zollo, M.; Andre, A.; Cossu, A.; Sini, M.C.; D’Angelo, A.; Marino, N.; Budroni, M.; Tanda, F.; Arrigoni, G.; Palmieri, G. Overexpression of h-prune in breast cancer is correlated with advanced disease status. Clin. Cancer Res. 2005, 11, 199–205. [Google Scholar]
- Hayashi, S.I.; Eguchi, H.; Tanimoto, K.; Yoshida, T.; Omoto, Y.; Inoue, A.; Yoshida, N.; Yamaguchi, Y. The expression and function of estrogen receptor alpha and beta in human breast cancer and its clinical application. Endocr. Relat. Cancer 2003, 10, 193–202. [Google Scholar] [CrossRef]
- Ryther, R.C.C.; Flynt, A.S.; Phillips, J.A.; Patton, J.G. siRNA therapeutics: Big potential from small RNAs. Gene Ther. 2004, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, D.; Toncheva, D. RNA Interference—Regulations and Application in Oncology. J. Cancer Mol. 2008, 4, 67–77. [Google Scholar]
- Shen, H.; Mittal, V.; Ferrari, M.; Chang, J. Delivery of gene silencing agents for breast cancer therapy. Breast Cancer Res. BCR 2013, 15, 205. [Google Scholar] [CrossRef]
- Bernstein, E.; Denli, A.M.; Hannon, G.J. The rest is silence. RNA 2001, 7, 1509–1521. [Google Scholar] [PubMed]
- Xu, C.-F.; Wang, J. Delivery systems for siRNA drug development in cancer therapy. Asian J. Pharm. Sci. 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Chowdhury, E.H. Strategies for tumor-directed delivery of siRNA. Expert Opin. Drug Deliv. 2011, 8, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzada, T.; Reid, G.; McKenzie, D. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 2018, 10, 69–86. [Google Scholar] [CrossRef]
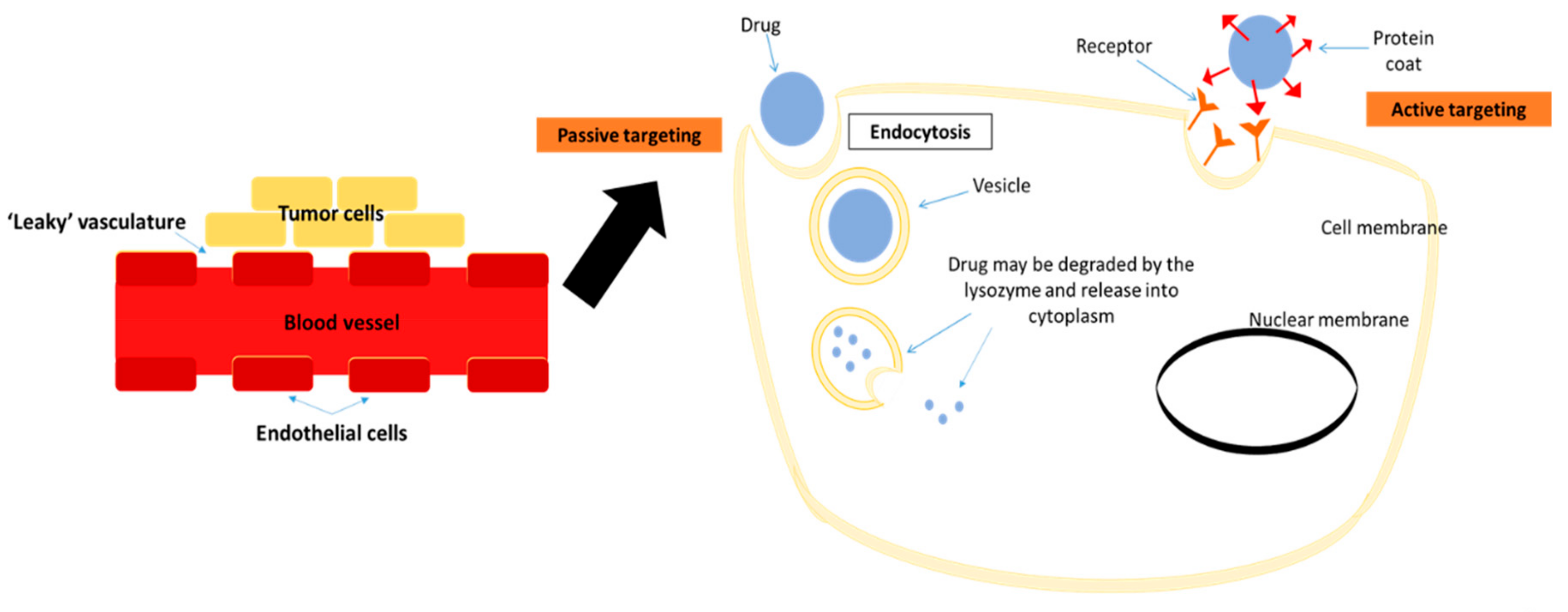
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Handb. Exp. Pharmacol. 2010, 3–53. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Upponi, J.R.; Torchilin, V.P. Passive vs. active targeting: An update of the EPR role in drug delivery to tumors. In Nano-Oncologicals: New Targeting and Delivery Approaches; Alonso, M.J., Garcia-Fuentes, M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 3–45. [Google Scholar]
- Huang, C.; Zhang, Y.; Yuan, H.; Gao, H.; Zhang, S. Role of nanoparticle geometry in endocytosis: Laying down to stand up. Nano Lett. 2013, 13, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Bora, R.S.; Gupta, D.; Mukkur, T.K.; Saini, K.S. RNA interference therapeutics for cancer: Challenges and opportunities (review). Mol. Med. Rep. 2012, 6, 9–15. [Google Scholar] [CrossRef]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef]
- Mohd Hafiz, M.R.; Zain, M.; Mohd Faiz, F.A.; Mohamed Saifulaman, M.S. Targeted RNAi of the Mitogen-activated Protein Kinase Pathway Genes in Acute Myeloid Leukemia Cells. Sains Malays. 2013, 42, 1131–1137. [Google Scholar]
- Bakhtiar, A.; Kamaruzman, N.I.; Othman, I.; Zain, A.; Chowdhury, E. Intracellular delivery of p53 gene and MAPK siRNA into breast cancer cells utilizing barium salt nanoparticles. J. Breast Cancer Res. Adv. 2017. [Google Scholar] [CrossRef]
- De Mello, L.J.; Souza, G.R.; Winter, E.; Silva, A.H.; Pittella, F.; Creczynski-Pasa, T.B. Knockdown of antiapoptotic genes in breast cancer cells by siRNA loaded into hybrid nanoparticles. Nanotechnology 2017, 28, 175101. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.; Liang, W. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef]
- Subramanian, N.; Kanwar, J.; Athalya, P.; Janakiraman, N.; Khetan, V.; Kanwar, R.; Eluchuri, S.; Krishnakumar, S. EpCAM aptamer mediated cancer cell specific delivery of EpCAM siRNA using polymeric nanocomplex. J. Biomed. Sci. 2015, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Morry, J.; Ngamcherdtrakul, W.; Gu, S.; Reda, M.; Castro, D.J.; Sangvanich, T.; Gray, J.W.; Yantasee, W. Targeted treatment of metastatic breast cancer by PLK1 siRNA delivered by an antioxidant nanoparticle platform. Mol. Cancer Ther. 2017, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, A.; Bondhopadhyay, B.; Mukherjee, M.; Bandyopadhyay, A.; Mukherjee, N.; Konar, K.; Bhattacharya, S.; Basu, A. Simultaneous knockdown of uPA and MMP9 can reduce breast cancer progression by increasing cell-cell adhesion and modulating EMT genes. Sci. Rep. 2016, 6, 21903. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Luo, Q.F.; Wei, C.K.; Li, D.F.; Fang, L. siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 92–100. [Google Scholar]
- Chua, M.; Tiash, S.; Fatemian, T.; Noordin, M.I.; Cheong, S.; Chowdhury, E.H. Carbonate apatite-facilitated intracellular delivery of c-ROS1 siRNA sensitizes MCF-7 breast cancer cells to cisplatin and paclitaxel. Cancer 2013, 1. [Google Scholar] [CrossRef]
- Qin, B.; Cheng, K. Silencing of the IKKε gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010, 12, R74. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, H.; Lin, S.Y.; Goss, J.A.; Brunicardi, F.C.; Li, K. siRNA-based targeting of cyclin E overexpression inhibits breast cancer cell growth and suppresses tumor development in breast cancer mouse model. PLoS ONE 2010, 5, e12860. [Google Scholar] [CrossRef]
- Gu, S.; Ngamcherdtrakul, W.; Reda, M.; Hu, Z.; Gray, J.W.; Yantasee, W. Lack of acquired resistance in HER2-positive breast cancer cells after long-term HER2 siRNA nanoparticle treatment. PLoS ONE 2018, 13, e0198141-e. [Google Scholar] [CrossRef]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef]
- Ku, S.H.; Jo, S.D.; Lee, Y.K.; Kim, K.; Kim, S.H. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 2016, 104, 16–28. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amin, A.R.; Wang, D.; Koenig, L.; Nannapaneni, S.; Chen, Z.; Wang, Z.; Sica, G.; Chen, Z.G.; Shin, D.M. RRM2 regulates Bcl-2 in head and neck and lung cancers: A potential target for cancer therapy. Clin. Cancer Res. 2013, 19, 3416–3428. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Aleku, M.; Schulz, P.; Keil, O.; Santel, A.; Schaeper, U.; Dieckhoff, B.; Janke, O.; Endruschat, J.; Durieux, B.; Röder, N.; et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008, 68, 9788–9798. [Google Scholar] [CrossRef]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Aleku, M.; Roder, N.; Mopert, K.; Durieux, B.; Janke, O.; Endruschat, J.; Dames, S.; Lange, C.; Eisermann, M.; et al. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin. Cancer Res. 2010, 16, 5469–5480. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; South, V.J.; Zhang, Y.; Davide, J.P.; Farrell, L.; Kohl, N.E.; Sepp-Lorenzino, L.; Lobell, R.B. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell 2005, 8, 49–59. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Lopez-Berestein, G.; Fu, S.; Tsimberidou, A.; Pant, S.; Piha-Paul, S.; Janku, F.; Hong, D.; Sulovic, S.; Meng, X.; et al. EphA2 gene targeting using neutral liposomal small interfering RNA (EPHARNA) delivery: A phase I clinical trial. J. Clin. Oncol. 2017, 35, TPS2604. [Google Scholar] [CrossRef]
- Triozzi, P.L. APN401 in Treating Patients with Recurrent or Metastatic Pancreatic Cancer, Colorectal Cancer, or Other Solid Tumors That Cannot Be Removed by Surgery; NCT03087591; NIH: Bethesda, MD, USA, 2017. [Google Scholar]
- Triozzi, P.; Kooshki, M.; Alistar, A.; Bitting, R.; Neal, A.; Lametschwandtner, G.; Loibner, H. Phase I clinical trial of adoptive cellular immunotherapy with APN401 in patients with solid tumors. J. Immunother. Cancer 2015, 3 (Suppl. 2), P175. [Google Scholar] [CrossRef]
- Li, Y.; Humphries, B.; Yang, C.; Wang, Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer-Challenges and Opportunities. Nanomaterials 2018, 8, 361. [Google Scholar] [CrossRef]
| Targeted Genes | Delivery Carrier | Cell Line | Animal Model | References |
|---|---|---|---|---|
| ER, BCL-2, ERBB2, and EGFR | Carbonate apatite | MCF-7, MDA-MB-231 | Balb/c | [82] |
| egfr1 and erbb2 | Carbonate apatite | MCF-7 | Balb/c | [114] |
| BCL-2 and BCL-XL | Calcium phosphate pEG-polyanion | MCF-7 | NA | [109] |
| MAPK | Barium salts nanoparticles | MCF-7 | Balb/c | [108] |
| VEGF | Polycation liposome-encapsulated calcium phosphate nanoparticles (PLCP) | MCF-7 | Balb/c | [110] |
| EpCAM | Polyethyleneimine | MCF-7 and WERI-Rb1 | NA | [111] |
| PLK1 | Mesoporous silica coated with PEI and PEG | Bt549 and MDA-MB-231 | SCID hairless SHO (Crl:SHO-Prkdc scid Hr hr) | [112] |
| uPA and MMP9 | Interferin transfection reagent | MDA-MB-231 | NA | [113] |
| CDK8 | Lipofectamine 2000 | MDA-MB-231 and MCF-7 | NA | [114] |
| c-ROS1 | Carbonate apatite | MCF-7 | NA | [115] |
| IKKε | Lipofectamine 2000 | SK-BR-3 and MCF-7 | NA | [116] |
| Cyclin E | Oligofectamine | SK-BR3, MDA-MB-157, MDA-MB-436, T47D, and MDA-MB-453 | Nude mice | [117] |
| HER2 | Mesoporous silica coated with cationic polymer and PEG | BT474 | NA | [118] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruzman, N.I.; Aziz, N.A.; Poh, C.L.; Chowdhury, E.H. Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer. Cancers 2019, 11, 632. https://doi.org/10.3390/cancers11050632
Kamaruzman NI, Aziz NA, Poh CL, Chowdhury EH. Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer. Cancers. 2019; 11(5):632. https://doi.org/10.3390/cancers11050632
Chicago/Turabian StyleKamaruzman, Nur Izyani, Noraini Abd Aziz, Chit Laa Poh, and Ezharul Hoque Chowdhury. 2019. "Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer" Cancers 11, no. 5: 632. https://doi.org/10.3390/cancers11050632
APA StyleKamaruzman, N. I., Aziz, N. A., Poh, C. L., & Chowdhury, E. H. (2019). Oncogenic Signaling in Tumorigenesis and Applications of siRNA Nanotherapeutics in Breast Cancer. Cancers, 11(5), 632. https://doi.org/10.3390/cancers11050632





