Development and Mechanistic Insight into the Enhanced Cytotoxic Potential of Parvifloron D Albumin Nanoparticles in EGFR-Overexpressing Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Optimization of BSA-Based NPs Preparation Method
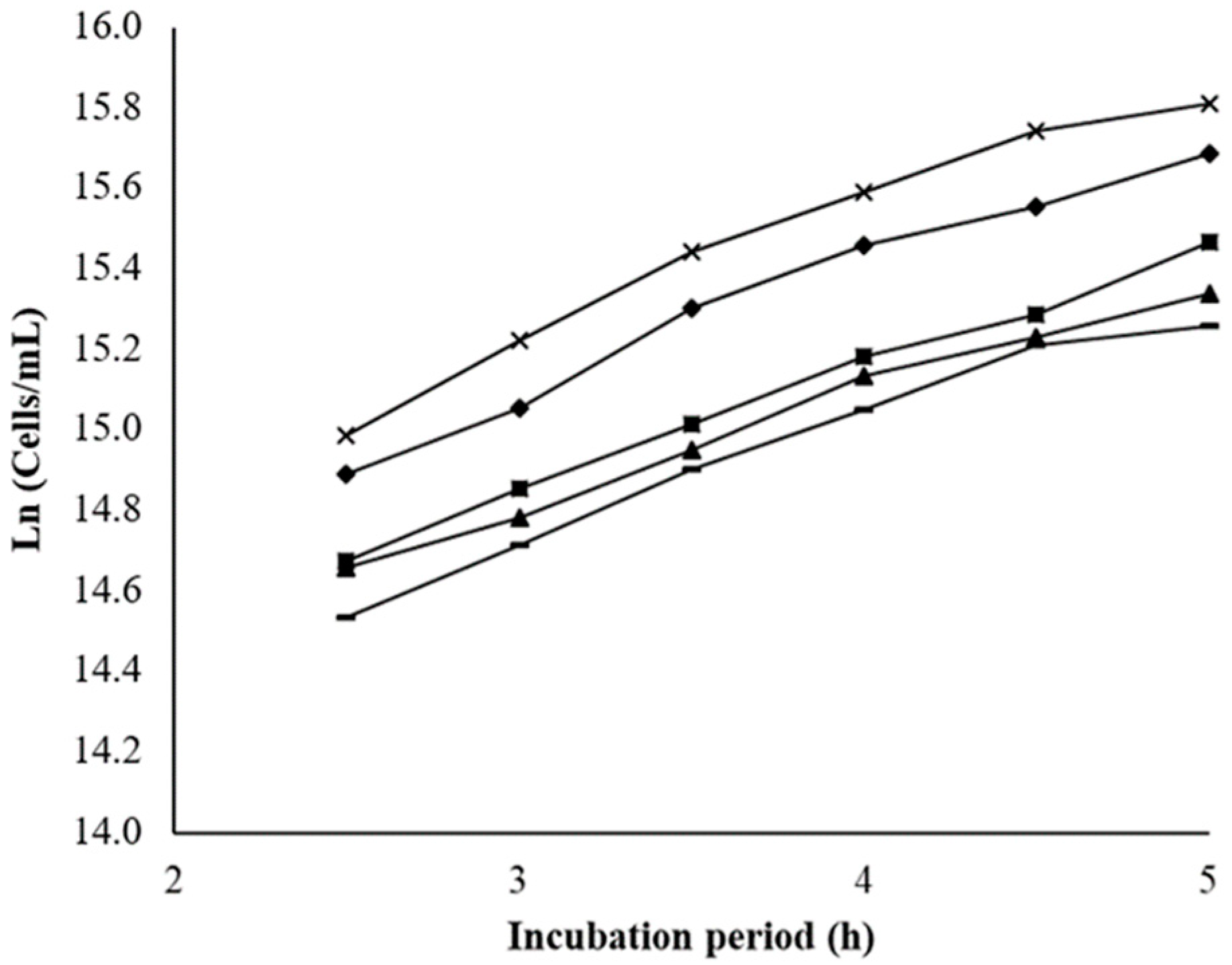
2.2. Cytotoxicity Assay in a Saccharomyces Cerevisiae Model
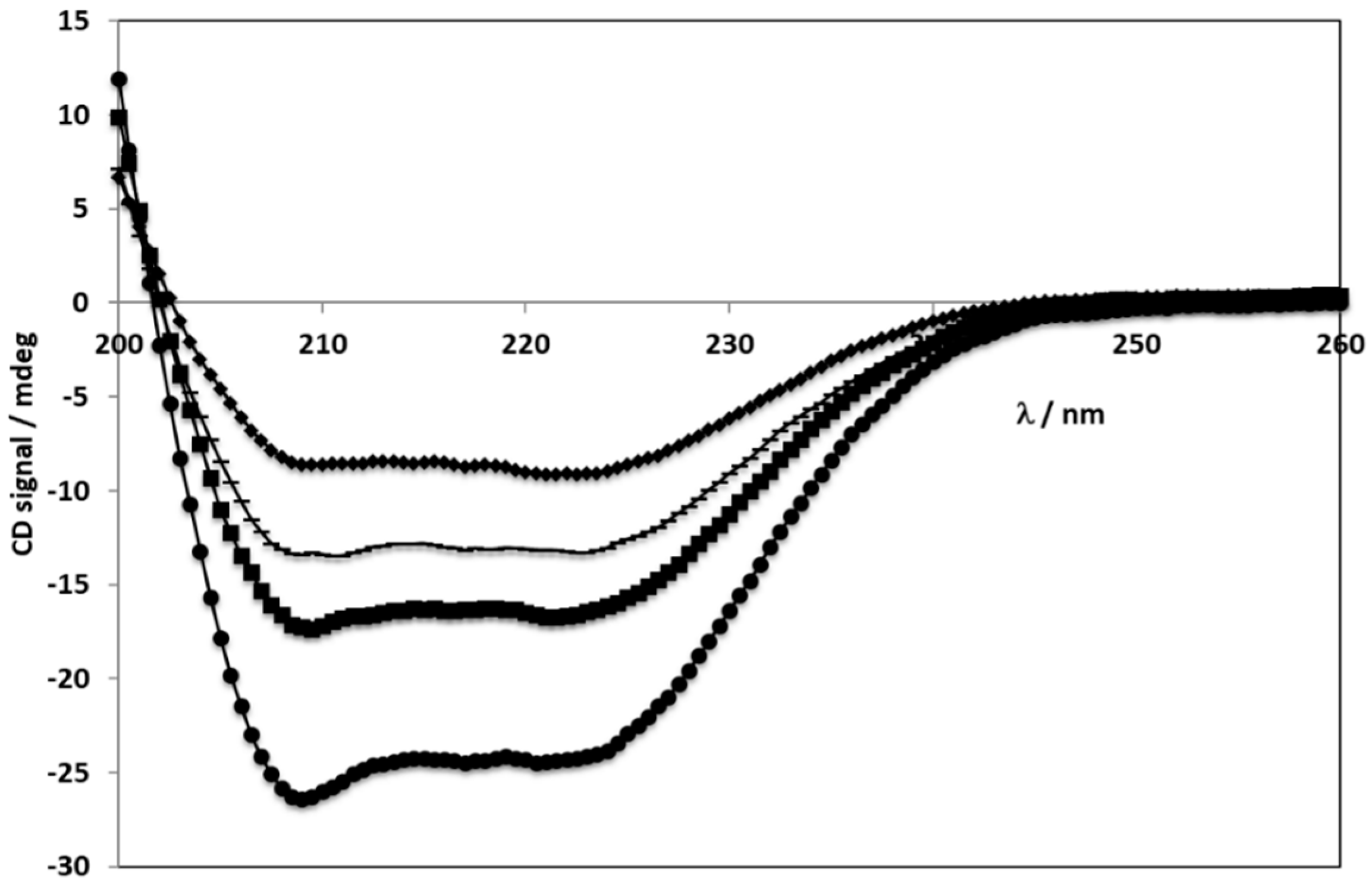
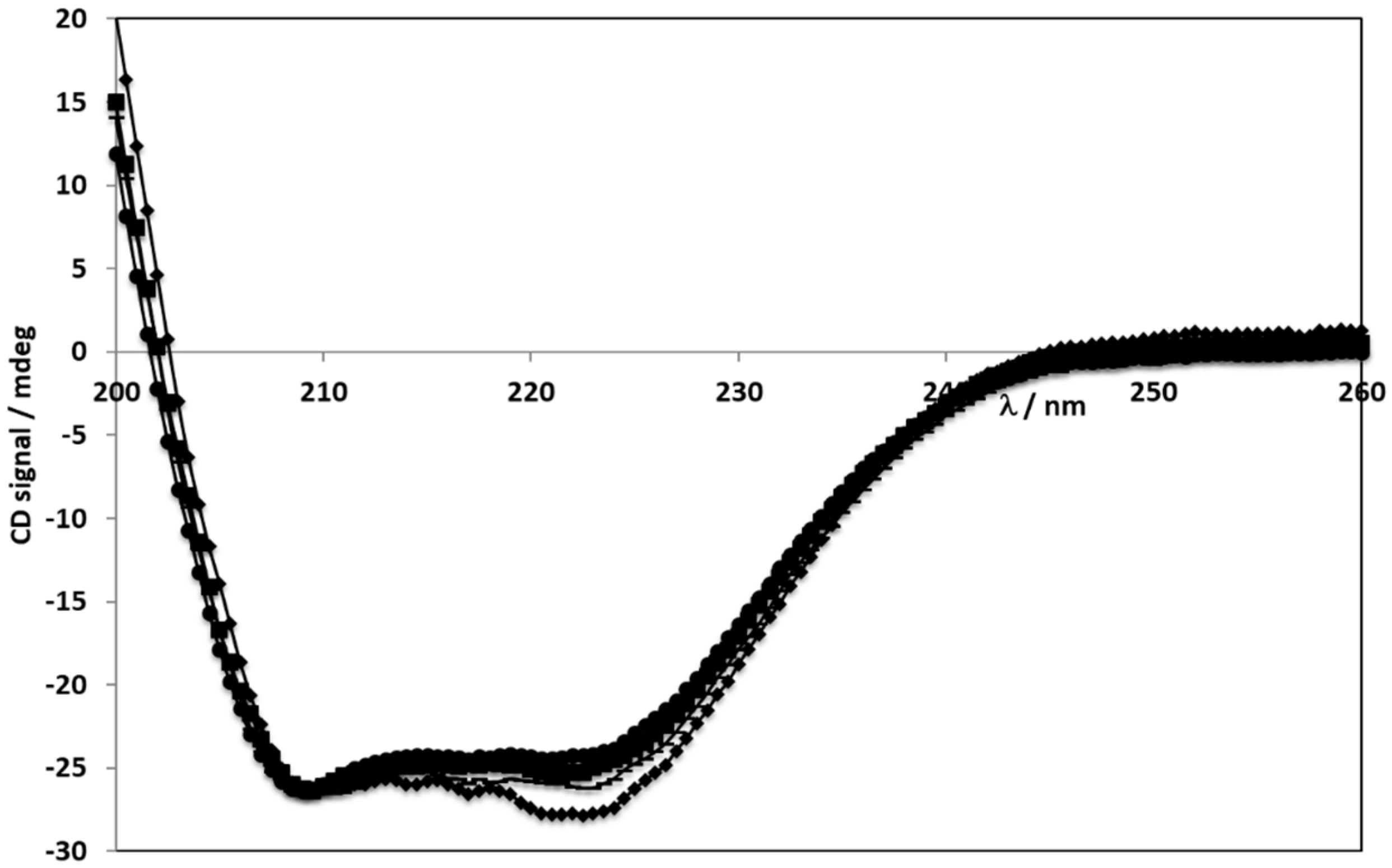
2.3. Circular Dichroism Spectroscopy
2.4. Hemolytic Activity of Potential Injectable PvD-loaded BSA NPs Dosage Form
2.5. PvD Encapsulation Efficiency
2.6. ERL and CET Conjugated BSA NPs Conjugation Quantification
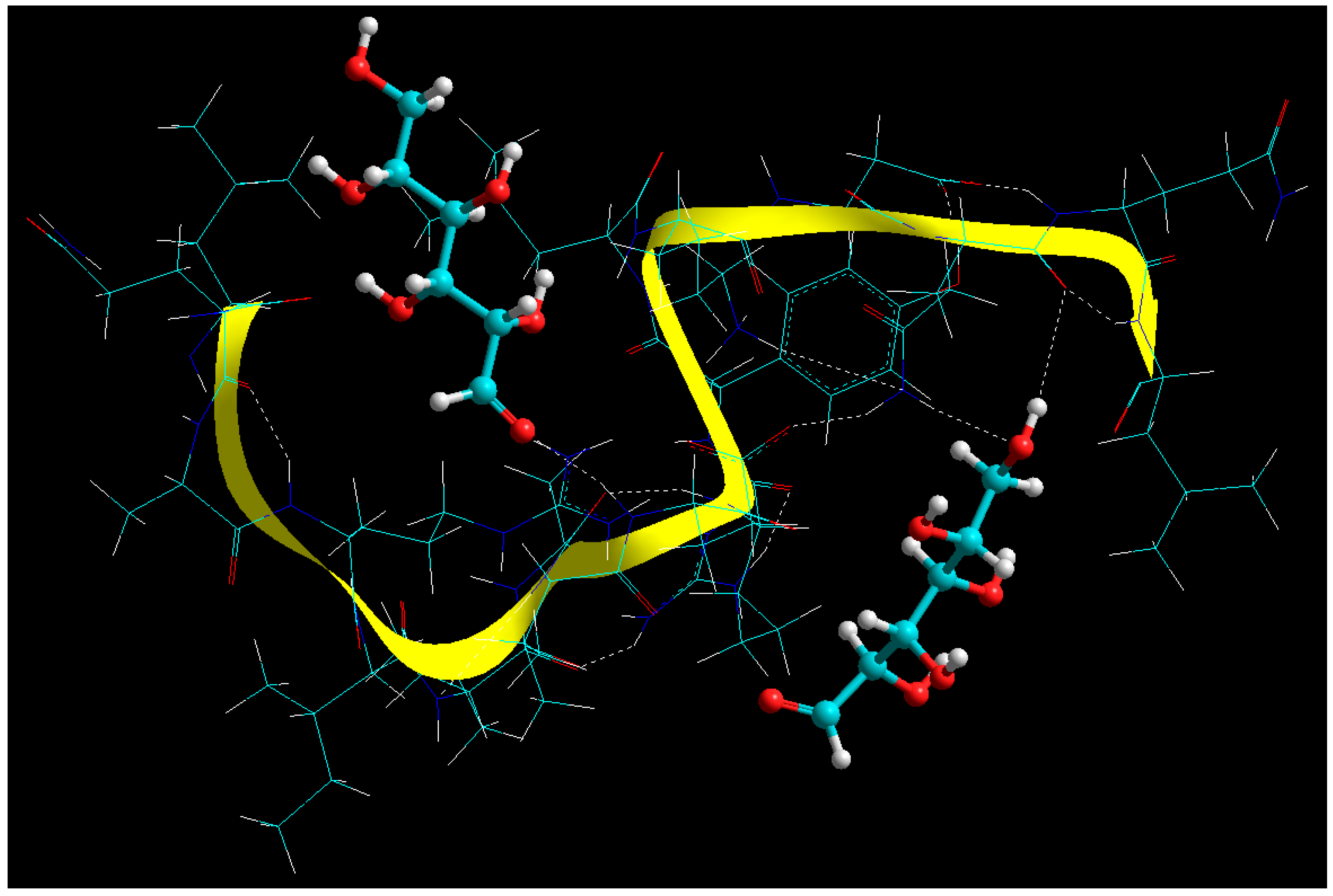
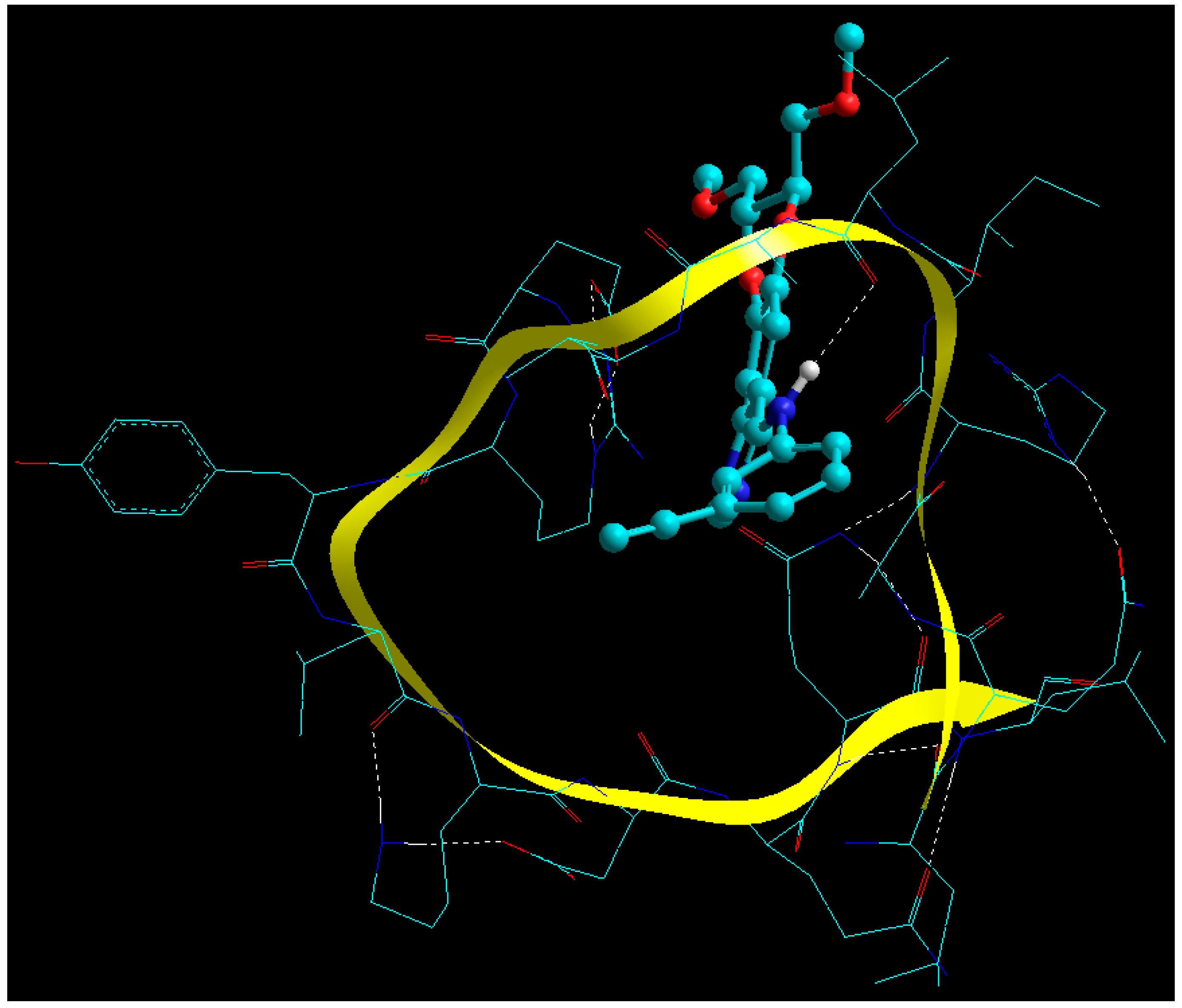
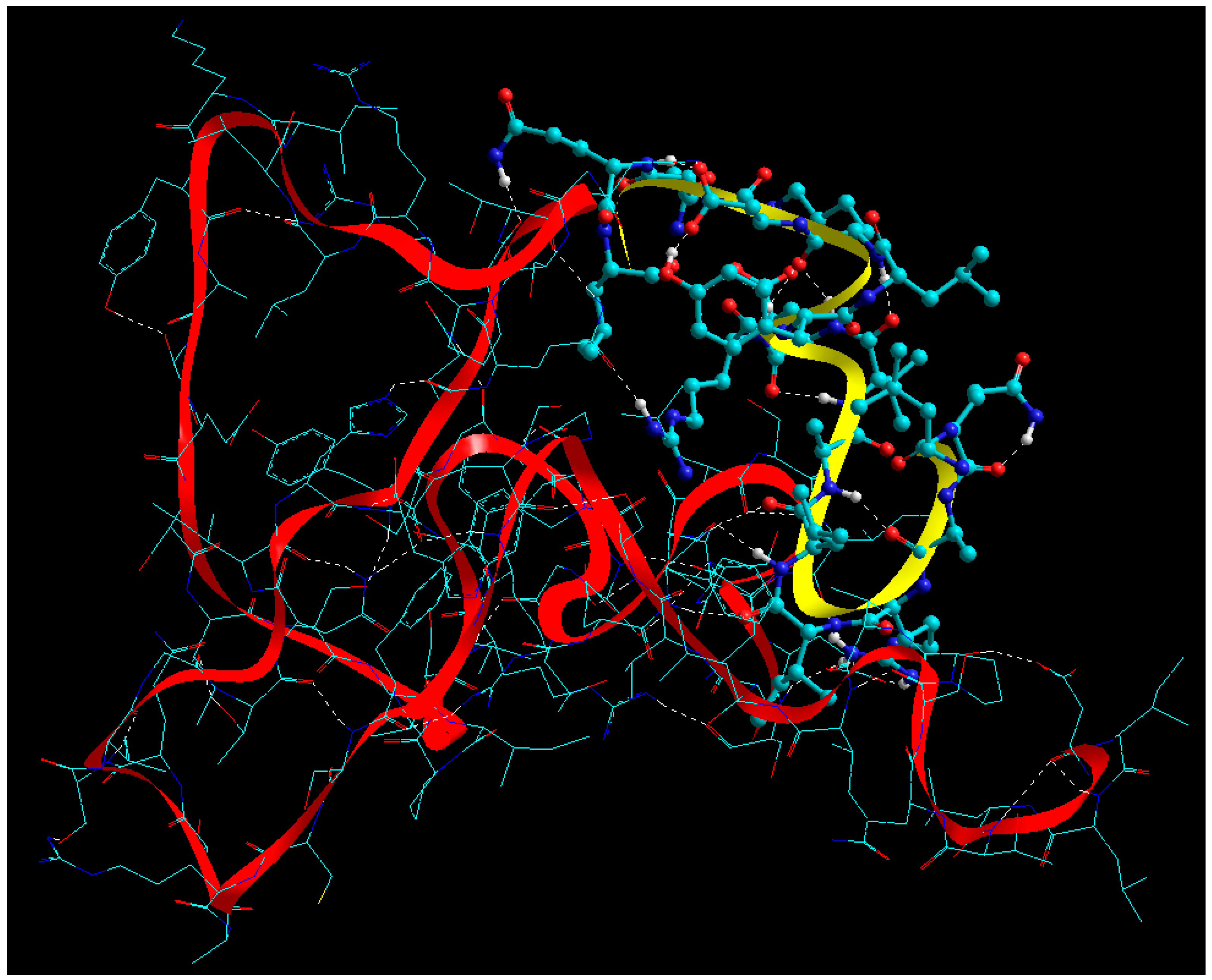
2.7. Static Lattice Atomistic Simulations (SLAS) in Vacuum
2.7.1. Molecular Mechanics Assisted Model Building and Energy Refinements
2.7.2. Fabrication of Glucose Cross-Linked BSA NPs
2.7.3. Functionalization of BSA NPs with ERL
2.7.4. Functionalization of BSA NPs with CET
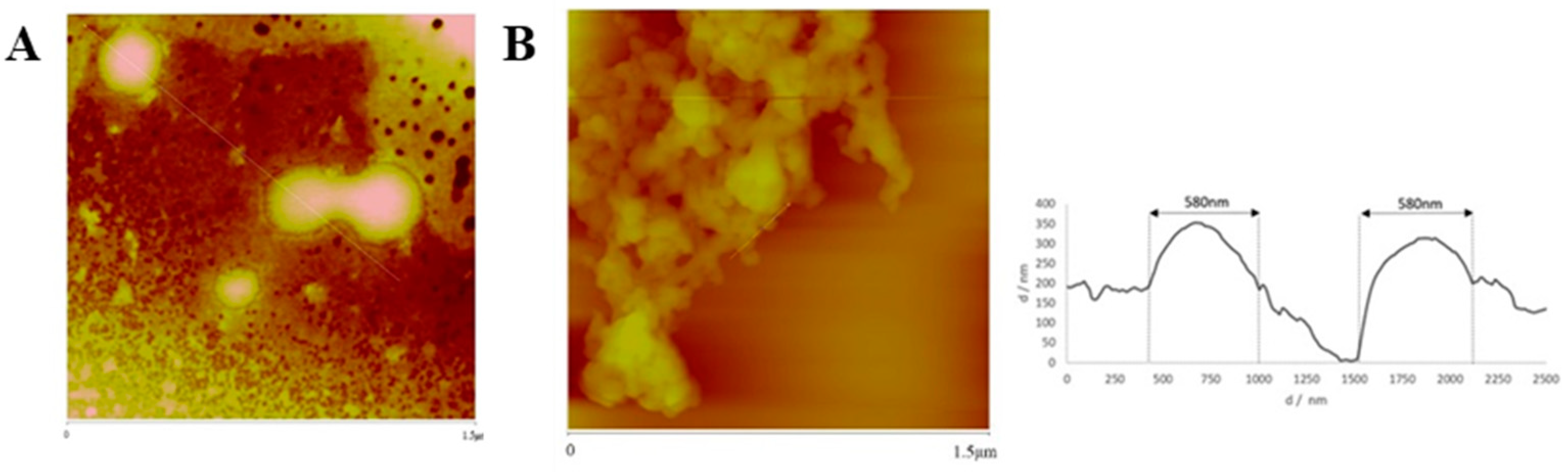
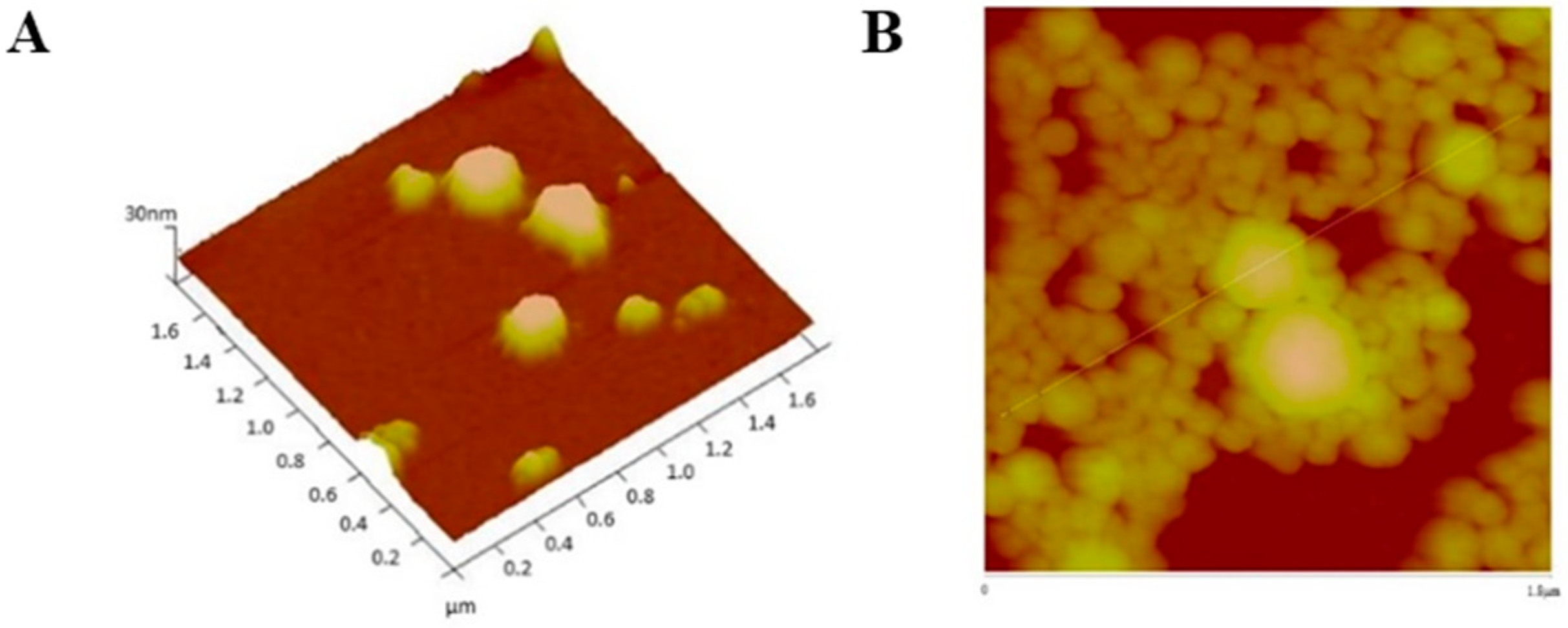
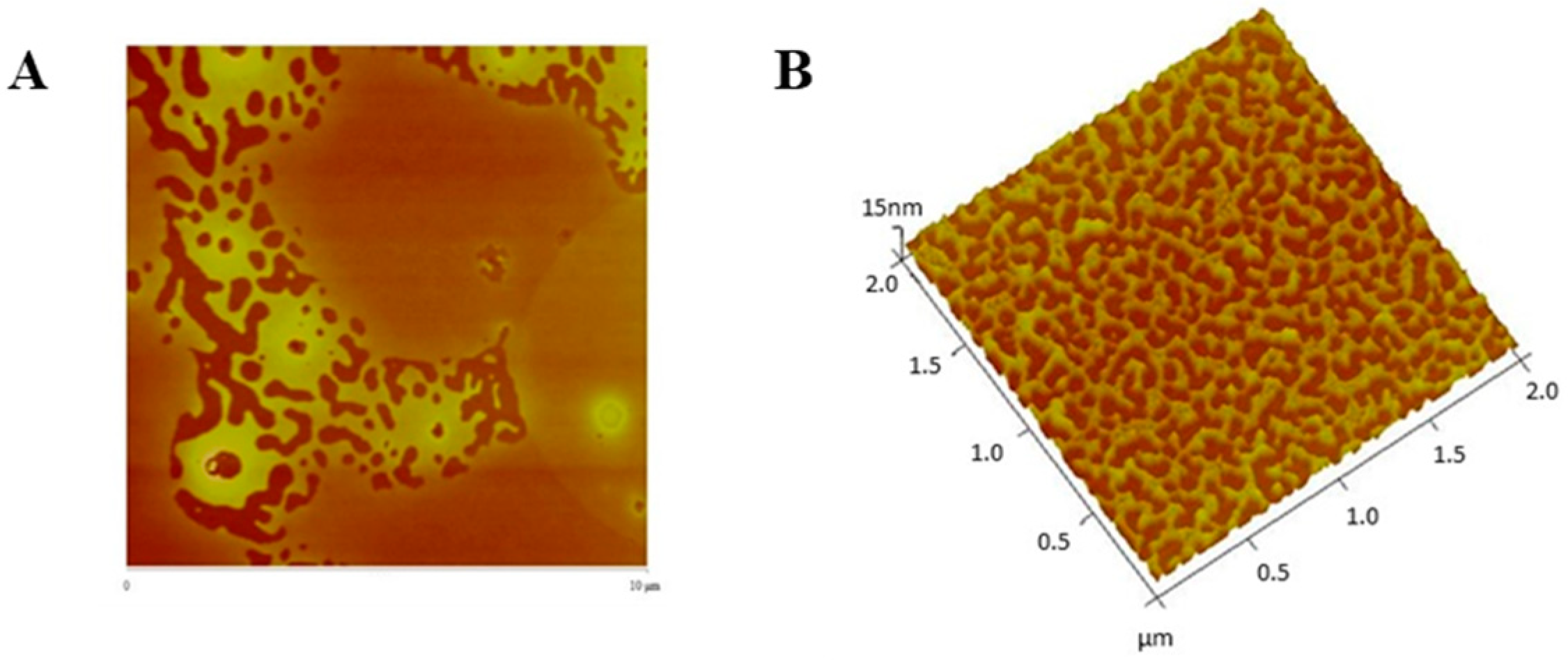
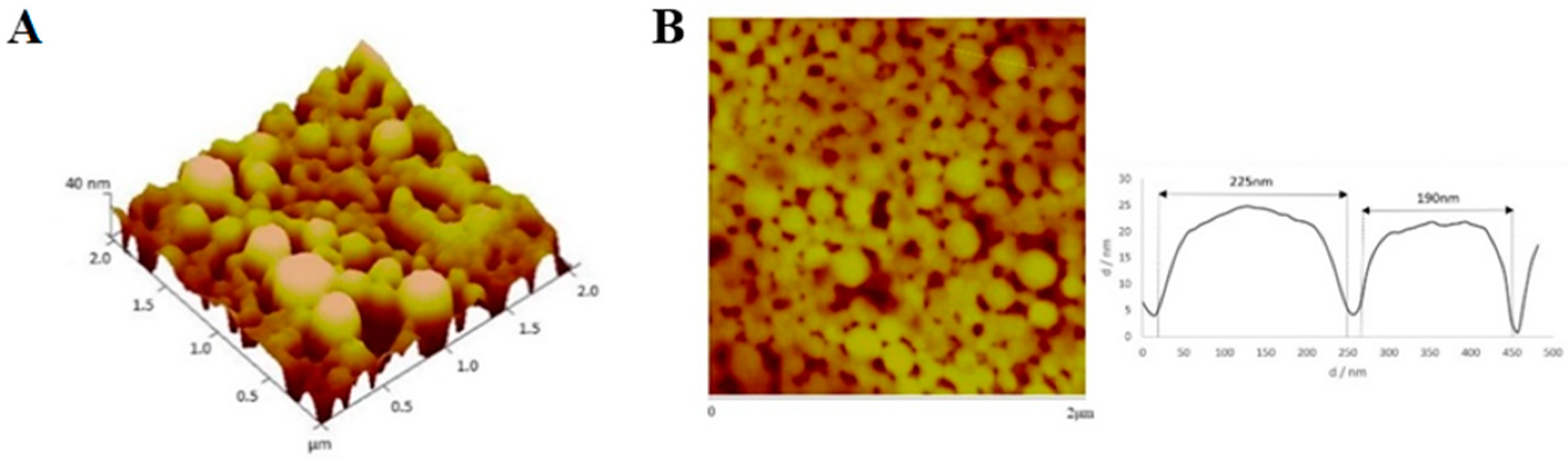
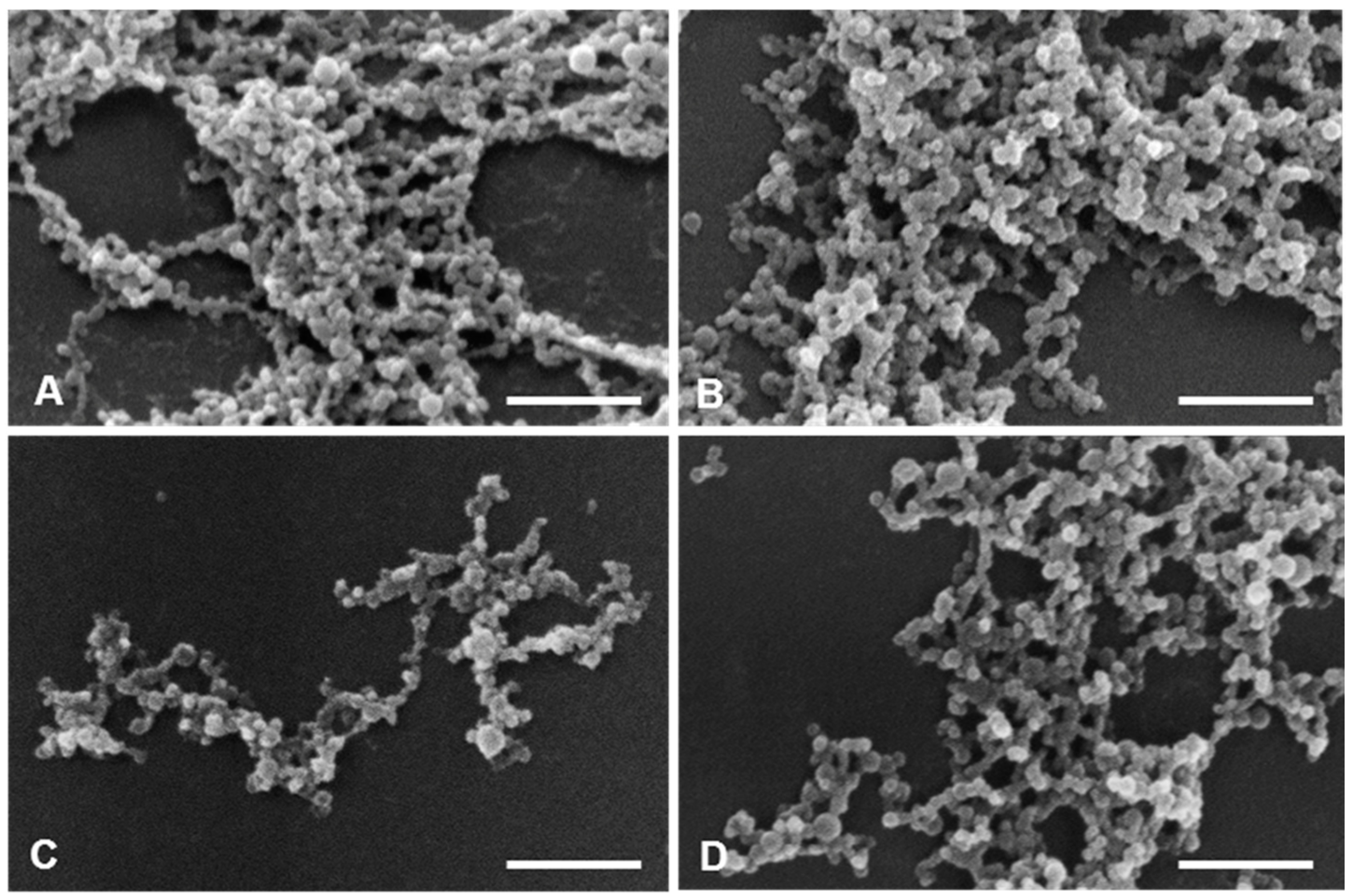
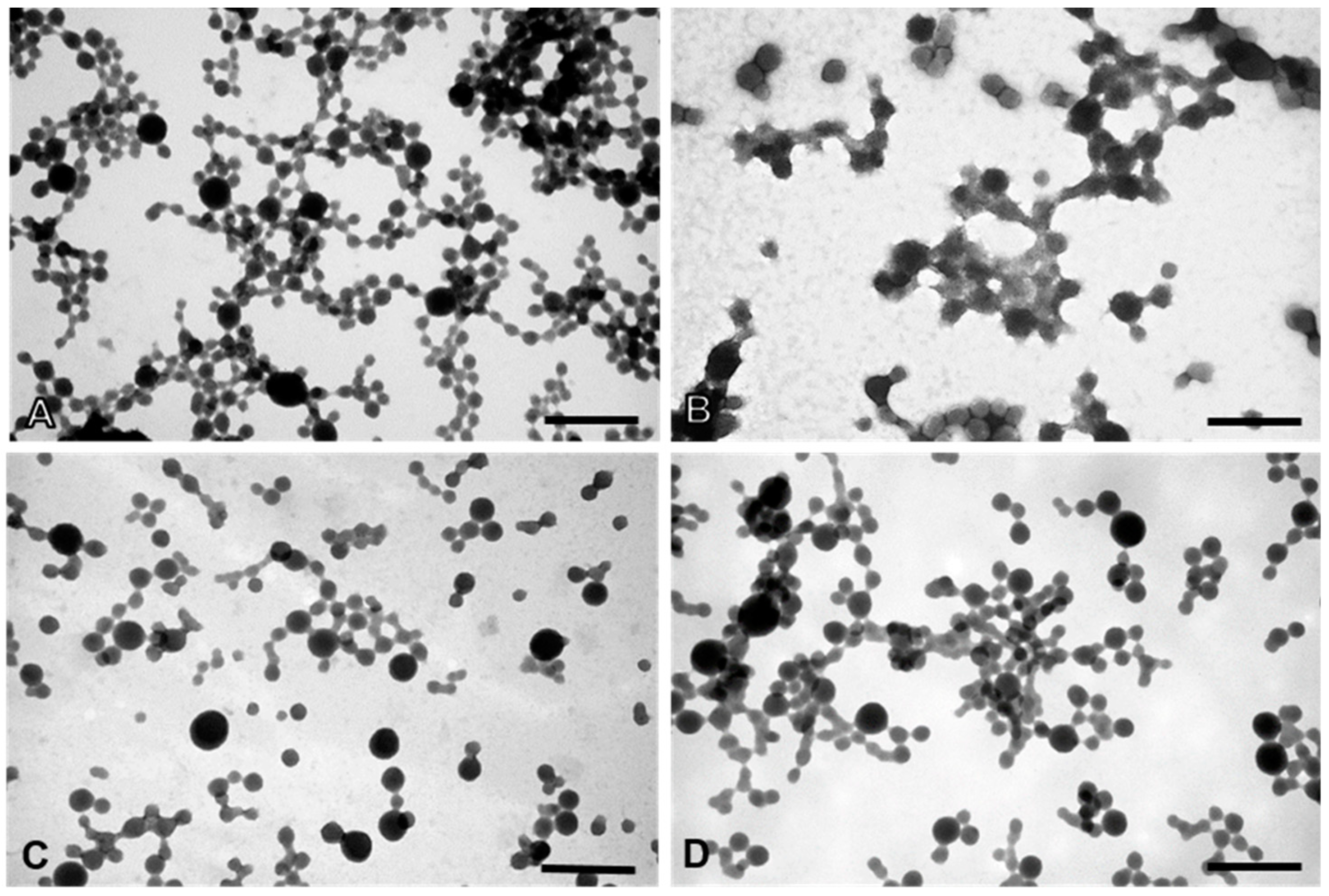
2.8. Physical and Morphological Characterization of the EGFR Inhibitors Conjugated BSA NPs
2.9. Cell Viability Studies of PvD-loaded BSA NPs
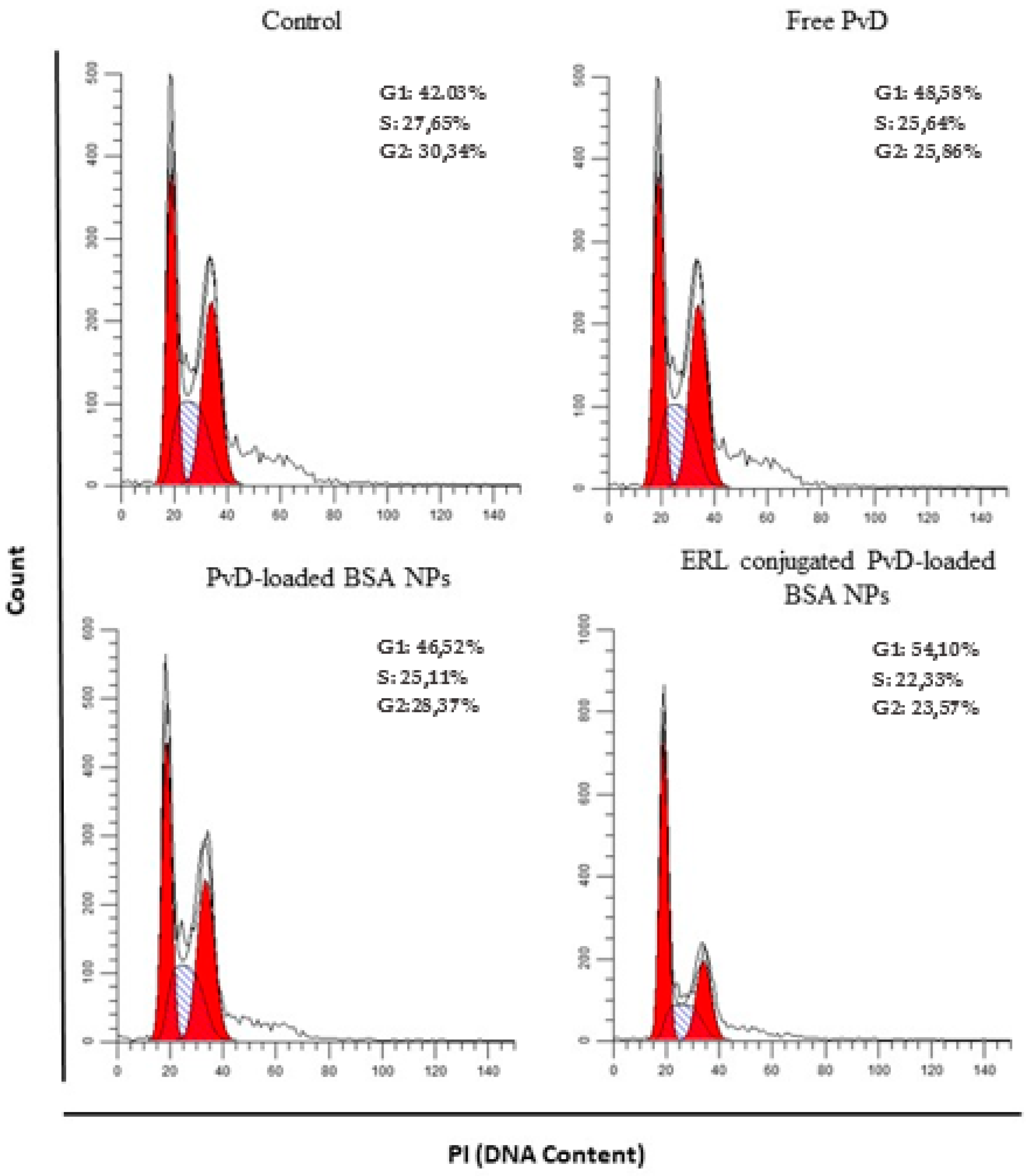
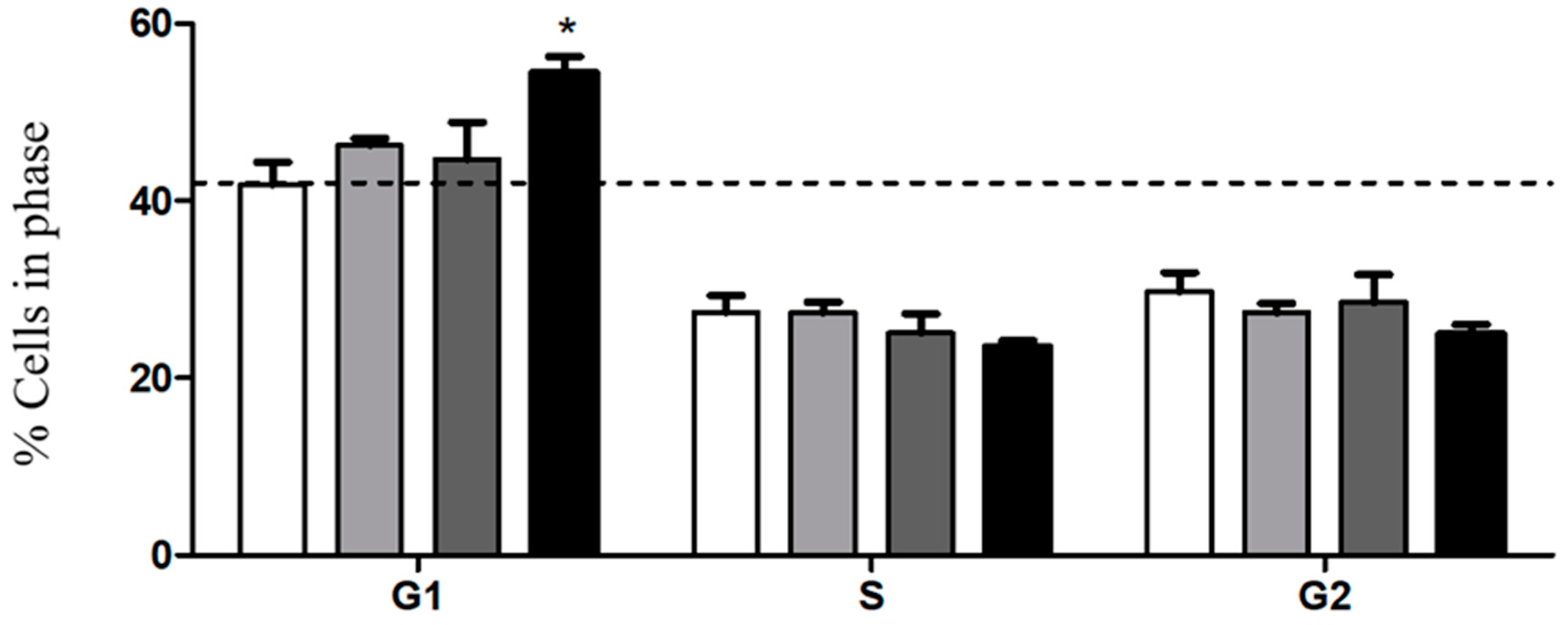
2.10. Cell Cycle Assays
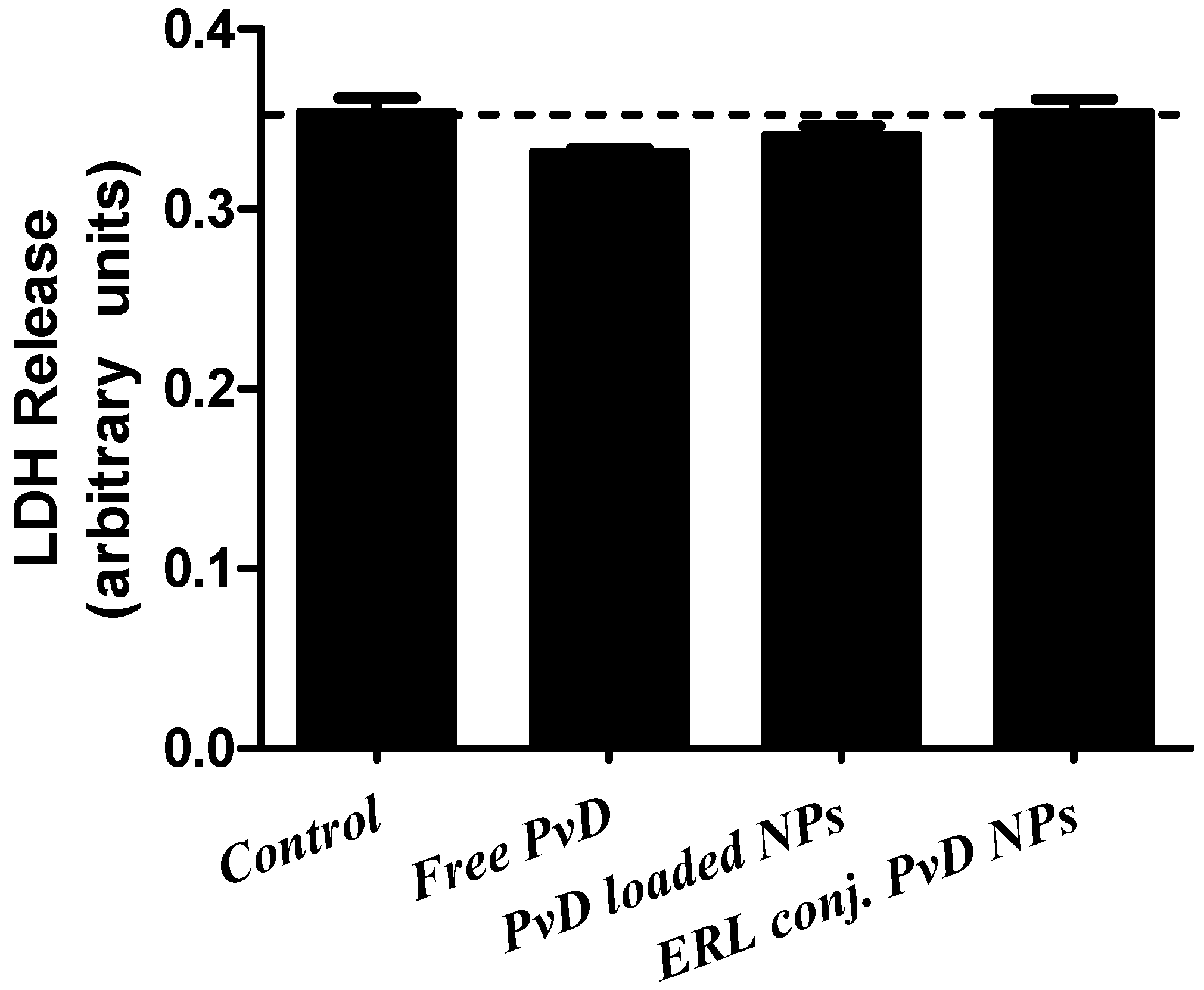
2.11. Lactate Dehydrogenase Assay
3. Discussion
4. Materials and Methods
4.1. Optimization of BSA-Based NPs Development
4.2. NPs Cytotoxicity Assays on Saccharomyces Cerevisiae Model
4.3. Evaluation of BSA NPs Secondary Structure by far UV Circular Dichroism
4.4. Haemolytic Activity
4.5. Synthesis of BSA NPs Conjugated with ERL or CET
4.6. HPLC Method
4.7. LC-MS/MS Analysis for CET Quantification
4.8. Static Lattice Atomistic Simulations
4.9. Physical and Morphological Characterization of the EGFR Inhibitors Conjugated BSA NPs (Size, Surface and Morphology): Dynamic Light Scattering (DLS) and Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
4.10. Cell Viability Studies of PvD-Loaded BSA NPs
4.11. Cell Cycle Assays
4.12. Lactate Dehydrogenase (LDH) Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Rebelo, A.; Molpeceres, J.; Rijo, P.; Pinto Reis, C. Pancreatic Cancer Therapy Review: From Classic Therapeutic Agents to Modern Nanotechnologies. Curr. Drug Metab. 2017, 18, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Chen, C.; Yao, Q.; Li, M. Targeted drug delivery in pancreatic cancer. Biochim. Biophys. Acta 2010, 1805, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Reis, C. Emerging therapeutic nanotechnologies in pancreatic cancer: Advances, risks and challenges. Ther. Deliv. 2018, 9, 691–694. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, J.; Teo, J.; Boyer, C.; Goldstein, D.; Kavallaris, M.; Phillips, P.A. Potential applications of nanotechnology for the diagnosis and treatment of pancreatic cancer. Front. Physiol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, C. Application of albumin-based nanoparticles in the management of cancer. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, W.; Di, Y.; Gu, J.; Guo, Z.; Li, H.; Fu, D.; Jin, C. Triple-functional albumin-based nanoparticles for combined chemotherapy and photodynamic therapy of pancreatic cancer with lymphatic metastases. Int. J. Nanomed. 2017, 12, 6771–6785. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, L.; Fan, X.; Zhang, G.; Li, Y.; Jiang, B. Bioorganic & Medicinal Chemistry Letters Identification of a diverse synthetic abietane diterpenoid library for anticancer activity. Bioorg. Med. Chem. Lett. 2016, 1–6. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Pinto, C.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Gali-muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015. [Google Scholar] [CrossRef]
- Fronza, M.; Lamy, E.; Gunther, S.; Heinzmann, B.; Laufer, S.; Merfort, I. Abietane diterpenes induce cytotoxic effects in human pancreatic cancer cell line MIA PaCa-2 through different modes of action. Phytochemistry 2012, 78, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.O.; Molpeceres, J.; Batanero, B.; Fernandes, A.S.; Saraiva, N.; Costa, J.G.; Rijo, P.; Figueiredo, I.V.; Faísca, P.; Reis, C.P. Functionalized diterpene parvifloron D-loaded hybrid nanoparticles for targeted delivery in melanoma therapy. Ther. Deliv. 2016, 7, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Santos-rebelo, A.; Garcia, C.; Eleut, C.; Bastos, A.; Coelho, C.; Coelho, M.A.N.; Viana, A.S.; Ascensão, L.; Pinto, F.; Gaspar, M.M.; et al. Development of Parvifloron D-Loaded Smart Nanoparticles to Target Pancreatic Cancer. Pharmaceutics 2018, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, O.; Perdomo, J.; Simões, M.F.; Rijo, P.; Quintana, J.; Estévez, F. The abietane diterpenoid parvifloron D from Plectranthus ecklonii is a potent apoptotic inducer in human leukemia cells. Phytomedicine 2015, 22, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, B.V.; Silva, P.B.; Aparecido dos Santos Ramos, M.; Maria Silveira Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Damgé, C. Nanotechnology as a promising strategy for alternative routes of insulin delivery. Methods Enzymol. 2012, 508, 271–294. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Design of Insulin-Loaded Alginate Nanoparticles: Influence of Calcium Ion on Polymer Gel Matrix Properties. Chem. Ind. Chem. Eng. Q. 2006, 12, 47–52. [Google Scholar] [CrossRef]
- Reis, C.P.; Figueiredo, I.V.; Carvalho, R.A.; Jones, J.; Nunes, P.; Soares, A.F.; Silva, C.F.; Ribeiro, A.J.; Veiga, F.J.; Damgé, C.; et al. Toxicological assessment of orally delivered nanoparticulate insulin. Nanotoxicology 2008, 2, 205–217. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Silva, C.; Martinho, N.; Rosado, C. Drug carriers for oral delivery of peptides and proteins: accomplishments and future perspectives. Ther. Deliv. 2013, 4, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Di, Y.; Xie, C.; Song, Y.; He, H.; Li, H.; Pu, X.; Lu, W.; Fu, D.; Jin, C. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int. J. Nanomed. 2015, 10, 6825–6834. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Released 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, G.; Duarte, D.; Reis, C.P. An Overview of Pharmaceutical Excipients: Safe or Not Safe? J. Pharm. Sci. 2016, 105, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L.; Ho, H.K. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov. Today 2018, 23, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Sahandi, P.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, C.; Zhao, X.; Zu, Y.; Wang, Y.; Zhang, B.; Zhao, D.; Zhao, Q.; Su, L.; Gao, Y.; et al. Preparation, characterization and targeting of micronized 10-hydroxycamptothecin-loaded folate-conjugated human serum albumin nanoparticles to cancer cells. Int. J. Nanomed. 2011, 6, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Maghsoudi, A.; Farahani, E.V. Optimization of PEGylation Conditions for BSA Nanoparticles Using Response Surface Methodology. AAPS PharmSciTech 2010, 11, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef]
- Kiani, K.; Shakeri, S.; Maryam, K. Preparation and in vitro investigation of antigastric cancer activities of carvacrol-loaded human serum albumin nanoparticles. IET Nanobiotechnol. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Piao, J.; Chen, Y.; Gao, S.; Lu, C.; Niu, L.; Xia, Y.; Hu, Y.; Ji, R.; et al. Studies on the preparation, characterization and intracellular kinetics of JD27-loaded human serum albumin nanoparticles. Procedia Eng. 2015, 102, 590–601. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential - What they are and what they are not? J. Control. Released 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Maximos, A.S. Circular dichroism of bovine serum albumin in divalent salt solutions. Int. J. Pept. Protein Res. 1989, 34, 46–51. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 3095–3103. [Google Scholar] [CrossRef]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casin, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Choonara, Y.E.; Pillay, V. In silico analytico-mathematical interpretation of biopolymeric assemblies: Quantification of energy surfaces and molecular attributes via atomistic simulations. Bioeng. Transl. Med. 2018, 1–10. [Google Scholar] [CrossRef]
- Vasan, S.; Zhang, X.; Zhang, X.; Kapurniotu, A.; Bernhagen, J.; Teichberg, S.; Basgen, J.; Wagle, D.; Shih, D.; Terlecky, I.; et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature 1996, 382, 275–278. [Google Scholar] [CrossRef]
- Govender, M.; Choonara, Y.E.; Van Vuuren, S.; Kumar, P. A Dual-Biotic System for the Concurrent Delivery of Antibiotics and Probiotics: In Vitro, Ex Vivo, In Vivo and In Silico Evaluation and Correlation. Pharm. Res. 2016, 33, 3057–3071. [Google Scholar] [CrossRef]
- Atef, A.; Ali, A.; Hsu, F.; Hsieh, C.; Shiau, C.; Chiang, C.; Wei, Z.; Chen, C.; Huang, H. Erlotinib-Conjugated Iron Oxide Nanoparticles as a Smart Cancer - Targeted Theranostic Probe for MRI. Nat. Publ. Gr. 2016, 1–16. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic Force Microscopy: A Powerful Tool to Address Scaffold Design in Tissue Engineering. J. Funct. Biomater. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Çalış, S.; Öztürk, A.K.; Arslan, F.B.; Eroğlu, H.; Çapan, Y. Nanopharmaceuticals as Drug-Delivery Systems: For, Against, and Current Applications Nanoscience and Nanotechnology. In Drug Delivery Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–154. [Google Scholar]
- Niknejada, H.; Mahmoudzadeh, R. Comparison of Different Crosslinking Methods for Preparation of Docetaxel-loaded Albumin Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 385–394. [Google Scholar]
- Roberto, A.; Caetano, P.P. A high-throughput screening method for general cytotoxicity part I: Chemical toxicity. Rev. Lusófona Ciências Tecnol. Saúde 2005, 2, 95–100. [Google Scholar]
- Liu, Y.; Chen, M.; Luo, Z.; Lin, J.; Song, L. Investigation on the site-selective binding of bovine serum albumin by erlotinib hydrochloride. J. Biomol. Struct. Dyn. 2012, 37–41. [Google Scholar] [CrossRef]
- Domotor, O.; Pelivan, K.; Borics, A.; Keppler, B.K.; Kowol, C.R.; Enyedy, E.A. Comparative studies on the human serum albumin binding of the clinically approved EGFR inhibitors gefitinib, erlotinib, afatinib, osimertinib and the investigational inhibitor KP2187. J. Pharm. Biomed. Anal. 2018, 154, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B. Design, Development and Evaluation of Erlotinib-Loaded Hybrid Nanoparticles for Targeted Drug Delivery to NonSmall Cell Lung Cancer Design, Development and Evaluation of Erlotinib-Loaded Hybrid. Theses Diss. 2015. [Google Scholar] [CrossRef]
- Becher, F.; Ciccolini, J.; Imbs, D.; Marin, C.; Dupuis, C.; Fakhry, N.; Pourroy, B.; Ghettas, A.; Junot, C.; Duffaud, F.; et al. A simple and rapid LC-MS / MS method for therapeutic drug monitoring of cetuximab: a GPCO- UNICANCER proof of concept study in head-and-neck cancer patients. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kumar, P.; Pillay, V.; Choonara, Y.E.; Modi, G.; Naidoo, D. In Silico Theoretical Molecular Modeling for Alzheimer’ s Disease: The Nicotine-Curcumin Paradigm in Neuroprotection and Neurotherapy. Int. J. Mol. Sci. 2011, 12, 694–724. [Google Scholar] [CrossRef]
- Burmistrova, O.; Simões, M.F.; Rijo, P.; Quintana, J.; Bermejo, J.; Estévez, F. Antiproliferative activity of abietane diterpenoids against human tumor cells. J. Nat. Prod. 2013, 76, 1413–1423. [Google Scholar] [CrossRef]
| Mean Size (nm) (± SD) | Zeta Potential | PdI | CE (%) | ||
|---|---|---|---|---|---|
| Cross-linking agent | Glutaraldehyde | 178 (± 43) | −1 | <0.220 | 54 |
| Glucose | 225 (± 60) | −14 | <0.250 | 97 | |
| Glucose + UV | 237 (± 70) | −4 | <0.120 | 44 | |
| UV | 600 (± 116) | −1 | <0.180 | 95 | |
| Stirring rate (rpm) | 100 | 4396 (± 398) | 0 | <0.710 | 98 |
| 500 | 225 (± 25) | −14 | <0.250 | 97 | |
| Cross-linking time | 24 h | 129 (± 34 | 11 | <0.100 | 56 |
| 30 min | 225 (± 25) | −14 | <0.250 | 97 | |
| Type of organic solvent | DMSO | 153 (± 25) | 0 | <0.010 | 69 |
| Ethanol | 145 (±42) | −2 | <0.330 | 50 | |
| Acetone | 244 (± 9) | −8 | <0.260 | 98 | |
| Hexane | 1346 (± 144) | −1 | <0.180 | 88 | |
| Aqueous: Organic ratio | 1:1 | 236 (± 62) | −1 | <0.230 | 78 |
| 2:1 | 898 (± 33) | 0 | <0.210 | 77 | |
| 3:1 | 244 (± 9) | −8 | <0.260 | 98 |
| BSA Nanoformulations | Mean Diameter (nm) ± SD | Mean Zeta Potential (mV) ± SD | PdI |
|---|---|---|---|
| ERL-CET conjugated PvD-loaded BSA NPs | 1466 (±155) | −48 (± 6) | <0.560 |
| ERL-CET conjugated empty BSA NPs | 502 (± 36) | −32 (± 6) | <1 |
| ERL conjugated PvD-loaded BSA NPs | 349 (± 59) | −39 (± 10) | <0.450 |
| ERL conjugated empty BSA NPs | 336 (± 91) | −36 (± 5) | <0.090 |
| CET conjugated PvD-loaded BSA NPs | 43 (± 4) | −43 (± 4) | <1 |
| CET conjugated empty BSA NPs | 42 (± 5) | −32 (± 6) | <1 |
| PvD-loaded BSA NPs | 280 (± 86) | −42 (± 4) | <0.370 |
| Empty BSA NPs | 393 (± 131) | −37 (± 6) | <0.120 |
| Formulation Tested | BxPC3 | PANC-1 |
|---|---|---|
| Free PvD | 10.6 ± 3.6 | 21.7 |
| Empty BSA NPs | >30 | >40 |
| PvD-loaded BSA NPs | >30 | >40 |
| ERL conjugated empty BSA NPs | <20 | >40 |
| ERL conjugated PvD-loaded BSA NPs | 21.5 ± 2.2 | 16.8 |
| CET conjugated empty BSA NPs | >30 | >40 |
| CET conjugated PvD-loaded BSA NPs | >30 | >40 |
| ERL-CET conjugated empty BSA NPs | <20 | >40 |
| ERL-CET conjugated PvD-loaded BSA NPs | 6.9 ± 1.1 | > 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Rebelo, A.; Kumar, P.; Pillay, V.; Choonara, Y.E.; Eleutério, C.; Figueira, M.; Viana, A.S.; Ascensão, L.; Molpeceres, J.; Rijo, P.; et al. Development and Mechanistic Insight into the Enhanced Cytotoxic Potential of Parvifloron D Albumin Nanoparticles in EGFR-Overexpressing Pancreatic Cancer Cells. Cancers 2019, 11, 1733. https://doi.org/10.3390/cancers11111733
Santos-Rebelo A, Kumar P, Pillay V, Choonara YE, Eleutério C, Figueira M, Viana AS, Ascensão L, Molpeceres J, Rijo P, et al. Development and Mechanistic Insight into the Enhanced Cytotoxic Potential of Parvifloron D Albumin Nanoparticles in EGFR-Overexpressing Pancreatic Cancer Cells. Cancers. 2019; 11(11):1733. https://doi.org/10.3390/cancers11111733
Chicago/Turabian StyleSantos-Rebelo, Ana, Pradeep Kumar, Viness Pillay, Yahya E. Choonara, Carla Eleutério, Mariana Figueira, Ana S. Viana, Lia Ascensão, Jesús Molpeceres, Patrícia Rijo, and et al. 2019. "Development and Mechanistic Insight into the Enhanced Cytotoxic Potential of Parvifloron D Albumin Nanoparticles in EGFR-Overexpressing Pancreatic Cancer Cells" Cancers 11, no. 11: 1733. https://doi.org/10.3390/cancers11111733
APA StyleSantos-Rebelo, A., Kumar, P., Pillay, V., Choonara, Y. E., Eleutério, C., Figueira, M., Viana, A. S., Ascensão, L., Molpeceres, J., Rijo, P., Correia, I., Amaral, J., Solá, S., Rodrigues, C. M. P., Gaspar, M. M., & Reis, C. P. (2019). Development and Mechanistic Insight into the Enhanced Cytotoxic Potential of Parvifloron D Albumin Nanoparticles in EGFR-Overexpressing Pancreatic Cancer Cells. Cancers, 11(11), 1733. https://doi.org/10.3390/cancers11111733












