The Stem Cell Phenotype of Aggressive Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
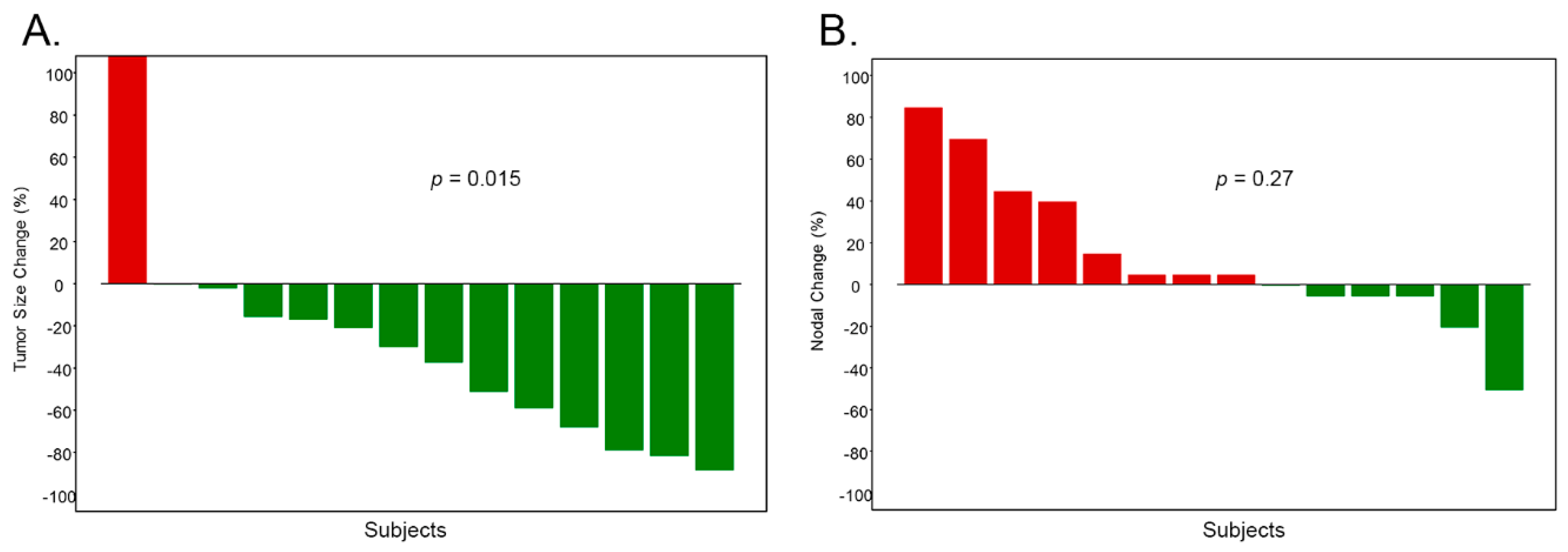
2.1. Nodal is Associated with Disease Progression
2.2. Nodal is Associated with Drug Resistance
3. Materials and Methods
3.1. Breast Cancer Patient Samples
3.2. Immunohistochemistry
3.3. Statistical Analyses and Clinical Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes in breast cancer. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Meijnen, P.; Peterse, J.L.; Antonini, N.; Rutgers, E.J.; van de Vijver, M.J. Immunohistochemical categorization of ductal carcinoma in situ of the breast. Br. J. Cancer 2008, 98, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Olenhuis, C.N.; Oosting, S.F.; Gietema, J.A.; de Vries, E.G. Prognostic versus predictive value of biomarkers in oncology. Eur. J. Cancer 2008, 44, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Santana-Davila, R.; Perez, E.A. Treatment options for patients with triple-negative breast cancer. J. Hematol. Oncol. 2010, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Margaryan, N.V.; Seftor, E.A.; Seftor, R.E.B.; Hendrix, M.J.C. Targeting the stem cell properties of adult breast cancer cells: Using combinatorial strategies to overcome drug resistance. Curr. Mol. Biol. Rep. 2017, 3, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kirschmann, D.A.; Seftor, E.A.; Nieva, D.R.; Mariano, E.A.; Hendrix, M.J.C. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res. Treat. 1999, 55, 127–136. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Kirschmann, D.A.; Seftor, R.E.B. Molecular biology of breast cancer metastasis. Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res. 2000, 2, 417–422. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Hess, A.R.; Seftor, R.E.B. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat. Rev. Cancer 2003, 3, 411–421. [Google Scholar] [CrossRef]
- Gong, W.; Baocun, S.; Zhao, X.; Zhang, D.; Sun, J.; Liu, T.; Gu, Q.; Dong, X.; Liu, F.; Wang, Y.; et al. Nodal signaling promotes vasculogenic mimicry formation in breast cancer via the Smad2/3 pathway. Oncotarget 2016, 7, 7012570167. [Google Scholar] [CrossRef]
- Bodenstine, T.M.; Chandler, G.S.; Reed, D.W.; Margaryan, N.V.; Gilgur, A.; Atkinson, J.; Ahmed, N.; Hyser, M.; Seftor, E.A.; Strizzi, L.; et al. Nodal expression in triple-negative breast cancer: Cellular effects of its inhibition following doxorubicin treatment. Cell Cycle 2016, 15, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodenstine, T.M.; Chandler, G.S.; Seftor, R.E.B.; Seftor, E.A.; Hendrix, M.J.C. Plasticity underlies tumor progression: Role of Nodal signaling. Cancer Metastasis Rev. 2016, 35, 21–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postovit, L.M.; Seftor, E.A.; Seftor, R.E.B.; Hendrix, M.J.C. Targeting Nodal in malignant melanoma cells. Expert Opin. Ther. Targets 2007, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, M.J.C.; Kandela, I.; Mazar, A.P.; Seftor, E.A.; Seftor, R.E.B.; Margaryan, N.V.; Strizzi, L.; Murphy, G.F.; Long, G.V.; Scoyler, R.A. Targeting melanoma with front-line therapy does not abrogate Nodal-expressing tumor cells. Lab. Investig. 2017, 97, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Sandomenico, A.; Margaryan, N.V.; Foca, A.; Sanguigno, L.; Bodenstine, T.M.; Chandler, G.S.; Reed, D.W.; Gilgur, A.; Seftor, E.A.; et al. Effects of a novel Nodal-targeting monoclonal antibody in melanoma. Oncotarget 2015, 6, 34071–34086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strizzi, L.; Hardy, K.M.; Margaryan, N.V.; Hillman, D.W.; Seftor, E.A.; Chen, B.; Geiger, X.J.; Thompson, E.A.; Lingle, W.L.; Andorfer, C.A.; et al. Potential for the embryonic morphogen Nodal as a prognostic and predictive biomarker in breast cancer. Breast Cancer Res. 2012, 14, R75. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.M.; Strizzi, L.; Margaryan, N.V.; Gupta, K.; Murphy, G.F.; Scolyer, R.A.; Hendrix, M.J.C. Targeting Nodal in conjunction with dacarbazine induces synergistic anticancer effects in metastatic melanoma. Mol. Cancer Res. 2015, 13, 670–680. [Google Scholar] [CrossRef]
- Kirsammer, G.; Strizzi, L.; Margaryan, N.V.; Gilgur, A.; Hyser, M.; Atkinson, J.; Kirschmann, D.A.; Seftor, E.A.; Hendrix, M.J.C. Nodal signaling promotes a tumorigenic phenotype in human breast cancer. Semin. Cancer Biol. 2014, 29, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Sun, B.; Sun, H.; Zhao, X.; Zhang, D.; Liu, T.; Zhao, N.; Gu, Q.; Dong, X.; Liu, F. Nodal signaling activates the Smad2/3 pathway to regulate stem cell-like properties in breast cancer cells. Am. J. Cancer Res. 2017, 7, 503–517. [Google Scholar]
- Hendrix, M.J.C.; Seftor, E.A.; Margaryan, N.V.; Seftor, R.E.B. Heterogeneity and Plasticity of Melanoma: Challenges of Current Therapies. Cutaneous Melanoma: Etiology and Therapy; Ward, H.W., Farma, J.M., Eds.; Codon Publications: Brisbane, Australia, 2017; Chapter 4. [Google Scholar]
- Gillet, J.P.; Efferth, T.; Remacle, J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim. Biophys. Acta 2007, 1775, 237–262. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Iancu, C.M.; Killian, P.H.; Kronski, E.; Mirisola, V.; Angelini, G.; Jochum, M.; Nerlich, A.G.; Pfeffer, U. Overexpression of the ATP binding cassette gene ABCA1 determines resistance to Curcumin in M14 melanoma cells. Mol. Cancer 2009, 8, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Fu, Z.; Shi, M.; Xia, K.; Ji, C.; Xu, P.; Lv, M.; Pan, B.; Dai, L.; Xie, H. Systematic analysis of gene expression pattern in has-miR-760 overexpressed resistance of MCF-7 human breast cancer cell to doxorubicin. Biomed. Pharmacother. 2015, 69, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Kang, Y.; Li, Y.; Zeng, Y.; Ding, G.; Shang, J. miR-33a expression sensitizes Lgr+ HCC-CSCs to doxorubicin via ABCA1. Neoplasia 2017, 641, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hedditch, E.L.; Gao, B.; Russell, A.J.; Lu, Y.; Emmanuel, C.; Beesley, J.; Johnatty, S.E.; Chen, X.; Harnett, P.; George, J.; et al. ABCA transporter gene expression and poor outcome in epithelial ovarian cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.; Cheng, S.; Wei, W.; Li, Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol. Med. Rep. 2015, 11, 525–632. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org (accessed on 4 December 2018).
- Zhao, Y.; Dong, Q.; Li, J.; Zhang, K.; Qin, J.; Zhao, J.; Sun, Q.; Want, Z.; Wartmann, T.; Jauch, K.W.; et al. Targeting cancer stem cells and their niche: Perspectives for future therapeutic targets and strategies. Semin. Cancer Biol. 2018, 53, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 2018, 20, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Ahrlund-Rickter, L.; Hendrix, M.J.C. Oncofetal signaling as a target for cancer therapy. Semin. Cancer Biol. 2014, 29, 1–2. [Google Scholar] [CrossRef]

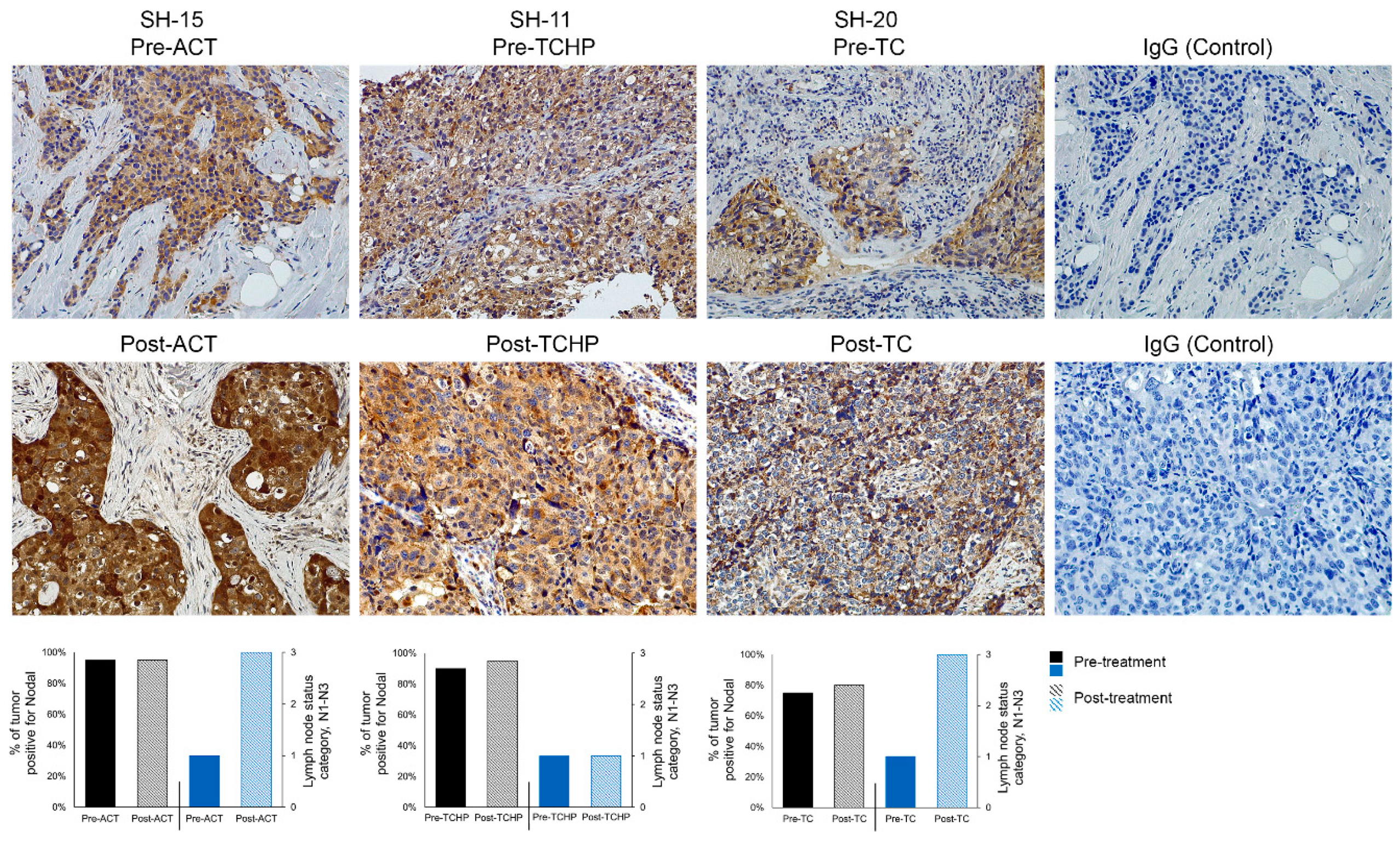
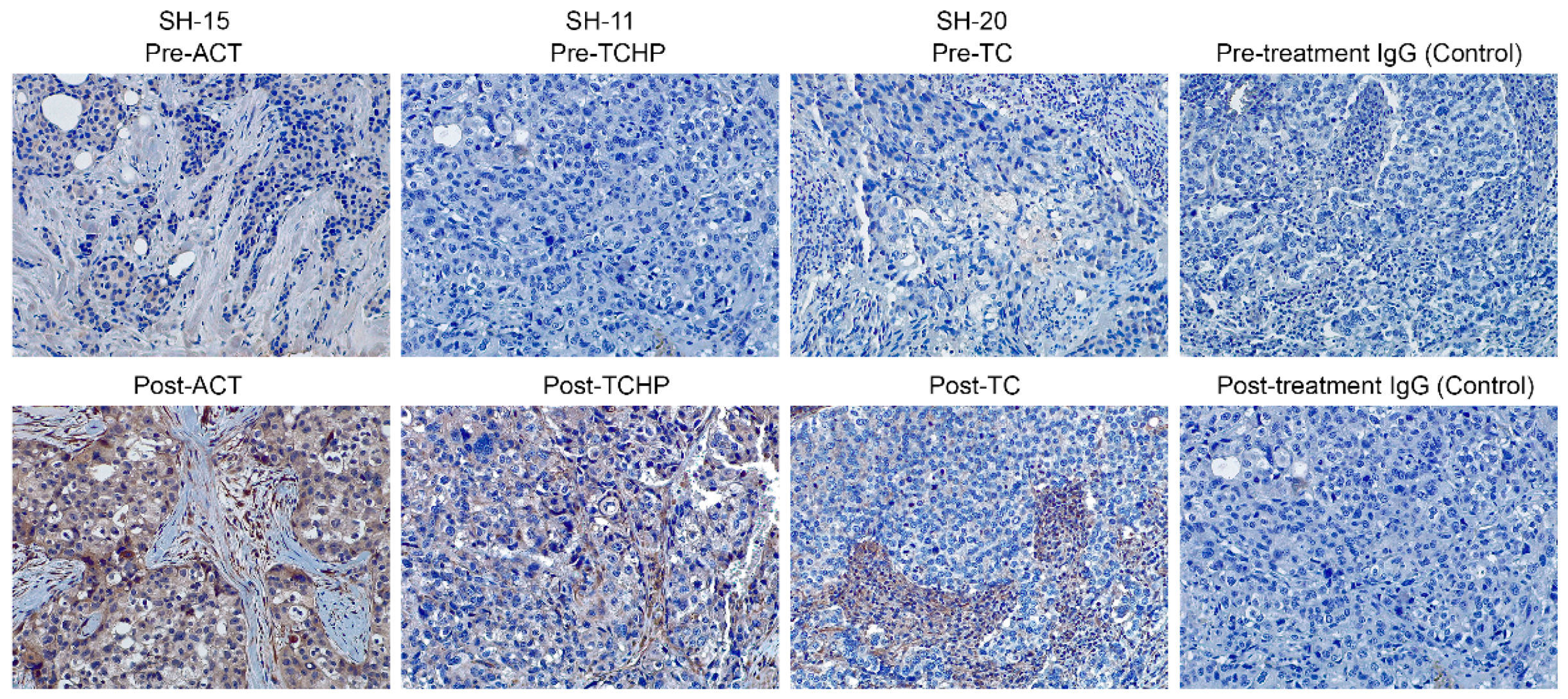
| Subject Number | Age at Diagnosis (years) | Histology | Grade | ER% | PR% | Her2/neu | Ki67 | Clinical Stage | Original Tumor Size | * Lymph Node Involvement Pre-Therapy | Pathologic Stage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SH-02 | 53 | Ductal | 3 | 0 | 0 | 1 | 56 | IIA | 2.8 | N0 | I |
| SH-04 | 69 | Ductal | 2 | 99 | 22 | 1 | 33 | IIIB | 4.9 | N1 | IIA |
| SH-05 | 67 | Ductal | 2 | 100 | 100 | 1 | 13 | IIIB | 5.1 | N1 | IIIB |
| SH-08 | 32 | Ductal | 3 | 0 | 0 | 1 | 86 | IIB | 5.1 | N0 | I |
| SH-09 | 56 | Ductal | 2 | 100 | 62 | 2 negatives FISH | 24 | IIB | 2.7 | N1 | IIA |
| SH-10 | 45 | Ductal | 2 | 99 | 96 | 1 | 31 | IIA | 1.8 | N1 | IIA |
| SH-11 | 58 | Ductal | 3 | 0 | 0 | 3 | 97 | IIB | 2.4 | N1 | IIB |
| SH-12 | 45 | Ductal | 3 | 95 | 88 | 2 positives FISH | 74 | IIA | 3.3 | N1 | I |
| SH-13 | 53 | Ductal | 3 | 0 | 0 | 1 | 90 | IIIA | 2.6 | N2 | IIA |
| SH-14 | 57 | Ductal | 2 | 100 | 100 | 1 | 43 | IIIC | 2.8 | N3 | IIB |
| SH-15 | 59 | Ductal | 2 | 100 | 34 | 1 | 25 | III | 3.5 | N1 | IIIC |
| SH-17 | 61 | Ductal | 3 | 5 | 0 | 1 | 89 | IIB | 3.4 | N1 | I |
| SH-19 | 59 | Ductal | 3 | 22 | 0 | 1 | 96 | IIB | 3.7 | N1 | IIA |
| SH-20 | 42 | Ductal | 3 | 5 | 3 | 1 | ND | IIB | 2.6 | N1 | IIIC |
| Subject Number | † %Nodal Expression in Tumor Pre-Treatment | † %Nodal Expression in Tumor Post-Treatment | † %ABCA1 Expression in Tumor Pre-Treatment | † %ABCA1 Expression in Tumor Post-Treatment | Post-Therapy Tumor Size | * Lymph Node Involvement after Therapy | Neo-Adjuvant Therapy (Drug Regiment) | Surgical Intervention | Distant Recurrence | Years Since Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| SH-02 | 25% | 95% | 30% | 80% | 0.9 | N0 | ACx4;Tx12 | Lump and SLN | No | 2 |
| SH-04 | 95% | 90% | 50% | 80% | 2.4 | N0 | ACx4;Tx10 | Mastectomy and ALND | No | 1 |
| SH-05 | 50% | 95% | 25% | 80% | 5 | N1 | TC x 4 | Mastectomy and ALND | No | 2 |
| SH-08 | 90% | 40% | 0% | 75% | 0.6 | N0 | ACx4;Tx12 | Mastectomy and SLN | No | 2 |
| SH-09 | 95% | 75% | 95% | 95% | 0.5 | N1 | ACx4;Tx12 | Mastectomy and ALND | No | 3 |
| SH-10 | 90% | 95% | 90% | 80% | 1.5 | N1 | ACx4;Tx12 | Central lumpectomy and SLN | No | 1 |
| SH-11 | 90% | 95% | 10% | 60% | 1.9 | N1 | TCHP | Lump and SLN | No | 1 |
| SH-12 | 95% | 90% | 10% | 80% | 0.7 | N0 | TCHP | Lump and SLN | No | 1 |
| SH-13 | 10% | 95% | 75% | 40% | 2.2 | N1 | ACx4;Tx12 | Lump and ALND | No | 1 |
| SH-14 | 75% | 90% | 20% | 20% | 2.8 | N1 | TCx4 | Mastectomy and ALND | No | 2 |
| SH-15 | 95% | 95% | 20% | 95% | 2.2 | N3 | ACx4;Tx12 | Mastectomy and ALND | No | 1 |
| SH-17 | 80% | 75% | 80% | 80% | 1.4 | N0 | ACx4;Tx12 | Lump and SLN | Yes | 2 |
| SH-19 | 50% | 90% | 10% | 90% | 2.6 | N0 | ACx4;Tx2 | Lump and ALND | No | 1 |
| SH-20 | 75% | 80% | 10% | 50% | 6.3 | N3 | TCx6 | Mastectomy and ALND | Yes | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margaryan, N.V.; Hazard-Jenkins, H.; Salkeni, M.A.; Smolkin, M.B.; Coad, J.A.; Wen, S.; Seftor, E.A.; Seftor, R.E.B.; Hendrix, M.J.C. The Stem Cell Phenotype of Aggressive Breast Cancer Cells. Cancers 2019, 11, 340. https://doi.org/10.3390/cancers11030340
Margaryan NV, Hazard-Jenkins H, Salkeni MA, Smolkin MB, Coad JA, Wen S, Seftor EA, Seftor REB, Hendrix MJC. The Stem Cell Phenotype of Aggressive Breast Cancer Cells. Cancers. 2019; 11(3):340. https://doi.org/10.3390/cancers11030340
Chicago/Turabian StyleMargaryan, Naira V., Hannah Hazard-Jenkins, Mohamad A. Salkeni, Matthew B. Smolkin, James A. Coad, Sijin Wen, Elisabeth A. Seftor, Richard E. B. Seftor, and Mary J. C. Hendrix. 2019. "The Stem Cell Phenotype of Aggressive Breast Cancer Cells" Cancers 11, no. 3: 340. https://doi.org/10.3390/cancers11030340
APA StyleMargaryan, N. V., Hazard-Jenkins, H., Salkeni, M. A., Smolkin, M. B., Coad, J. A., Wen, S., Seftor, E. A., Seftor, R. E. B., & Hendrix, M. J. C. (2019). The Stem Cell Phenotype of Aggressive Breast Cancer Cells. Cancers, 11(3), 340. https://doi.org/10.3390/cancers11030340






