Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Nanostructured Lipid Carriers Efficiently Encapsulate Verteporfin
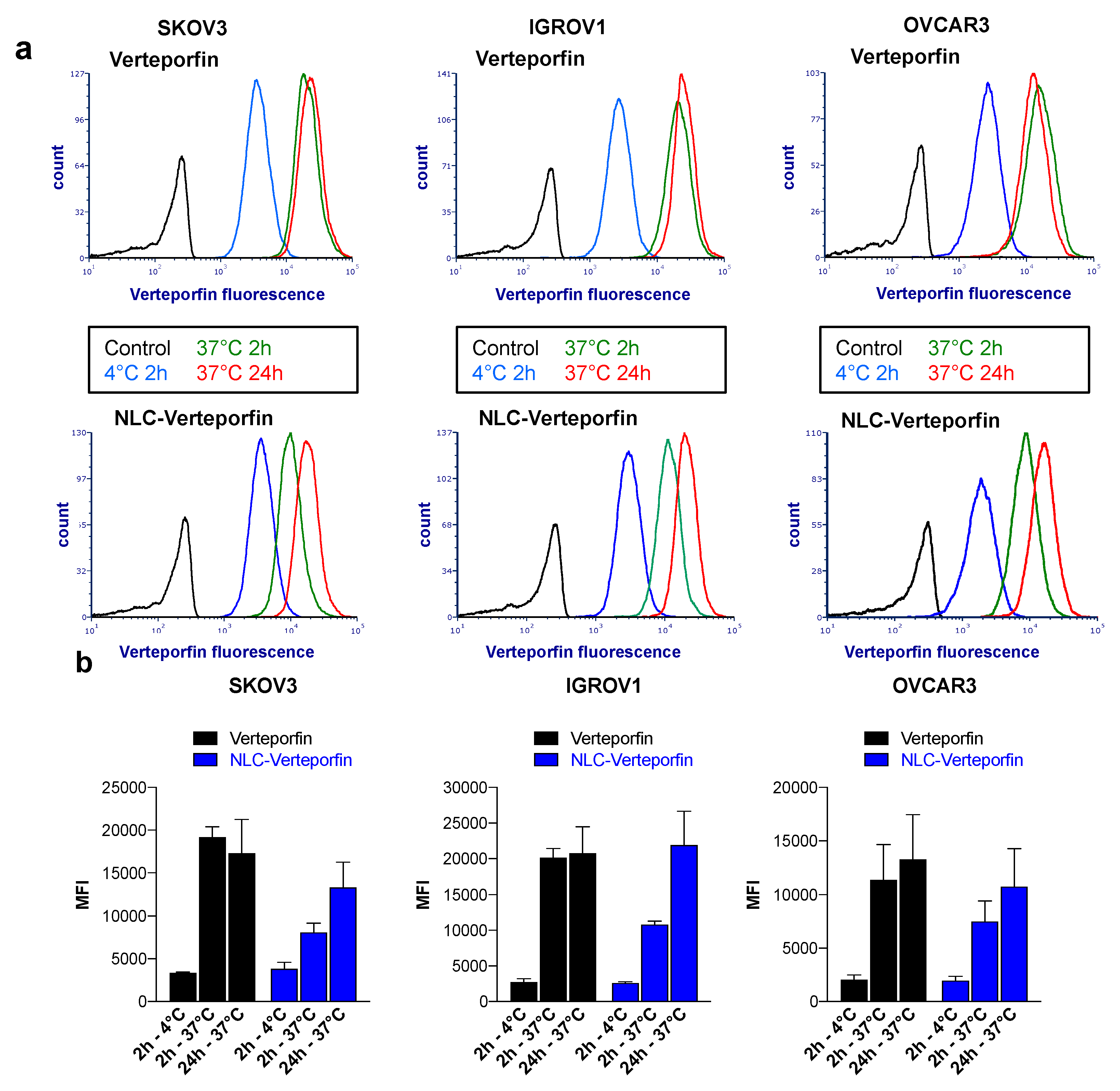
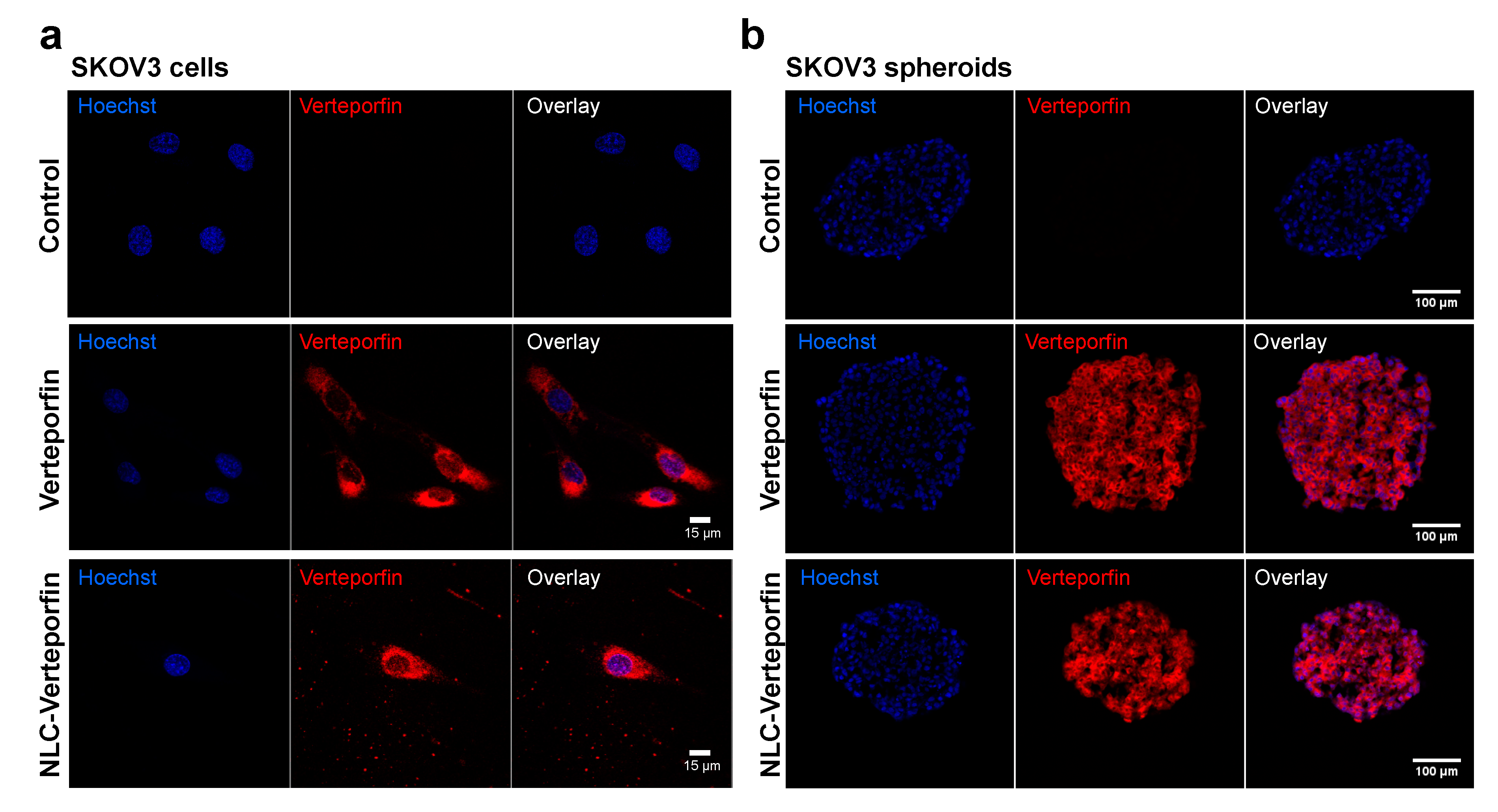
2.2. Verteporfin and NLC-Verteporfin Bind and are Internalized in Ovarian Cancer Cells
2.3. Verteporfin and NLC-Verteporfin Induce Phototoxicity in Ovarian Cancer Cells and Spheroids
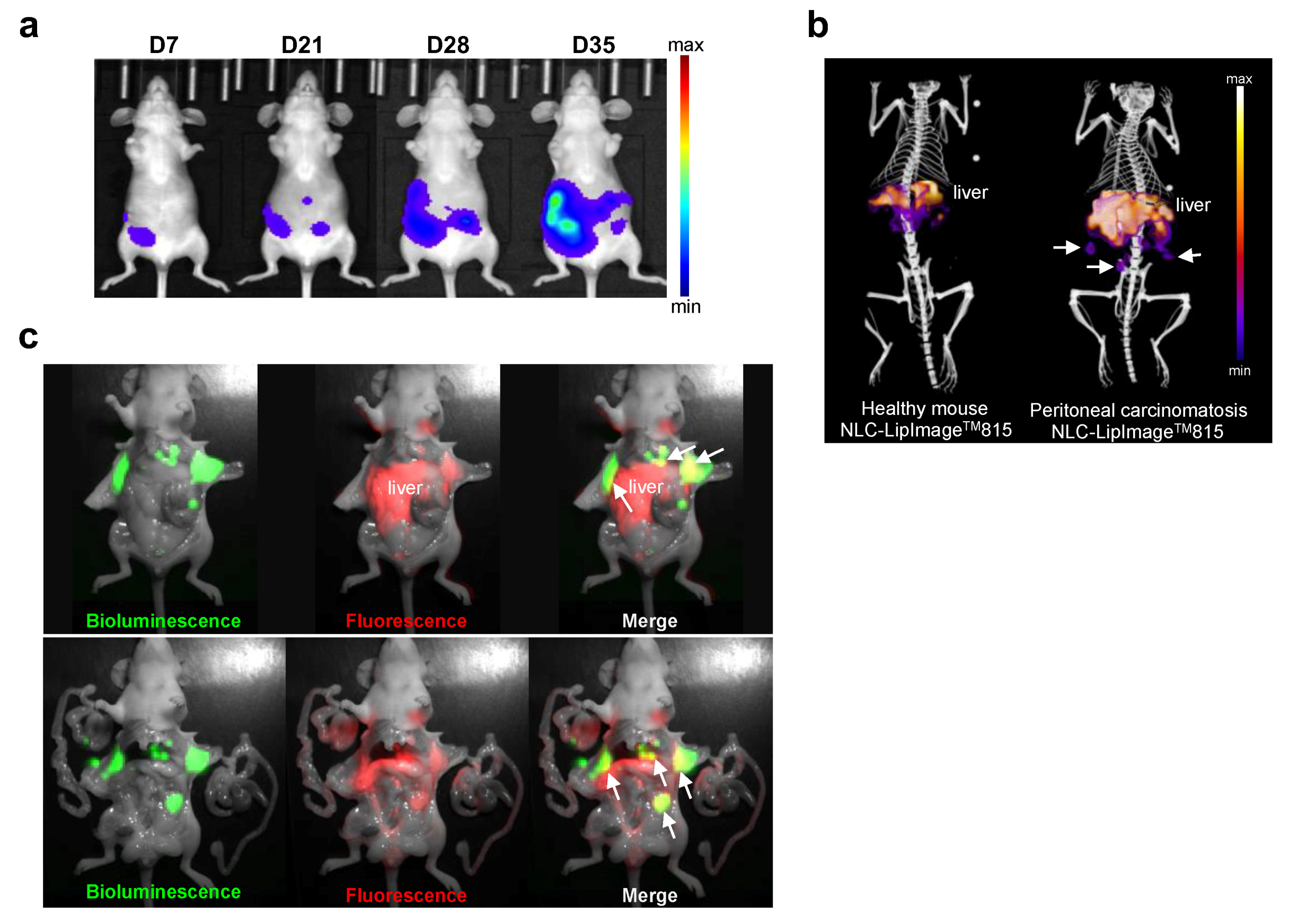
2.4. NLC Accumulate in Ovarian Tumors In Vivo
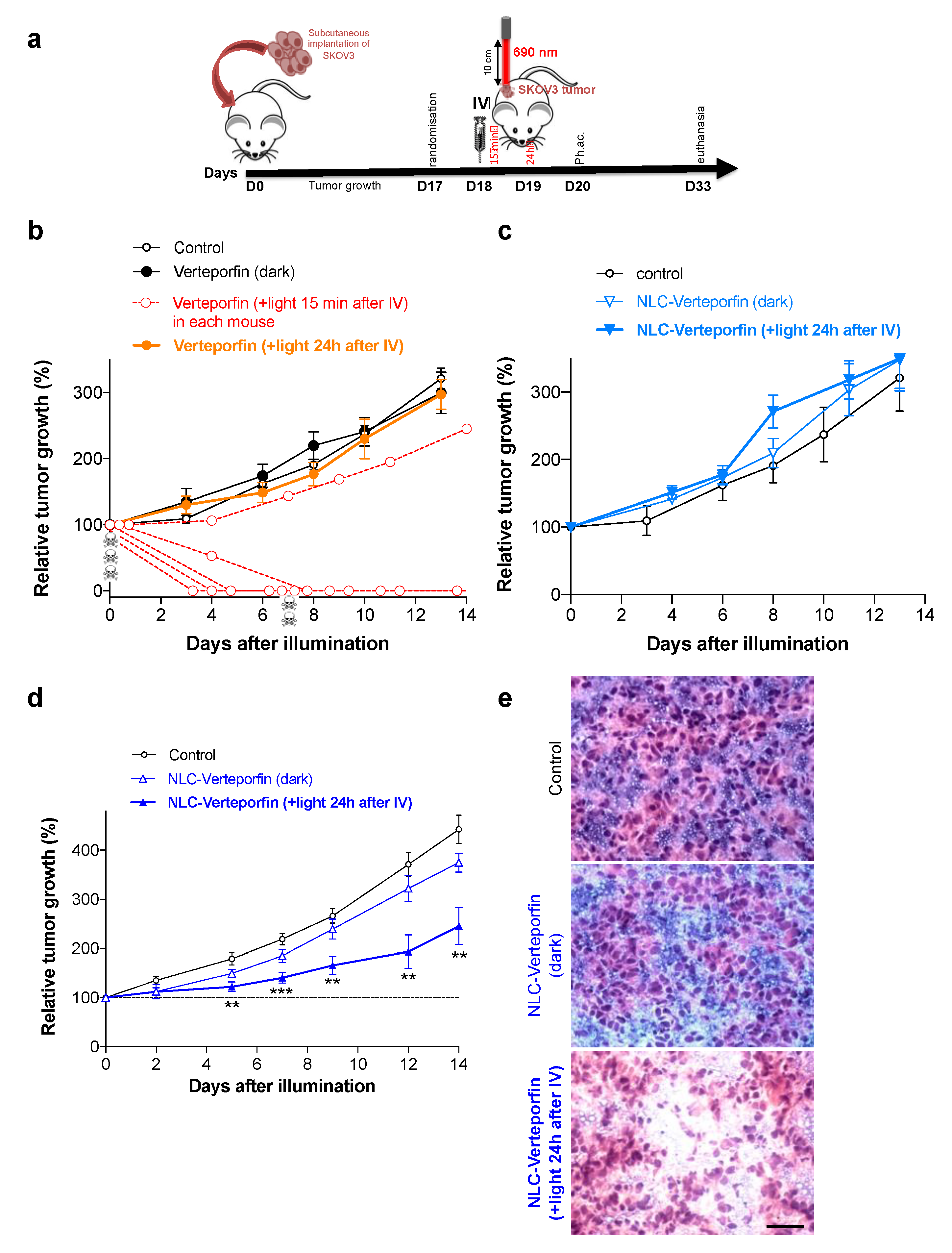
2.5. NLC-Verteporfin Improves PDT, Free Verteporfin is Highly Toxic In Vivo
2.6. NLC Accumulate in Disseminated Ovarian Tumor Nodules
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation of Nanostructured Lipid Carriers
4.3. Dynamic Light Scattering
4.4. HPLC Analyses
4.5. Absorbance and Fluorescence Spectrum
4.6. Cell Lines and Culture
4.6.1. Two-Dimensions (2D) Cell Culture
4.6.2. Three-Dimensions (3D) Cell Culture
4.7. Flow Cytometry
4.8. Fluorescence Microscopy
4.9. In Vitro Cytotoxicity Assays
4.10. In Vivo Experiments
4.10.1. Subcutaneous Ovarian Tumor Model
4.10.2. Pharmacokinetics Studies on Blood Plasma Samples
4.10.3. Biodistribution of Fluorescent NLC In Vivo
4.10.4. Orthotopic Murine Model of Peritoneal Carcinomatosis from Ovarian Cancer
4.10.5. PDT In Vivo
4.10.6. Photoacoustic Imaging
4.10.7. Histology
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Almerie, M.Q.; Gossedge, G.; Wright, K.E.; Jayne, D.G. Treatment of peritoneal carcinomatosis with photodynamic therapy: Systematic review of current evidence. Photodiagnosis Photodyn. Ther. 2017, 20, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Demkow, U. Immunological aspects of antitumor photodynamic therapy outcome. Cent. Eur. J. Immunol. 2015, 40, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, e1560. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Du, S.; Gao, P.; Zheng, J. Verteporfin suppresses the proliferation, epithelial-mesenchymal transition and stemness of head and neck squamous carcinoma cells via inhibiting YAP1. J. Cancer 2019, 10, 4196–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akens, M.K.; Hardisty, M.R.; Wilson, B.C.; Schwock, J.; Whyne, C.M.; Burch, S.; Yee, A.J. Defining the therapeutic window of vertebral photodynamic therapy in a murine pre-clinical model of breast cancer metastasis using the photosensitizer BPD-MA (Verteporfin). Breast Cancer Res. Treat. 2010, 119, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Tonello, S.; Estevao, B.M.; Gianotti, E.; Marchese, L.; Reno, F. Verteporfin based silica nanoparticle for in vitro selective inhibition of human highly invasive melanoma cell proliferation. J. Photochem. Photobiol. B 2017, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Miletto, I.; Gianotti, E.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Reno, F. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J. Photochem. Photobiol. B 2019, 197, 111533. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.L.; Gravier, J.; Henry, M.; Sancey, L.; Bejaud, J.; Pancani, E.; Wiber, M.; Texier, I.; Coll, J.L.; Benoit, J.P.; et al. Conventional versus stealth lipid nanoparticles: Formulation and in vivo fate prediction through FRET monitoring. J. Control. Release 2014, 188, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsjarvi, S.; Dufort, S.; Gravier, J.; Texier, I.; Yan, Q.; Bibette, J.; Sancey, L.; Josserand, V.; Passirani, C.; Benoit, J.P.; et al. Influence of size, surface coating and fine chemical composition on the in vitro reactivity and in vivo biodistribution of lipid nanocapsules versus lipid nanoemulsions in cancer models. Nanomedicine 2013, 9, 375–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, F.P.; Creusat, G.; Frochot, C.; Moussaron, A.; Verhille, M.; Vanderesse, R.; Thomann, J.S.; Boisseau, P.; Texier, I.; Couffin, A.C.; et al. Preparation and characterization of mTHPC-loaded solid lipid nanoparticles for photodynamic therapy. J. Photochem. Photobiol. B 2014, 130, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Advanced cell culture techniques for cancer drug discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Gravier, J.; Navarro, F.P.; Delmas, T.; Mittler, F.; Couffin, A.C.; Vinet, F.; Texier, I. Lipidots: Competitive organic alternative to quantum dots for in vivo fluorescence imaging. J. Biomed. Opt. 2011, 16, 096013. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Vollaire, J.; Baulin, V.; Werner, M.; Orlowska, A.; Josserand, V.; Mahuteau-Betzer, F.; et al. The pyrrolopyrimidine colchicine-binding site agent PP-13 reduces the metastatic dissemination of invasive cancer cells in vitro and in vivo. Biochem. Pharmacol. 2019, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Couvet, M.; Coll, J.L. Unsuccessful mitosis in multicellular tumour spheroids. Oncotarget 2017, 8, 28769–28784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmalanandhan, V.S.; Duren, A.; Hendricks, P.; Vielhauer, G.; Sittampalam, G.S. Activity of anticancer agents in a three-dimensional cell culture model. Assay Drug Dev. Technol. 2010, 8, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Nath, S.; Obaid, G.; Ruhi, M.K.; Moore, K.; Bano, S.; Kessel, D.; Hasan, T. A Combination of Visudyne and a Lipid-anchored Liposomal Formulation of Benzoporphyrin Derivative Enhances Photodynamic Therapy Efficacy in a 3D Model for Ovarian Cancer. Photochem. Photobiol. 2019, 95, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.S.; Obeidin, V.A.; Yigitbasi, T.; Tumer, S.S.; Yigit, P. Verteporfin mediated sequence dependent combination therapy against ovarian cancer cell line. J. Photochem. Photobiol. B 2018, 183, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Battaglia Parodi, M.; La Spina, C.; Berchicci, L.; Petruzzi, G.; Bandello, F. Photosensitizers and photodynamic therapy: Verteporfin. Dev. Ophthalmol. 2016, 55, 330–336. [Google Scholar] [CrossRef]
- Gravier, J.; Sancey, L.; Hirsjarvi, S.; Rustique, E.; Passirani, C.; Benoit, J.P.; Coll, J.L.; Texier, I. FRET imaging approaches for in vitro and in vivo characterization of synthetic lipid nanoparticles. Mol. Pharm. 2014, 11, 3133–3144. [Google Scholar] [CrossRef] [PubMed]
- Sayag, D.; Cabon, Q.; Texier, I.; Navarro, F.P.; Boisgard, R.; Virieux-Watrelot, D.; Carozzo, C.; Ponce, F. Phase-0/phase-I study of dye-loaded lipid nanoparticles for near-infrared fluorescence imaging in healthy dogs. Eur. J. Pharm. Biopharm. 2016, 100, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Pellosi, D.S.; Paula, L.B.; de Melo, M.T.; Tedesco, A.C. Targeted and Synergic Glioblastoma Treatment: Multifunctional Nanoparticles Delivering Verteporfin as Adjuvant Therapy for Temozolomide Chemotherapy. Mol. Pharm. 2019, 16, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Bazylinska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, M.; Pei, Y.; Huang, Y.; Chen, Y.; Ma, F.; Lu, H.; Chen, J. Core-matched nanoassemblies for targeted co-delivery of chemotherapy and photosensitizer to treat drug-resistant cancer. Acta Biomater. 2019, 88, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Fraker, D.L.; Mick, R.; Metz, J.; Busch, T.M.; Smith, D.; Zhu, T.; Rodriguez, C.; Dimofte, A.; Spitz, F.; et al. A phase II trial of intraperitoneal photodynamic therapy for patients with peritoneal carcinomatosis and sarcomatosis. Clin. Cancer Res. 2006, 12, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Miller, A.; Singh, A.K.; Seshadri, M. Photoacoustic Imaging as an Early Biomarker of Radio Therapeutic Efficacy in Head and Neck Cancer. Theranostics 2018, 8, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Fujwara, K.; Yoshino, K.; Enomoto, T.; Fujita, M.; Ueda, Y.; Miyatake, T.; Kimura, T.; Muraji, M.; Fujita, H.; Kimura, T.; et al. Usefulness of computed tomography in predicting cytoreductive surgical outcomes for ovarian cancer. Arch. Gynecol. Obstet. 2011, 284, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Glaser, G.; Torres, M.; Kim, B.; Aletti, G.; Weaver, A.; Mariani, A.; Hartmann, L.; Cliby, W. The use of CT findings to predict extent of tumor at primary surgery for ovarian cancer. Gynecol. Oncol. 2013, 130, 280–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquart, A.; Keramidas, M.; Vollaire, J.; Boisgard, R.; Pottier, G.; Rustique, E.; Mittler, F.; Navarro, F.P.; Boutet, J.; Coll, J.L.; et al. LipImage 815: Novel dye-loaded lipid nanoparticles for long-term and sensitive in vivo near-infrared fluorescence imaging. J. Biomed. Opt. 2013, 18, 101311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ramakrishnan, S.K.; Triner, D.; Centofanti, B.; Maitra, D.; Gyorffy, B.; Sebolt-Leopold, J.S.; Dame, M.K.; Varani, J.; Brenner, D.E.; et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci. Signal 2015, 8, ra98. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Mazzaferro, S.; Lavaud, J.; Vanwonterghem, L.; Henry, M.; Arboleas, M.; Vollaire, J.; Josserand, V.; Coll, J.L.; Lecommandoux, S.; et al. Targeting CD44 receptor-positive lung tumors using polysaccharide-based nanocarriers: Influence of nanoparticle size and administration route. Nanomedicine 2016, 12, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, J.; Henry, M.; Coll, J.L.; Josserand, V. Exploration of melanoma metastases in mice brains using endogenous contrast photoacoustic imaging. Int. J. Pharm. 2017, 532, 704–709. [Google Scholar] [CrossRef] [PubMed]


| Properties | NLC-Verteporfin | NLC-LipImageTM815 | |
|---|---|---|---|
| T0 | T0 + 3 Months | ||
| Hydrodynamic diameter (nm) a | 47.9 ± 1.0 | 54.4 ± 0.6 | 46.1 ± 0.7 |
| Polydispersity index a | 0.12 ± 0.02 | 0.18 ± 0.01 | 0.13 ± 0.01 |
| Zeta potential (mV) a | −3.7 ± 0.9 | −2.0 ± 1.3 | −4.2 ± 4.3 |
| Verteporfin (µg/mL) b | 1026.2 ± 15.6 | 1037.5 ± 0.7 | / |
| Verteporfin concentration (µM) b | 1428 | 1443 | / |
| LipiImageTM815 concentration (µM) | / | / | 302 |
| Cell Lines | Culture Conditions | Verteporfin IC50 (nmol·L−1) | NLC-Verteporfin IC50 (nmol·L−1) | |
|---|---|---|---|---|
| SKOV3 | 2D | 2 h | 17.8 ± 0.9 | 7.3 ± 0.4 |
| 24 h | 23.8 ± 0.9 | 8.8 ± 0.5 | ||
| 3D | 2 h | 41.2 ± 4.2 | 29.3 ± 7.1 | |
| 24 h | 9.3 ± 0.7 | 9.7 ± 1.1 | ||
| OVCAR3 | 2D | 2 h # | 117.5 ± 14.4 | 116.4 ± 22.7 |
| 24 h | 94.9 ± 15.4 | 97.5 ± 17.4 | ||
| 3D | 2 h | 28.9 ± 0.8 | 36.6 ± 3.2 | |
| 24 h | 9.7 ± 1.0 | 5.3 ± 0.5 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michy, T.; Massias, T.; Bernard, C.; Vanwonterghem, L.; Henry, M.; Guidetti, M.; Royal, G.; Coll, J.-L.; Texier, I.; Josserand, V.; et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers 2019, 11, 1760. https://doi.org/10.3390/cancers11111760
Michy T, Massias T, Bernard C, Vanwonterghem L, Henry M, Guidetti M, Royal G, Coll J-L, Texier I, Josserand V, et al. Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers. 2019; 11(11):1760. https://doi.org/10.3390/cancers11111760
Chicago/Turabian StyleMichy, Thierry, Thibault Massias, Claire Bernard, Laetitia Vanwonterghem, Maxime Henry, Mélanie Guidetti, Guy Royal, Jean-Luc Coll, Isabelle Texier, Véronique Josserand, and et al. 2019. "Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo" Cancers 11, no. 11: 1760. https://doi.org/10.3390/cancers11111760
APA StyleMichy, T., Massias, T., Bernard, C., Vanwonterghem, L., Henry, M., Guidetti, M., Royal, G., Coll, J.-L., Texier, I., Josserand, V., & Hurbin, A. (2019). Verteporfin-Loaded Lipid Nanoparticles Improve Ovarian Cancer Photodynamic Therapy In Vitro and In Vivo. Cancers, 11(11), 1760. https://doi.org/10.3390/cancers11111760






