Imaging of Preclinical Endometrial Cancer Models for Monitoring Tumor Progression and Response to Targeted Therapy
Abstract
1. Introduction
2. Literature Search
3. Imaging in Endometrial Cancer
3.1. MRI
3.2. PET
3.3. CT
3.4. SPECT
3.5. Optical Imaging
4. Emerging Novel Imaging Techniques Relevant for Preclinical EC Models
4.1. Radioligands for Visualization of Target-Specific Expression in EC
4.2. Oncologic PET Tracers Relevant for EC
4.3. Advanced MRI Sequences Relevant for EC
4.4. Advanced Image Analyses
5. Imaging-Related Challenges in Preclinical Endometrial Cancer Models
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Compliance with ethical standards
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yang, S.; Zhang, J.; Qian, L.; Chen, X. Overweight, obesity and endometrial cancer risk: Results from a systematic review and meta-analysis. Int. J. Biol. Markers 2014, 29, e21–e29. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O‘Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Cook, N.; Jodrell, D.I.; Tuveson, D.A. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today 2012, 17, 253–260. [Google Scholar] [CrossRef]
- Konings, G.F.; Saarinen, N.; Delvoux, B.; Kooreman, L.; Koskimies, P.; Krakstad, C.; Fasmer, K.E.; Haldorsen, I.S.; Zaffagnini, A.; Hakkinen, M.R.; et al. Development of an Image-Guided Orthotopic Xenograft Mouse Model of Endometrial Cancer with Controllable Estrogen Exposure. Int. J. Mol. Sci. 2018, 19, 2547. [Google Scholar] [CrossRef]
- Moiola, C.P.; Lopez-Gil, C.; Cabrera, S.; Garcia, A.; Van Nyen, T.; Annibali, D.; Fonnes, T.; Vidal, A.; Villanueva, A.; Matias-Guiu, X.; et al. Patient-Derived Xenograft Models for Endometrial Cancer Research. Int. J. Mol. Sci. 2018, 19, 2431. [Google Scholar] [CrossRef]
- Boone, J.D.; Dobbin, Z.C.; Straughn, J.M., Jr.; Buchsbaum, D.J. Ovarian and cervical cancer patient derived xenografts: The past, present, and future. Gynecol. Oncol. 2015, 138, 486–491. [Google Scholar] [CrossRef]
- Bashir, U.; Weeks, A.; Goda, J.S.; Siddique, M.; Goh, V.; Cook, G.J. Measurement of 18F-FDG PET tumor heterogeneity improves early assessment of response to bevacizumab compared with the standard size and uptake metrics in a colorectal cancer model. Nucl. Med. Commun. 2019, 40, 611–617. [Google Scholar] [CrossRef]
- De Jong, M.; Essers, J.; van Weerden, W.M. Imaging preclinical tumour models: Improving translational power. Nat. Rev. Cancer 2014, 14, 481–493. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Popa, M.; Fonnes, T.; Brekke, N.; Kopperud, R.; Visser, N.C.; Rygh, C.B.; Pavlin, T.; Salvesen, H.B.; McCormack, E.; et al. Multimodal Imaging of Orthotopic Mouse Model of Endometrial Carcinoma. PLoS ONE 2015, 10, e0135220. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Reijnen, C.; IntHout, J.; Massuger, L.; Strobbe, F.; Kusters-Vandevelde, H.V.N.; Haldorsen, I.S.; Snijders, M.; Pijnenborg, J.M.A. Diagnostic Accuracy of Clinical Biomarkers for Preoperative Prediction of Lymph Node Metastasis in Endometrial Carcinoma: A Systematic Review and Meta-Analysis. Oncologist 2019, 24, e880–e890. [Google Scholar] [CrossRef]
- Nougaret, S.; Horta, M.; Sala, E.; Lakhman, Y.; Thomassin-Naggara, I.; Kido, A.; Masselli, G.; Bharwani, N.; Sadowski, E.; Ertmer, A.; et al. Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2019, 29, 792–805. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Salvesen, H.B. What Is the Best Preoperative Imaging for Endometrial Cancer? Curr. Oncol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef]
- Rossi, E.C.; Jackson, A.; Ivanova, A.; Boggess, J.F. Detection of sentinel nodes for endometrial cancer with robotic assisted fluorescence imaging: Cervical versus hysteroscopic injection. Int. J. Gynecol. Cancer 2013, 23, 1704–1711. [Google Scholar] [CrossRef]
- Laios, A.; Volpi, D.; Tullis, I.D.; Woodward, M.; Kennedy, S.; Pathiraja, P.N.; Haldar, K.; Vojnovic, B.; Ahmed, A.A. A prospective pilot study of detection of sentinel lymph nodes in gynaecological cancers using a novel near infrared fluorescence imaging system. BMC Res. Notes 2015, 8, 608. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Fujiwara, S.; Tanaka, Y.; Sasaki, H.; Tsunetoh, S.; Yamamoto, K.; Yamada, T.; Ohmichi, M. The detection of sentinel lymph nodes in laparoscopic surgery can eliminate systemic lymphadenectomy for patients with early stage endometrial cancer. Int. J. Clin. Oncol. 2018, 23, 305–313. [Google Scholar] [CrossRef]
- Bedyńska, M.; Szewczyk, G.; Klepacka, T.; Sachadel, K.; Maciejewski, T.; Szukiewicz, D.; Fijałkowska, A. Sentinel lymph node mapping using indocyanine green in patients with uterine and cervical neoplasms: Restrictions of the method. Arch. Gynecol. Obstet. 2019, 299, 1373–1384. [Google Scholar] [CrossRef]
- Galban, C.J.; Hoff, B.A.; Chenevert, T.L.; Ross, B.D. Diffusion MRI in early cancer therapeutic response assessment. NMR Biomed. 2017, 30. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Gruner, R.; Husby, J.A.; Magnussen, I.J.; Werner, H.M.; Salvesen, O.O.; Bjorge, L.; Stefansson, I.; Akslen, L.A.; Trovik, J.; et al. Dynamic contrast-enhanced MRI in endometrial carcinoma identifies patients at increased risk of recurrence. Eur. Radiol. 2013, 23, 2916–2925. [Google Scholar] [CrossRef]
- Fasmer, K.E.; Bjornerud, A.; Ytre-Hauge, S.; Gruner, R.; Tangen, I.L.; Werner, H.M.; Bjorge, L.; Salvesen, O.O.; Trovik, J.; Krakstad, C.; et al. Preoperative quantitative dynamic contrast-enhanced MRI and diffusion-weighted imaging predict aggressive disease in endometrial cancer. Acta Radiol. 2018, 59, 1010–1017. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Z.; Zhou, S.F.; Lu, N. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Des. Dev. Ther. 2014, 8, 1745–1751. [Google Scholar] [CrossRef]
- Bian, X.; Gao, J.; Luo, F.; Rui, C.; Zheng, T.; Wang, D.; Wang, Y.; Roberts, T.M.; Liu, P.; Zhao, J.J.; et al. PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy. Oncogene 2018, 37, 341–351. [Google Scholar] [CrossRef]
- Contreras, C.M.; Akbay, E.A.; Gallardo, T.D.; Haynie, J.M.; Sharma, S.; Tagao, O.; Bardeesy, N.; Takahashi, M.; Settleman, J.; Wong, K.K.; et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis. Models Mech. 2010, 3, 181–193. [Google Scholar] [CrossRef]
- Husby, J.A.; Reitan, B.C.; Biermann, M.; Trovik, J.; Bjorge, L.; Magnussen, I.J.; Salvesen, O.O.; Salvesen, H.B.; Haldorsen, I.S. Metabolic Tumor Volume on 18F-FDG PET/CT Improves Preoperative Identification of High-Risk Endometrial Carcinoma Patients. J. Nucl. Med. 2015, 56, 1191–1198. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.; Li, B.; Song, Z.; Chen, P.; Ouyang, L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. J. Cancer 2019, 10, 3303–3314. [Google Scholar] [CrossRef]
- Monsivais, D.; Peng, J.; Kang, Y.; Matzuk, M.M. Activin-like kinase 5 (ALK5) inactivation in the mouse uterus results in metastatic endometrial carcinoma. Proc. Natl. Acad. Sci. USA 2019, 116, 3883–3892. [Google Scholar] [CrossRef]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Jeffrey Lowery, W.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wang, J.; Avanzato, V.A.; Bakkum-Gamez, J.N.; Russell, S.J.; Bell, J.C.; Peng, K.W. Oncolytic vaccinia virotherapy for endometrial cancer. Gynecol. Oncol. 2014, 132, 722–729. [Google Scholar] [CrossRef]
- Kocher, B.; Piwnica-Worms, D. Illuminating cancer systems with genetically engineered mouse models and coupled luciferase reporters in vivo. Cancer Discov. 2013, 3, 616–629. [Google Scholar] [CrossRef]
- Wang, Y.; Tseng, J.C.; Sun, Y.; Beck, A.H.; Kung, A.L. Noninvasive imaging of tumor burden and molecular pathways in mouse models of cancer. Cold. Spring Harb. Protoc. 2015, 2015, 135–144. [Google Scholar] [CrossRef][Green Version]
- Cabrera, S.; Llaurado, M.; Castellvi, J.; Fernandez, Y.; Alameda, F.; Colas, E.; Ruiz, A.; Doll, A.; Schwartz, S., Jr.; Carreras, R.; et al. Generation and characterization of orthotopic murine models for endometrial cancer. Clin. Exp. Metastasis 2012, 29, 217–227. [Google Scholar] [CrossRef]
- Yeramian, A.; Garcia, V.; Bergada, L.; Domingo, M.; Santacana, M.; Valls, J.; Martinez-Alonso, M.; Carceller, J.A.; Cussac, A.L.; Dolcet, X.; et al. Bioluminescence Imaging to Monitor the Effects of the Hsp90 Inhibitor NVP-AUY922 on NF-kappaB Pathway in Endometrial Cancer. Mol. Imaging Biol. 2016, 18, 545–556. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Gao, L.; Gao, X.; Li, N.; Song, Y.; Huang, X.; Lin, S.; Wang, X. Comparative Study of the Effects of Ferrochelatase-siRNA Transfection Mediated by Ultrasound Microbubbles and Polyethyleneimine in Combination with Low-dose ALA to Enhance PpIX Accumulation in Human Endometrial Cancer Xenograft Nude Mice Models. Photochem. Photobiol. 2019, 95, 1045–1051. [Google Scholar] [CrossRef]
- Fong, P.; Meng, L.R. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med. Sci. Monit. Basic Res. 2014, 20, 146–152. [Google Scholar] [CrossRef]
- Philp, L.; Chan, H.; Rouzbahman, M.; Overchuk, M.; Chen, J.; Zheng, G.; Bernardini, M.Q. Use of Porphysomes to detect primary tumour, lymph node metastases, intra-abdominal metastases and as a tool for image-guided lymphadenectomy: Proof of concept in endometrial cancer. Theranostics 2019, 9, 2727–2738. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, P.; Zhang, F.; Xu, E.; Symonds, L.; Ohlson, C.E.; Bronson, R.T.; Maira, S.M.; Di Tomaso, E.; Li, J.; et al. A genetic mouse model of invasive endometrial cancer driven by concurrent loss of Pten and Lkb1 Is highly responsive to mTOR inhibition. Cancer Res. 2014, 74, 15–23. [Google Scholar] [CrossRef]
- Halle, M.K.; Tangen, I.L.; Berg, H.F.; Hoivik, E.A.; Mauland, K.K.; Kusonmano, K.; Berg, A.; Hurtado, A.; Kalland, K.H.; Oyan, A.M.; et al. HER2 expression patterns in paired primary and metastatic endometrial cancer lesions. Br. J. Cancer 2018, 118, 378–387. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O‘Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using (89)Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Massicano, A.V.F.; Lee, S.; Crenshaw, B.K.; Aweda, T.A.; El Sayed, R.; Super, I.; Bose, R.; Marquez-Nostra, B.V.; Lapi, S.E. Imaging of HER2 with [(89)Zr]pertuzumab in Response to T-DM1 Therapy. Cancer Biother. Radiopharm. 2019, 34, 209–217. [Google Scholar] [CrossRef]
- Jiang, D.; Im, H.J.; Sun, H.; Valdovinos, H.F.; England, C.G.; Ehlerding, E.B.; Nickles, R.J.; Lee, D.S.; Cho, S.Y.; Huang, P.; et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1296–1305. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, L.; Zhang, S. Preoperative serum CA125: A useful marker for surgical management of endometrial cancer. BMC Cancer 2015, 15, 396. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sevak, K.K.; Monette, S.; Carlin, S.D.; Knight, J.C.; Wuest, F.R.; Sala, E.; Zeglis, B.M.; Lewis, J.S. Preclinical 89Zr Immuno-PET of High-Grade Serous Ovarian Cancer and Lymph Node Metastasis. J. Nucl. Med. 2016, 57, 771–776. [Google Scholar] [CrossRef]
- Wadehra, M.; Natarajan, S.; Seligson, D.B.; Williams, C.J.; Hummer, A.J.; Hedvat, C.; Braun, J.; Soslow, R.A. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer 2006, 107, 90–98. [Google Scholar] [CrossRef]
- Habeeb, O.; Goodglick, L.; Soslow, R.A.; Rao, R.G.; Gordon, L.K.; Schirripa, O.; Horvath, S.; Braun, J.; Seligson, D.B.; Wadehra, M. Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer 2010, 116, 4718–4726. [Google Scholar] [CrossRef]
- Fu, M.; Brewer, S.; Olafsen, T.; Wu, A.M.; Gordon, L.K.; Said, J.; Braun, J.; Wadehra, M. Positron emission tomography imaging of endometrial cancer using engineered anti-EMP2 antibody fragments. Mol. Imaging Biol. 2013, 15, 68–78. [Google Scholar] [CrossRef]
- Smith, H.O.; Leslie, K.K.; Singh, M.; Qualls, C.R.; Revankar, C.M.; Joste, N.E.; Prossnitz, E.R. GPR30: A novel indicator of poor survival for endometrial carcinoma. Am. J. Obstet. Gynecol. 2007, 196, 386.e1–386.e11. [Google Scholar] [CrossRef]
- Krakstad, C.; Trovik, J.; Wik, E.; Engelsen, I.B.; Werner, H.M.; Birkeland, E.; Raeder, M.B.; Oyan, A.M.; Stefansson, I.M.; Kalland, K.H.; et al. Loss of GPER identifies new targets for therapy among a subgroup of ERalpha-positive endometrial cancer patients with poor outcome. Br. J. Cancer 2012, 106, 1682–1688. [Google Scholar] [CrossRef]
- Nayak, T.K.; Ramesh, C.; Hathaway, H.J.; Norenberg, J.P.; Arterburn, J.B.; Prossnitz, E.R. GPER-targeted, 99mTc-labeled, nonsteroidal ligands demonstrate selective tumor imaging and in vivo estrogen binding. Mol. Cancer Res. 2014, 12, 1635–1643. [Google Scholar] [CrossRef]
- Aide, N.; Kinross, K.; Cullinane, C.; Roselt, P.; Waldeck, K.; Neels, O.; Dorow, D.; McArthur, G.; Hicks, R.J. 18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model. J. Nucl. Med. 2010, 51, 1559–1564. [Google Scholar] [CrossRef]
- Jensen, M.M.; Erichsen, K.D.; Johnbeck, C.B.; Bjorkling, F.; Madsen, J.; Jensen, P.B.; Sehested, M.; Hojgaard, L.; Kjaer, A. [18F]FDG and [18F]FLT positron emission tomography imaging following treatment with belinostat in human ovary cancer xenografts in mice. BMC Cancer 2013, 13, 168. [Google Scholar] [CrossRef]
- Munk Jensen, M.; Erichsen, K.D.; Bjorkling, F.; Madsen, J.; Jensen, P.B.; Sehested, M.; Hojgaard, L.; Kjaer, A. Imaging of treatment response to the combination of carboplatin and paclitaxel in human ovarian cancer xenograft tumors in mice using FDG and FLT PET. PLoS ONE 2013, 8, e85126. [Google Scholar] [CrossRef]
- Trencsenyi, G.; Marian, T.; Lajtos, I.; Krasznai, Z.; Balkay, L.; Emri, M.; Mikecz, P.; Goda, K.; Szaloki, G.; Juhasz, I.; et al. 18FDG, [18F]FLT, [18F]FAZA, and 11C-methionine are suitable tracers for the diagnosis and in vivo follow-up of the efficacy of chemotherapy by miniPET in both multidrug resistant and sensitive human gynecologic tumor xenografts. Biomed Res. Int. 2014, 2014, 787365. [Google Scholar] [CrossRef]
- Perumal, M.; Stronach, E.A.; Gabra, H.; Aboagye, E.O. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose- and 3‘-deoxy-3‘-[18F]fluorothymidine-positron emission tomography as biomarkers of therapy response in platinum-resistant ovarian cancer. Mol. Imaging Biol. 2012, 14, 753–761. [Google Scholar] [CrossRef]
- Shah, C.; Miller, T.W.; Wyatt, S.K.; McKinley, E.T.; Olivares, M.G.; Sanchez, V.; Nolting, D.D.; Buck, J.R.; Zhao, P.; Ansari, M.S.; et al. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin. Cancer Res. 2009, 15, 4712–4721. [Google Scholar] [CrossRef]
- Elmi, A.; Makvandi, M.; Weng, C.C.; Hou, C.; Clark, A.S.; Mach, R.H.; Mankoff, D.A. Cell-Proliferation Imaging for Monitoring Response to CDK4/6 Inhibition Combined with Endocrine-Therapy in Breast Cancer: Comparison of [(18)F]FLT and [(18)F]ISO-1 PET/CT. Clin. Cancer Res. 2019, 25, 3063–3073. [Google Scholar] [CrossRef]
- Salvesen, H.B.; Haldorsen, I.S.; Trovik, J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012, 13, e353–e361. [Google Scholar] [CrossRef]
- Trovik, J.; Wik, E.; Werner, H.M.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Yoshida, Y.; Kiyono, Y.; Kurokawa, T.; Kudo, T.; Fujibayashi, Y.; Kotsuji, F.; Okazawa, H. Functional oestrogen receptor alpha imaging in endometrial carcinoma using 16alpha-[(1)(8)F]fluoro-17beta-oestradiol PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 37–45. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Yoshida, Y.; Kudo, T.; Kiyono, Y.; Kurokawa, T.; Kobayashi, M.; Tsuchida, T.; Fujibayashi, Y.; Kotsuji, F.; Okazawa, H. Functional images reflect aggressiveness of endometrial carcinoma: Estrogen receptor expression combined with 18F-FDG PET. J. Nucl. Med. 2009, 50, 1598–1604. [Google Scholar] [CrossRef]
- Heidari, P.; Deng, F.; Esfahani, S.A.; Leece, A.K.; Shoup, T.M.; Vasdev, N.; Mahmood, U. Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin. Cancer Res. 2015, 21, 1340–1347. [Google Scholar] [CrossRef]
- He, S.; Wang, M.; Yang, Z.; Zhang, J.; Zhang, Y.; Luo, J.; Zhang, Y. Comparison of 18F-FES, 18F-FDG, and 18F-FMISO PET Imaging Probes for Early Prediction and Monitoring of Response to Endocrine Therapy in a Mouse Xenograft Model of ER-Positive Breast Cancer. PLoS ONE 2016, 11, e0159916. [Google Scholar] [CrossRef]
- Berg, A.; Fasmer, K.E.; Mauland, K.K.; Ytre-Hauge, S.; Hoivik, E.A.; Husby, J.A.; Tangen, I.L.; Trovik, J.; Halle, M.K.; Woie, K.; et al. Tissue and imaging biomarkers for hypoxia predict poor outcome in endometrial cancer. Oncotarget 2016, 7, 69844–69856. [Google Scholar] [CrossRef]
- Han, K.; Shek, T.; Vines, D.; Driscoll, B.; Fyles, A.; Jaffray, D.; Keller, H.; Metser, U.; Pintilie, M.; Xie, J.; et al. Measurement of Tumor Hypoxia in Patients With Locally Advanced Cervical Cancer Using Positron Emission Tomography with (18)F-Fluoroazomyin Arabinoside. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1202–1209. [Google Scholar] [CrossRef]
- Pinker, K.; Andrzejewski, P.; Baltzer, P.; Polanec, S.H.; Sturdza, A.; Georg, D.; Helbich, T.H.; Karanikas, G.; Grimm, C.; Polterauer, S.; et al. Multiparametric [18F]Fluorodeoxyglucose/ [18F]Fluoromisonidazole Positron Emission Tomography/ Magnetic Resonance Imaging of Locally Advanced Cervical Cancer for the Non-Invasive Detection of Tumor Heterogeneity: A Pilot Study. PLoS ONE 2016, 11, e0155333. [Google Scholar] [CrossRef]
- Busk, M.; Mortensen, L.S.; Nordsmark, M.; Overgaard, J.; Jakobsen, S.; Hansen, K.V.; Theil, J.; Kallehauge, J.F.; D‘Andrea, F.P.; Steiniche, T.; et al. PET hypoxia imaging with FAZA: Reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 186–197. [Google Scholar] [CrossRef]
- Busk, M.; Munk, O.L.; Jakobsen, S.; Wang, T.; Skals, M.; Steiniche, T.; Horsman, M.R.; Overgaard, J. Assessing hypoxia in animal tumor models based on pharmocokinetic analysis of dynamic FAZA PET. Acta Oncol. 2010, 49, 922–933. [Google Scholar] [CrossRef]
- Wang, H.; Galban, S.; Wu, R.; Bowman, B.M.; Witte, A.; Vetter, K.; Galban, C.J.; Ross, B.D.; Cho, K.R.; Rehemtulla, A. Molecular imaging reveals a role for AKT in resistance to cisplatin for ovarian endometrioid adenocarcinoma. Clin. Cancer Res. 2013, 19, 158–169. [Google Scholar] [CrossRef]
- Cebulla, J.; Huuse, E.M.; Pettersen, K.; van der Veen, A.; Kim, E.; Andersen, S.; Prestvik, W.S.; Bofin, A.M.; Pathak, A.P.; Bjorkoy, G.; et al. MRI reveals the in vivo cellular and vascular response to BEZ235 in ovarian cancer xenografts with different PI3-kinase pathway activity. Br. J. Cancer 2015, 112, 504–513. [Google Scholar] [CrossRef][Green Version]
- Ellingsen, C.; Hompland, T.; Galappathi, K.; Mathiesen, B.; Rofstad, E.K. DCE-MRI of the hypoxic fraction, radioresponsiveness, and metastatic propensity of cervical carcinoma xenografts. Radiother. Oncol. 2014, 110, 335–341. [Google Scholar] [CrossRef]
- Ellingsen, C.; Walenta, S.; Hompland, T.; Mueller-Klieser, W.; Rofstad, E.K. The Microenvironment of Cervical Carcinoma Xenografts: Associations with Lymph Node Metastasis and Its Assessment by DCE-MRI. Transl. Oncol. 2013, 6, 607–617. [Google Scholar] [CrossRef]
- Ovrebo, K.M.; Ellingsen, C.; Hompland, T.; Rofstad, E.K. Dynamic contrast-enhanced magnetic resonance imaging of the metastatic potential of tumors: A preclinical study of cervical carcinoma and melanoma xenografts. Acta Oncol. 2013, 52, 604–611. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. 1), 11S–20S. [Google Scholar] [CrossRef]
- Veronese, M.; Rizzo, G.; Aboagye, E.O.; Bertoldo, A. Parametric imaging of (1)(8)F-fluoro-3-deoxy-3-L-fluorothymidine PET data to investigate tumour heterogeneity. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1781–1792. [Google Scholar] [CrossRef]
- Kristian, A.; Holtedahl, J.E.; Torheim, T.; Futsaether, C.; Hernes, E.; Engebraaten, O.; Maelandsmo, G.M.; Malinen, E. Dynamic 2-Deoxy-2-[(18)F]Fluoro-D-Glucose Positron Emission Tomography for Chemotherapy Response Monitoring of Breast Cancer Xenografts. Mol. Imaging Biol. 2017, 19, 271–279. [Google Scholar] [CrossRef]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular imaging of hypoxia. J. Nucl. Med. 2008, 49 (Suppl. 2), 129S–148S. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Convert, L.; Lebel, R.; Gascon, S.; Fontaine, R.; Pratte, J.F.; Charette, P.; Aimez, V.; Lecomte, R. Real-Time Microfluidic Blood-Counting System for PET and SPECT Preclinical Pharmacokinetic Studies. J. Nucl. Med. 2016, 57, 1460–1466. [Google Scholar] [CrossRef]
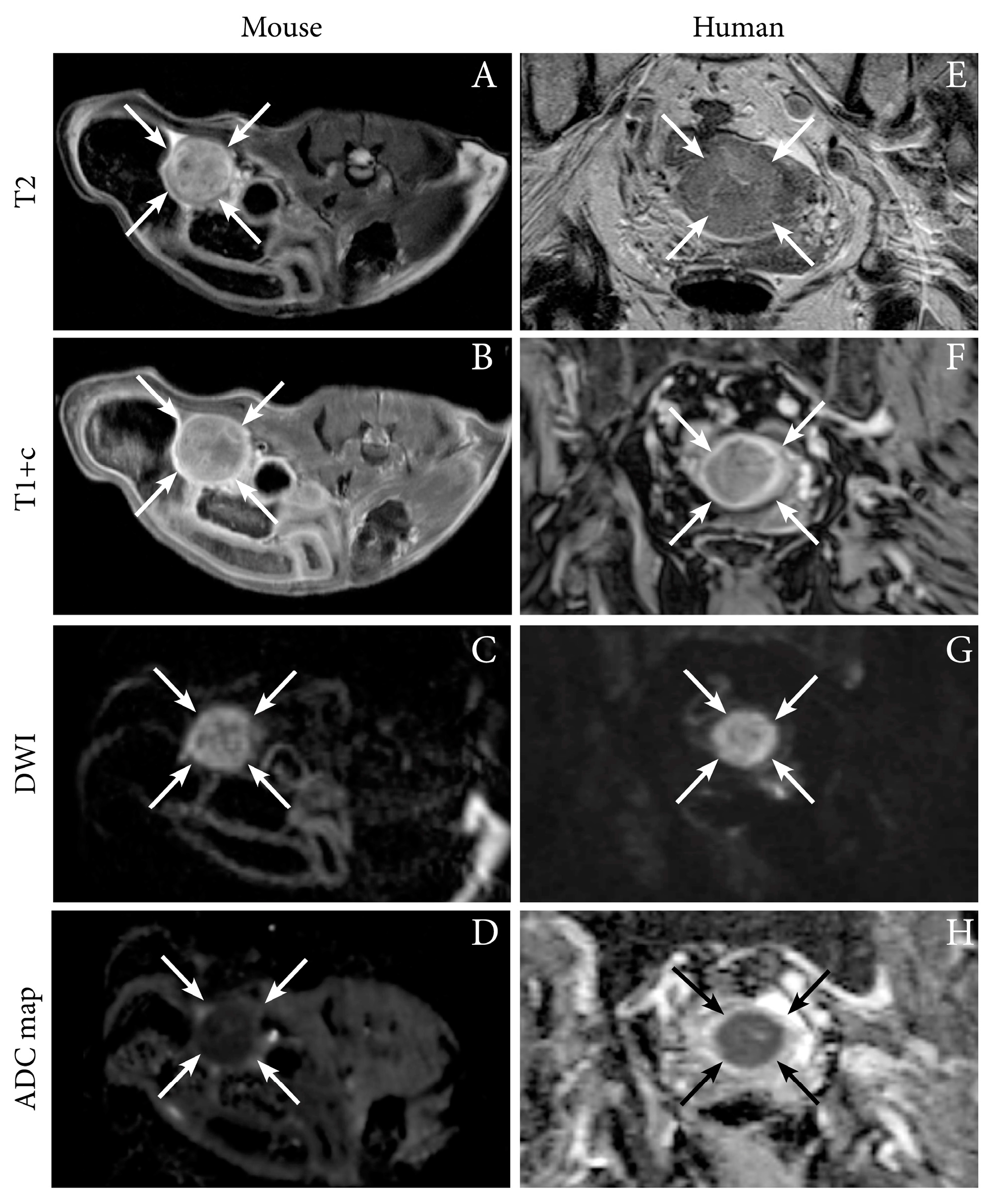
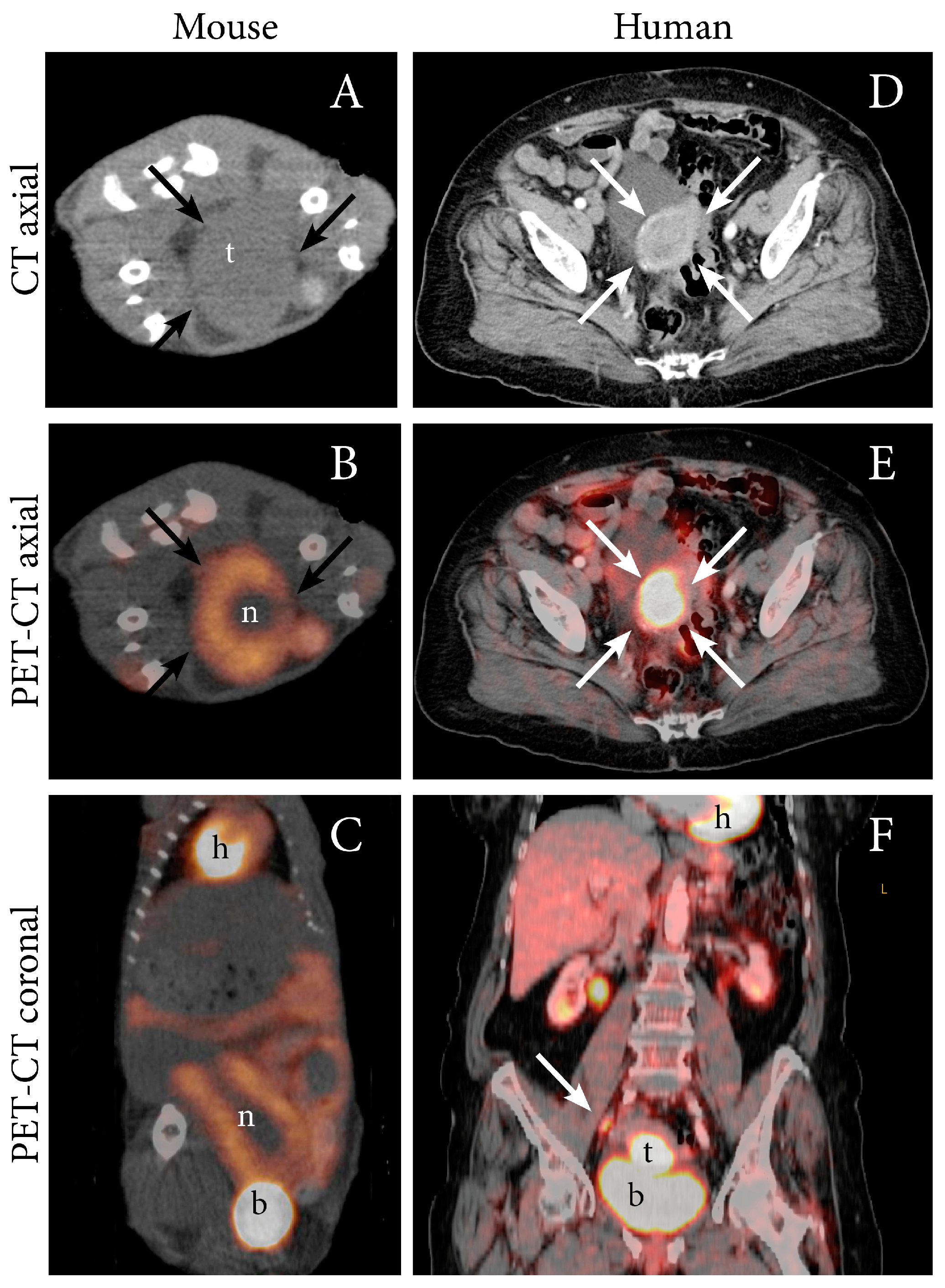
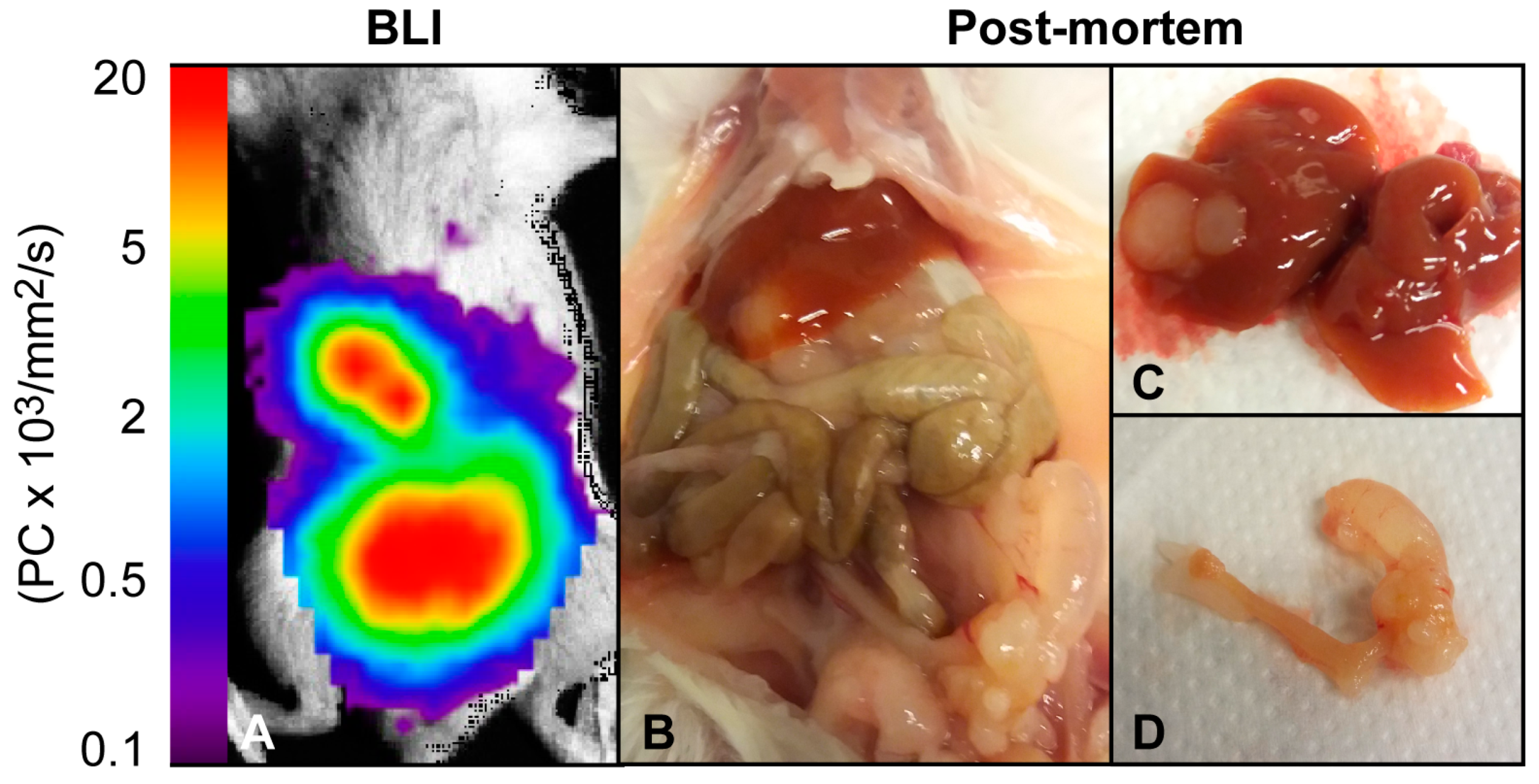
| Imaging Modality/ Sequence | Study Purpose | Imaging Characteristics | Animal Model | Ref |
|---|---|---|---|---|
| MRI T1, T1+C, T2, DW, ADC | Present preclinical imaging findings using multiple imaging techniques | Tumor can be delineated using anatomic sequences and exhibits restricted diffusion with low ADC-values | Ishikawa cells, orthotopic, NSG mice | [12] |
| T2 | Explore therapeutic effect of combined PI3K (BKM120) and PARP-inhibitor (Olaparib) treatment | Tumor volume decreased after combined treatment (synergistic effect) | Genetic mouse model (PTEN/Lkb1- deficient [40]) | [25] |
| T2 | Present a novel genetic mouse model | Tumor volume dependent on rapamycin treatment (mTOR inhibitor). | Genetic mouse model (Lkb1-deficient) | [26] |
| CT (CE-CT) | Present an estrogen-controllable mouse model with image-guided monitoring of tumor-growth | Correlation between CT-assessed tumor volume and tumor weight at necroscopy | Ishikawa cells, orthotopic, estrogen-controllable, athymic nude mice | [7] |
| CT | Present a novel genetic mouse model | Detection of lung metastases and regression of metastases post-therapy (ovariectomy) | Genetic mouse model, hormone dependent (Alk5-deficient) | [30] |
| PET FDG | Present preclinical imaging findings using multiple techniques | Growth of primary tumor and metastases can be detected Total lesion glycolysis was calculated (SUVmean x MTV) | Ishikawa cells and PDX-model, orthotopic, NSG mice | [12] |
| FDG | Explore effect of PI3K-inhibtor (ellagic acid) | Decreased SUVmax in metastases (lungs) after treatment | Cell lines KLE and AN3CA injected iv. in BALB/C nude mice | [29] |
| SPECT (NIS reporter gene) | Explore effect of oncolytic therapy | Tumor volume decreased after therapy | Cell lines AN3CA and ARK-2, subcutaneous athymic mice | [32] |
| Optical BLI | Generation and characterization of a mouse model | BLI signal increases over time. Metastatic growth detected | Hec1A cells, orthotopic, athymic nude mice | [35] |
| Present preclinical imaging findings using multiple techniques | BLI signal increases over time. Metastatic growth detected | Ishikawa cells, orthotopic, NSG mice | [12] | |
| Present an estrogen-controllable mouse model with image-guided monitoring of tumor-growth | BLI signal increases in estrogen-treated mice | Ishikawa cells, orthotopic, athymic nude mice | [7] | |
| Evaluate effect of Hsp90-inhibtor (NVP-AUY922) | NVP-AUY922 treatment reduces activity in the NF-κB pathway detected by BLI signal | Ishikawa cells, subcutaneous, (unknown mice strain | [36] | |
| FLI | Optimization of fluorescent signal | Low dose ALA permits detection of tumor by FLI following knockdown of FECH by ultrasound microbubbles and polyethyleneimine | Hec1a cells, subcutaneous, BALB/c- nude mice | [37] |
| Investigate mTOR treatment in tumors of different PTEN-status (+/-) | Decreased GFP signal in PTEN- compared to PTEN+ for rapamycin-treated tumors | Hec1a (PTEN+) and Ishikawa (PTEN-) subcutaneous, BALB/c-nude mice | [38] | |
| Explore fluorescence-guided resection of tumor and metastases | Detection and surgical removal of fluorescent tumor tissue with high sensitivity and specificity | VX2 rabbit tumor cells, orthotopic, White New Zealand rabbits | [39] |
| Target/Modality | Clinical Relevance/Finding | Tracer | Preclinical Animal Model/Finding |
|---|---|---|---|
| HER2–PET | HER2 positivity predicts aggressive disease and poor outcome [41]. | 89Zr-pertuzumab | Uptake in human HER2+ breast cancer [42,43] and mouse HER2+ xenografts (BT-474) including evaluation of tumor size change after treatment with HER2-targeted antibody-drug conjugate (T-DM1) [44]. |
| 64Cu-NOTA-pertuzumab | High specificity to HER2 expression and delineation of tumor and metastases in orthotopic and subcutaneous ovarian cancer xenografts [45]. | ||
| EMP2–PET | High EMP2 expression predicts aggressive disease [48,49]. | 64Cu-DOTA-EMP2 | High uptake and delineation of subcutaneous tumors of EMP2-overexpressing Hec1a-cells [50]. |
| CA125–PET | High serum CA125 predicts lymph node metastases [46]. | 89Zr-DFO-mAb-B43.13 | Delineation of subcutaneous ovarian cancer xenografts (OVCAR3)[47]. |
| GPER–SPECT | High GPER expression is associated with poor survival [51,52]. | 99mTc-GPER | Uptake in subcutaneous EC (Hec50) and breast cancer (MCF7/HER2–18) xenografts [53]. |
| Target | Imaging Modality/Sequence | Clinical Relevance | Clinical Findings | Preclinical Application and Findings |
|---|---|---|---|---|
| Tumor proliferation | FLT-PET | Sustained proliferation is a hallmark of cancer, including EC. | No human studies performed in EC. | Growth of primary tumor and metastases can be detected and monitored longitudinally in EC mouse models [12]. |
| FLT can detect treatment response in breast- and ovarian cancer models [54,55,56,57,58,59,60]. | ||||
| Estrogen status | FES-PET | Estrogen drives development of type 1/endometrioid EC, receptor status can predict survival [61,62]. | FES-FDG ratio can predict grade in EC, FES-PET avidity is linked to ERα expression [63,64]. | Shown to predict early treatment response to fulvestrant in ER+ breast cancer xenografts [65,66]. |
| Tumor hypoxia | FMISO-PET FAZA-PET DCE-MRI (Ktrans) | Hypoxia predicts poor survival in EC [67]. | FMISO- and FAZA-PET depict hypoxic regions in cervical cancer [68,69]. | FMISO- and FAZA-PET depict growth of subcutaneous ovarian xenografts and enable monitoring of treatment response (chemotherapy) [57]. Low tumor values of Ktrans is associated with hypoxia in cervical cancer models [74,75,76]. |
| Tumor heterogeneity and vascularity | DW- and DCE MRI | DW- and DCE-MRI are valuable supplements to conventional diagnostic MRI sequences [22,23]. | DCE-parameters (Fb, Ktrans and Ve) are lower in tumor than normal myometrium, tumor ADC is negatively correlated to tumor volume [23]. | DWI (ADC value is negatively correlated to Ki67 proliferation index) to assess treatment response by PI3K-inhibitor perifosine and cisplatin in ovarian xenografts [72]. DWI (↑ADC value) and DCE (↑Ve ) to demonstrate BEZ235 (dual PI3K/mTOR inhibitor) treatment response in ovarian xenografts. [73]. |
| Pharmaco- kinetic modeling, dynamic PET | More accurate quantification and better characterization of tumor heterogeneity in breast cancer [78]. | Rate constants K1 and K2 (perfusion) was higher and K3 was lower (metabolism) in breast cancer xenografts treated with chemotherapy; this response was not detectable by traditional SUV analyses [79]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espedal, H.; Fonnes, T.; Fasmer, K.E.; Krakstad, C.; Haldorsen, I.S. Imaging of Preclinical Endometrial Cancer Models for Monitoring Tumor Progression and Response to Targeted Therapy. Cancers 2019, 11, 1885. https://doi.org/10.3390/cancers11121885
Espedal H, Fonnes T, Fasmer KE, Krakstad C, Haldorsen IS. Imaging of Preclinical Endometrial Cancer Models for Monitoring Tumor Progression and Response to Targeted Therapy. Cancers. 2019; 11(12):1885. https://doi.org/10.3390/cancers11121885
Chicago/Turabian StyleEspedal, Heidi, Tina Fonnes, Kristine E. Fasmer, Camilla Krakstad, and Ingfrid S. Haldorsen. 2019. "Imaging of Preclinical Endometrial Cancer Models for Monitoring Tumor Progression and Response to Targeted Therapy" Cancers 11, no. 12: 1885. https://doi.org/10.3390/cancers11121885
APA StyleEspedal, H., Fonnes, T., Fasmer, K. E., Krakstad, C., & Haldorsen, I. S. (2019). Imaging of Preclinical Endometrial Cancer Models for Monitoring Tumor Progression and Response to Targeted Therapy. Cancers, 11(12), 1885. https://doi.org/10.3390/cancers11121885





