Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics
Abstract
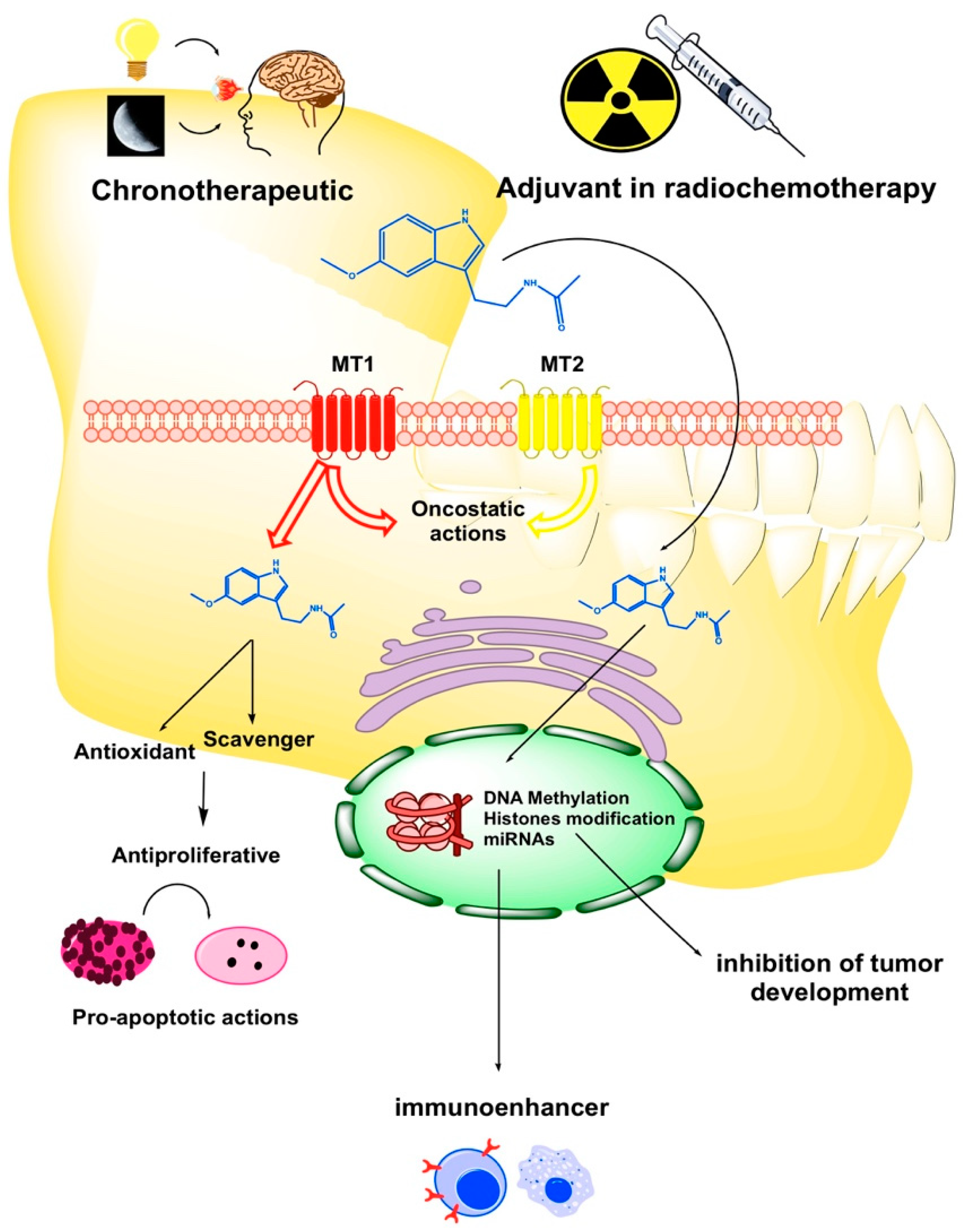
:1. Introduction
2. Epidemiology of Oral Cancer
3. The Role of Melatonin in the Oral Cavity: Functionality and Alterations in Oral Cancer
4. Responses Mediated by Melatonin Receptors in Oral Cancer
5. Melatonin in Combination with Conventional Treatment for Oral Cancer and Safety Profile
6. Epigenetic Regulation of Melatonin in Oral Cancer
6.1. Epigenetic Methylation of DNA
6.2. Epigenetic Modification of Chromatin Structure
6.3. Non-Coding micro-RNAs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Boyle, G.M.; Porceddu, S.; Theile, D.R.; Parsons, P.G.; Coman, W.B. Head and neck cancer: Past, present and future. Expert Rev. Anticancer Ther. 2006, 6, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Van der Waal, I.; de Bree, R.; Brakenhoff, R.; Coebergh, J.W. Early diagnosis in primary oral cancer: Is it possible? Med. Oral Patol. Oral Cir. Bucal 2011, 16, e300–e305. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Razzouk, S. Translational genomics and head and neck cancer: Toward precision medicine. Clin. Genet. 2014, 86, 412–421. [Google Scholar] [CrossRef]
- Viet, C.T.; Schmidt, B.L. Understanding oral cancer in the genome era. Head Neck 2010, 32, 1246–1268. [Google Scholar] [CrossRef]
- Li, C.C.; Shen, Z.; Bavarian, R.; Yang, F.; Bhattacharya, A. Oral cancer: Genetics and the role of precision medicine. Dent. Clin. N. Am. 2018, 62, 29–46. [Google Scholar] [CrossRef]
- Lo, W.L.; Kao, S.Y.; Chi, L.Y.; Wong, Y.K.; Chang, R.C. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: Factors affecting survival. J. Oral Maxillofac. Surg. 2003, 61, 751–758. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Wei, D.; Yap, Y.; Chen, F. Moving cancer diagnostics from bench to bedside. Trends Biotechnol. 2007, 25, 166–173. [Google Scholar] [CrossRef]
- Cutando, A.; Gomez-Moreno, G.; Arana, C.; Acuna-Castroviejo, D.; Reiter, R.J. Melatonin: Potential functions in the oral cavity. J. Periodontol. 2007, 78, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.L.; Porkka-Heiskanen, T.; Alila, A.; Stenberg, D.; Johansson, G. Correlation between salivary and serum melatonin: Dependence on serum melatonin levels. J. Pineal Res. 1990, 9, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Praninskiene, R.; Dumalakiene, I.; Kemezys, R.; Mauricas, M.; Jucaite, A. Diurnal melatonin patterns in children: Ready to apply in clinical practice? Pediatr. Neurol. 2012, 46, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Merolla, F.; Varricchio, S.; Salzano, G.; Zarrilli, G.; Mascolo, M.; Strazzullo, V.; Di Crescenzo, R.M.; Celetti, A.; Ilardi, G. Epigenetics of oral and oropharyngeal cancers. Biomed. Rep. 2018, 9, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Uttamani, J.R.; Naqvi, A.R.; Nares, S. microRNAs: Emerging players in oral cancers and inflammatory disorders. Tumour Biol. 2017, 39, 1010428317698379. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Q.; Guerra-Librero, A.; Fernández-Gil, B.I.; Florido, J.; Garcia-Lopez, S.; Martínez-Ruiz, L.; Mendivil-Perez, M.; Soto-Mercado, V.; Acuna-Castroviejo, D.; Ortega-Arellano, H.; et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2018, 64, e12461. [Google Scholar] [CrossRef]
- Fernández-Gil, B.I.; Guerra-Librero, A.; Shen, Y.Q.; Florido, J.; Martínez-Ruiz, L.; García-López, S.; Adan, C.; Rodríguez-Santana, C.; Acuna-Castroviejo, D.; Quinones-Hinojosa, A.; et al. Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ROS generation, apoptosis, and autophagy. Oxid. Med. Cell Longev. 2019, 2019, 7187128. [Google Scholar] [CrossRef]
- Bondy, S.C.; Campbell, A. Mechanisms underlying tumor suppressive properties of melatonin. Int. J. Mol. Sci. 2018, 19, 2205. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Liu, X.Y.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X. Melatonin in the oral cavity: Physiological and pathological implications. J. Periodontal Res. 2015, 50, 9–17. [Google Scholar] [CrossRef]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; La Vecchia, C.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef]
- Gatta, G.; Botta, L.; Sanchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Group, E.W. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- D’Souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Sauvaget, C.; de Camargo Cancela, M.; Sankaranarayanan, R. Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 2011, 23, 633–641. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- D’Souza, G.; Anantharaman, D.; Gheit, T.; Abedi-Ardekani, B.; Beachler, D.C.; Conway, D.I.; Olshan, A.F.; Wunsch-Filho, V.; Toporcov, T.N.; Ahrens, W.; et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral Oncol. 2016, 62, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Monteiro, L.S.; Amaral, J.B.; Vizcaino, J.R.; Lopes, C.A.; Torres, F.O. A clinical-pathological and survival study of oral squamous cell carcinomas from a population of the North of Portugal. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e120–e126. [Google Scholar] [CrossRef] [Green Version]
- Weijers, M.; Leemans, C.R.; Aartman, I.H.; Karagozoglu, K.H.; van der Waal, I. Oral cancer trends in a single head-and-neck cancer center in the Netherlands; decline in T-stage at the time of admission. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e914–e918. [Google Scholar] [CrossRef]
- Tiwana, M.S.; Wu, J.; Hay, J.; Wong, F.; Cheung, W.; Olson, R.A. 25 year survival outcomes for squamous cell carcinomas of the head and neck: Population-based outcomes from a Canadian province. Oral Oncol. 2014, 50, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Wu, C.C.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Locoregionally recurrent head and neck squamous cell carcinoma: Incidence, survival, prognostic factors, and treatment outcomes. Oncotarget 2017, 8, 55600–55612. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.L.; Kligerman, J.; Matos de Sa, G.; Arcuri, R.A.; Freitas, E.Q.; Farias, T.; Matos, F.; Lima, R.A. Elective neck dissection versus observation in stage I squamous cell carcinomas of the tongue and floor of the mouth. Otolaryngol. Head Neck Surg. 2001, 125, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Rinaldo, A.; Robbins, K.T.; Leemans, C.R.; Shah, J.P.; Shaha, A.R.; Andersen, P.E.; Kowalski, L.P.; Pellitteri, P.K.; Clayman, G.L.; et al. Changing concepts in the surgical management of the cervical node metastasis. Oral Oncol. 2003, 39, 429–435. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Dhar, H. Oral cancers: Current status. Oral Oncol. 2018, 87, 64–69. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Gil-Martin, E.; Egea, J.; Reiter, R.J.; Romero, A. The emergence of melatonin in oncology: Focus on colorectal cancer. Med. Res. Rev. 2019. [Google Scholar] [CrossRef]
- Zare, H.; Shafabakhsh, R.; Reiter, R.J.; Asemi, Z. Melatonin is a potential inhibitor of ovarian cancer: Molecular aspects. J. Ovarian Res. 2019, 12, 26. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.L.; Deng, M.J.; Wen, X.J.; Mo, Y.Y.; Chen, F.M.; Zou, C.L.; Duan, W.F.; Li, L.; Nie, X. Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral cancer. Oxid. Med. Cell Longev. 2018, 2018, 3510970. [Google Scholar] [CrossRef]
- Gonçalves Ndo, N.; Rodrigues, R.V.; Jardim-Perassi, B.V.; Moschetta, M.G.; Lopes, J.R.; Colombo, J.; Zuccari, D.A. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med. Chem. 2014, 14, 1302–1311. [Google Scholar] [CrossRef]
- Cutando, A.; López-Valverde, A.; De Vicente, J.; Gimenez, J.L.; CarcÍa, I.A.; De Diego, R.G. Action of melatonin on squamous cell carcinoma and other tumors of the oral cavity (Review). Oncol. Lett. 2014, 7, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.W.; Zheng, Z.Q.; Wang, Z.X.; Zhou, G.Q.; Chen, L.; Mao, Y.P.; Lin, A.H.; Reiter, R.J.; Ma, J.; Chen, Y.P.; et al. Pan-cancer genomic analyses reveal prognostic and immunogenic features of the tumor melatonergic microenvironment across 14 solid cancer types. J. Pineal Res. 2019, 66, e12557. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Kaur, G. Potential role of melatonin in prevention and treatment of oral carcinoma. Indian J. Dent. 2014, 5, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Isola, M.; Lilliu, M.A. Melatonin localization in human salivary glands. J. Oral Pathol. Med. 2016, 45, 510–515. [Google Scholar] [CrossRef]
- Cengiz, M.I.; Cengiz, S.; Wang, H.L. Melatonin and oral cavity. Int. J. Dent. 2012, 2012, 491872. [Google Scholar] [CrossRef]
- Shimozuma, M.; Tokuyama, R.; Tatehara, S.; Umeki, H.; Ide, S.; Mishima, K.; Saito, I.; Satomura, K. Expression and cellular localizaion of melatonin-synthesizing enzymes in rat and human salivary glands. Histochem. Cell Biol. 2011, 135, 389–396. [Google Scholar] [CrossRef]
- Lin, F.Y.; Lin, C.W.; Yang, S.F.; Lee, W.J.; Lin, Y.W.; Lee, L.M.; Chang, J.L.; Weng, W.C.; Lin, C.H.; Chien, M.H. Interactions between environmental factors and melatonin receptor type 1A polymorphism in relation to oral cancer susceptibility and clinicopathologic development. PLoS ONE 2015, 10, e0121677. [Google Scholar] [CrossRef]
- Nakamura, E.; Kozaki, K.; Tsuda, H.; Suzuki, E.; Pimkhaokham, A.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Amagasa, T.; Inazawa, J.; et al. Frequent silencing of a putative tumor suppressor gene melatonin receptor 1 A (MTNR1A) in oral squamous-cell carcinoma. Cancer Sci. 2008, 99, 1390–1400. [Google Scholar] [CrossRef]
- Cutando, A.; Aneiros-Fernandez, J.; Lopez-Valverde, A.; Arias-Santiago, S.; Aneiros-Cachaza, J.; Reiter, R.J. A new perspective in oral health: Potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. Arch. Oral Biol. 2011, 56, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Pi, H.; Li, M.; Ren, Z.; He, Z.; Zhu, F.; Tian, L.; Tu, M.; Xie, J.; Liu, M.; et al. Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis in tongue squamous cell carcinoma. J. Pineal Res. 2018, 64, e12457. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, F.; Acuna-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; López, L.C.; Venegas, C.; García, J.A.; López, A.; Volt, H.; Luna-Sánchez, M.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2015, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Foley, H.M.; Steel, A.E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 2019, 42, 65–81. [Google Scholar] [CrossRef]
- William, W.N., Jr. Oral premalignant lesions: Any progress with systemic therapies? Curr. Opin. Oncol. 2012, 24, 205–210. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Mandala, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef]
- Lu, Y.X.; Chen, D.L.; Wang, D.S.; Chen, L.Z.; Mo, H.Y.; Sheng, H.; Bai, L.; Wu, Q.N.; Yu, H.E.; Xie, D.; et al. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of Erk and Akt pathway. Cell Death Dis. 2016, 7, e2432. [Google Scholar] [CrossRef]
- Onseng, K.; Johns, N.P.; Khuayjarernpanishk, T.; Subongkot, S.; Priprem, A.; Hurst, C.; Johns, J. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J. Altern. Complement. Med. 2017, 23, 957–963. [Google Scholar] [CrossRef]
- Ha, P.K.; Califano, J.A. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006, 7, 77–82. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenetics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, M.J.; Hong, S.P.; Hong, S.D. Inactivation patterns of p16/INK4A in oral squamous cell carcinomas. Exp. Mol. Med. 2004, 36, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Uzawa, K.; Endo, Y.; Kato, Y.; Nakashima, D.; Ogawara, K.; Shiba, M.; Bukawa, H.; Yokoe, H.; Tanzawa, H. Plasma membrane Ca2+ ATPase isoform 1 down-regulated in human oral cancer. Oncol. Rep. 2006, 15, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R. The epigenetics of oral cancer. Int. J. Oral Maxillofac. Surg. 2006, 35, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.M.; Mydlarz, W.K.; Mithani, S.K.; Califano, J.A. DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. Int. J. Cancer 2007, 121, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, Y.; Schussel, J.L.; Brait, M. Tobacco and alcohol-induced epigenetic changes in oral carcinoma. Curr. Opin. Oncol. 2018, 30, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Liloglou, T.; Rogers, S.N.; Brown, J.S.; Vaughan, E.D.; Lowe, D.; Field, J.K.; Risk, J.M. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: Quantitative evaluation using pyrosequencing. Br. J. Cancer 2006, 94, 561–568. [Google Scholar] [CrossRef]
- Shaw, R.J.; Hall, G.L.; Lowe, D.; Bowers, N.L.; Liloglou, T.; Field, J.K.; Woolgar, J.A.; Risk, J.M. CpG island methylation phenotype (CIMP) in oral cancer: Associated with a marked inflammatory response and less aggressive tumour biology. Oral Oncol. 2007, 43, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Liu, Y.; Wang, K.; He, Z.; Gong, Z.; Zhao, Z.; Yang, Y.; Gao, X.; Li, F.; Wu, H.; et al. Biomarkers: Paving stones on the road towards the personalized precision medicine for oral squamous cell carcinoma. BMC Cancer 2018, 18, 911. [Google Scholar] [CrossRef]
- Morandi, L.; Gissi, D.; Tarsitano, A.; Asioli, S.; Gabusi, A.; Marchetti, C.; Montebugnoli, L.; Foschini, M.P. CpG location and methylation level are crucial factors for the early detection of oral squamous cell carcinoma in brushing samples using bisulfite sequencing of a 13-gene panel. Clin. Epigenet. 2017, 9, 85. [Google Scholar] [CrossRef]
- Bai, G.; Song, J.; Yuan, Y.; Chen, Z.; Tian, Y.; Yin, X.; Niu, Y.; Liu, J. Systematic analysis of differentially methylated expressed genes and site-speci fi c methylation as potential prognostic markers in head and neck cancer. J. Cell Physiol. 2019, 234, 22687–22702. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, M.; Ni, S.; Li, Q.; Ye, D.; Li, J.; Shen, Z.; Deng, H. DNA methylation biomarkers for head and neck squamous cell carcinoma. Epigenetics 2018, 13, 398–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arayataweegool, A.; Srisuttee, R.; Mahattanasakul, P.; Tangjaturonsasme, N.; Kerekhanjanarong, V.; Kitkumthorn, N.; Mutirangura, A. Head and neck squamous cell carcinoma drives long interspersed element-1 hypomethylation in the peripheral blood mononuclear cells. Oral Dis. 2019, 25, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Haim, A.; Zubidat, A.E. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140121. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Jud, C.; Munch, M.; Kobialka, S.; Wirz-Justice, A.; Albrecht, U. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur. J. Neurosci. 2006, 23, 1082–1086. [Google Scholar] [CrossRef]
- Rana, S.; Mahmood, S. Circadian rhythm and its role in malignancy. J. Circadian Rhythm. 2010, 8, 3. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef]
- Schwimmer, H.; Metzer, A.; Pilosof, Y.; Szyf, M.; Machnes, Z.M.; Fares, F.; Harel, O.; Haim, A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 2014, 31, 144–150. [Google Scholar] [CrossRef]
- Zubidat, A.E.; Fares, B.; Fares, F.; Haim, A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: Interaction with melatonin and epigenetic pathways. Cancer Control. 2018, 25, 1073274818812908. [Google Scholar] [CrossRef]
- Agbaria, S.; Haim, A.; Fares, F.; Zubidat, A.E. Epigenetic modification in 4T1 mouse breast cancer model by artificial light at night and melatonin—the role of DNA-methyltransferase. Chronobiol. Int. 2019, 36, 629–643. [Google Scholar] [CrossRef]
- Smiraglia, D.J.; Smith, L.T.; Lang, J.C.; Rush, L.J.; Dai, Z.; Schuller, D.E.; Plass, C. Differential targets of CpG island hypermethylation in primary and metastatic head and neck squamous cell carcinoma (HNSCC). J. Med. Genet. 2003, 40, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Von Zeidler, S.V.; Miracca, E.C.; Nagai, M.A.; Birman, E.G. Hypermethylation of the p16 gene in normal oral mucosa of smokers. Int. J. Mol. Med. 2004, 14, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Mallery, S.R.; Knobloch, T.J.; Song, H.; Lloyd, M.; Casto, B.C.; Weghorst, C.M. Alterations of p16(INK4a) and p14(ARF) in patients with severe oral epithelial dysplasia. Cancer Res. 2002, 62, 5295–5300. [Google Scholar] [PubMed]
- Kulkarni, V.; Saranath, D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004, 40, 145–153. [Google Scholar] [CrossRef]
- Eberharter, A.; Becker, P.B. Histone acetylation: A switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002, 3, 224–229. [Google Scholar] [CrossRef]
- Webber, L.P.; Wagner, V.P.; Curra, M.; Vargas, P.A.; Meurer, L.; Carrard, V.C.; Squarize, C.H.; Castilho, R.M.; Martins, M.D. Hypoacetylation of acetyl-histone H3 (H3K9ac) as marker of poor prognosis in oral cancer. Histopathology 2017, 71, 278–286. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Qi, B.; Zhang, W.; Cheng, J.; Wang, Y. High expression of the histone demethylase LSD1 associates with cancer cell proliferation and unfavorable prognosis in tongue cancer. J. Oral Pathol. Med. 2015, 44, 159–165. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lin, C.K.; Tsao, C.H.; Hsieh, C.C.; Lin, G.J.; Ma, K.H.; Shieh, Y.S.; Sytwu, H.K.; Chen, Y.W. Melatonin exerts anti-oral cancer effect via suppressing LSD1 in patient-derived tumor xenograft models. Oncotarget 2017, 8, 33756–33769. [Google Scholar] [CrossRef]
- Chung, Y.L.; Lee, M.Y.; Pui, N.N. Epigenetic therapy using the histone deacetylase inhibitor for increasing therapeutic gain in oral cancer: Prevention of radiation-induced oral mucositis and inhibition of chemical-induced oral carcinogenesis. Carcinogenesis 2009, 30, 1387–1397. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E.; Guerra-Librero, A.; Florido, J.; Shen, Y.Q.; Fernández-Gil, B.; Acuna-Castroviejo, D.; Escames, G. Oral mucositis: Melatonin gel an effective new treatment. Int. J. Mol. Sci. 2017, 18, 1003. [Google Scholar] [CrossRef]
- Yeh, C.M.; Lin, C.W.; Yang, J.S.; Yang, W.E.; Su, S.C.; Yang, S.F. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget 2016, 7, 21952–21967. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.Y.; Lin, C.W.; Chien, M.H.; Reiter, R.J.; Su, S.C.; Hsieh, Y.H.; Yang, S.F. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J. Pineal Res. 2016, 61, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhausser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Wright, A.; Wood, H.; Rabbitts, P. MicroRNAs and head and neck cancer: Reviewing the first decade of research. Eur. J. Cancer 2014, 50, 2619–2635. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Miloro, M.; Zhou, X. Review of microRNA proposed target genes in oral cancer. Part II. J. Oral Maxillofac. Res. 2011, 2, e2. [Google Scholar] [CrossRef]
- Manasa, V.G.; Kannan, S. Impact of microRNA dynamics on cancer hallmarks: An oral cancer scenario. Tumour Biol. 2017, 39, 1010428317695920. [Google Scholar] [CrossRef]
- Schneider, A.; Victoria, B.; Lopez, Y.N.; Suchorska, W.; Barczak, W.; Sobecka, A.; Golusinski, W.; Masternak, M.M.; Golusinski, P. Tissue and serum microRNA profile of oral squamous cell carcinoma patients. Sci. Rep. 2018, 8, 675. [Google Scholar] [CrossRef]
- Min, A.; Zhu, C.; Peng, S.; Rajthala, S.; Costea, D.E.; Sapkota, D. MicroRNAs as important players and biomarkers in oral carcinogenesis. Biomed. Res. Int. 2015, 2015, 186904. [Google Scholar] [CrossRef]
- Lin, S.C.; Liu, C.J.; Lin, J.A.; Chiang, W.F.; Hung, P.S.; Chang, K.W. miR-24 up-regulation in oral carcinoma: Positive association from clinical and in vitro analysis. Oral Oncol. 2010, 46, 204–208. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, S.J.; Youn, J.P.; Hwang, S.Y.; Park, C.S.; Park, Y.S. MicroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. J. Pineal Res. 2011, 51, 345–352. [Google Scholar] [CrossRef]
- Mori, F.; Ferraiuolo, M.; Santoro, R.; Sacconi, A.; Goeman, F.; Pallocca, M.; Pulito, C.; Korita, E.; Fanciulli, M.; Muti, P.; et al. Multitargeting activity of miR-24 inhibits long-term melatonin anticancer effects. Oncotarget 2016, 7, 20532–20548. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Kim, S.J.; Yoon, H.J.; Yu, S.Y.; Yang, H.; Jeong, S.I.; Hwang, S.Y.; Park, C.S.; Park, Y.S. Genome-wide profiling in melatonin-exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J. Pineal Res. 2013, 54, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hunsaker, M.; Barba, G.; Kingsley, K.; Howard, K.M. Differential microRNA expression of miR-21 and miR-155 within oral cancer extracellular vesicles in response to melatonin. Dent. J. 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Avissar, M.; Christensen, B.C.; Kelsey, K.T.; Marsit, C.J. MicroRNA expression ratio is predictive of head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Lamperska, K.M.; Kozlowski, P.; Kolenda, T.; Teresiak, A.; Blizniak, R.; Przybyla, W.; Masternak, M.M.; Golusinski, P.; Golusinski, W. Unpredictable changes of selected miRNA in expression profile of HNSCC. Cancer Biomark. 2016, 16, 55–64. [Google Scholar] [CrossRef]
- Cervigne, N.K.; Reis, P.P.; Machado, J.; Sadikovic, B.; Bradley, G.; Galloni, N.N.; Pintilie, M.; Jurisica, I.; Perez-Ordonez, B.; Gilbert, R.; et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum. Mol. Genet. 2009, 18, 4818–4829. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Chen, Z.; Cabay, R.J.; Zhang, L.; Luan, X.; Chen, D.; Yu, T.; Wang, A.; Zhou, X. microRNA-21 and microRNA-375 from oral cytology as biomarkers for oral tongue cancer detection. Oral Oncol. 2016, 57, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Zeljic, K.; Jovanovic, I.; Jovanovic, J.; Magic, Z.; Stankovic, A.; Supic, G. MicroRNA meta-signature of oral cancer: Evidence from a meta-analysis. Upsala J. Med. Sci. 2018, 123, 43–49. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Luo, Z.Q.; Zhang, L.J.; Li, J.; Liu, J.Q. Integrated analysis and microRNA expression profiling identified seven miRNAs associated with progression of oral squamous cell carcinoma. J. Cell. Physiol. 2017, 232, 2178–2185. [Google Scholar] [CrossRef]
- Gissi, D.B.; Morandi, L.; Gabusi, A.; Tarsitano, A.; Marchetti, C.; Cura, F.; Palmieri, A.; Montebugnoli, L.; Asioli, S.; Foschini, M.P.; et al. A noninvasive test for microRNA expression in oral squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 1789. [Google Scholar] [CrossRef]
- Shah, S.; Jadhav, K.; Shah, V.; Gupta, N.; Dagrus, K. miRNA 21: Diagnostic prognostic and therapeutic marker for oral cancer. Microrna 2016, 5, 175–179. [Google Scholar] [CrossRef]
- Yap, T.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; Seers, C.; McCullough, M. Predicting the presence of oral squamous cell carcinoma using commonly dysregulated MicroRNA in oral swirls. Cancer Prev. Res. 2018, 11, 491–502. [Google Scholar] [CrossRef] [PubMed]
| Model | Melatonin Combined with Other Drugs | Effect | Reference | |
|---|---|---|---|---|
| Cal 27 and SCC-9 human squamous cell carcinoma lines | MLT (0.1, 0.5 or 1 mM) plus rapamycin (20 nM) | Enhanced cytotoxic effects of rapamycin in HNSCC cells | [16] | |
| In vitro studies | Cal 27 and SCC-9 human squamous cell carcinoma lines | MLT (0.1, 0.5, 1 and 5 mM) combined with 8 Gy irradiation and 10 µM Cisplatin | Improved effectiveness of chemo- and radiotherapy | [17] |
| OSCC cells | MLT (0,5 and 5 mM) combined with 5-FU (1 and 10 µM) | Potentiated cytotoxicity of 5-FU | [57] | |
| Male Wistar rats | 7.5 Gy to oral mucosa for 5 days plus 3% MLT gel for 21 days post-irradiation | Inhibited radiotherapy-induced mucositis | [53] | |
| In vivo studies | OSCC-xenografted mice | MLT (20 mg/kg/day) combined with 5-FU (20 mg/kg, twice per week) for 4 weeks | Inhibited OSCC tumor growth | [57] |
| Cal 27 cells -xenografted rats | Rapamycin (1 mg/kg i.p.) for 10 days plus injection of MLT (300 mg/kg s.c.) 1 day before each rapamycin administration | Ameliorated the toxicity of rapamycin in normal cells | [16] | |
| Clinical trial in 27 patients with metastatic solid tumor in HNC | MLT (20 mg/day orally every day) combined with 5-FU and Cisplatin | Increased 1-year survival and tumor regression rate; and side effects reduced | [56] | |
| Human studies | Clinical trial in 39 patients with HNC under concurrent chemoradiation | MLT gargle (20 mg) before irradiation, and MLT capsules (20 mg) taken before bedtime during 7 weeks of concurrent chemoradiation | Delayed onset of oral mucositis and reduced amount of morphine required to alleviate the pain vs controls | [58] |
| Oncologic Treatment | Pathological Complication | Melatonin Treatment | Clinical Observations | References |
|---|---|---|---|---|
| Concurrent chemoradiation (5 days/week of radiation for 7 weeks; total dose ≥50 Gy), plus cisplatin chemotherapy | Oral mucositis | Oral gargle (20 mg/10 mL or 20 mg oral dose) | Adjuvant MLT delayed the onset of oral mucositis, reducing the palliative morphine required to control pain. | [58] |
| Male Wistar rats irradiated under anesthesia with a dose of 7.5 Gy/day for 5 days | Oral mucositis | 45 mg/day for 21 days postirradiation, either by local mouth application (MLT gel; 48 h before each irradiation, 3 times/day) or by s.c. injection each day | MLT prevented mucosal disruption and ulcer formation by blunting inflammasome signaling activation in the tongue | [53] |
| Epigenetic Control | Experimental Model | Melatonin Treatment | Main Findings | References |
|---|---|---|---|---|
| DNA methylation | OSCC cell lines | The loss by homozygous deletion or silencing by CpG hypermethylation of the MLT receptor 1A (MTNR1A) gene was associated with cancer status and tumor phenotype | [50] | |
| Histone modification | Patient-derived tumor xenografts models overexpressing LSD1. Mouse-based subcutaneous OC SCC25-xenograft model. OSCC cell lines. | 20 mg/kg daily, i.p., for 24 and 42 days. 0–20 mM for 24 h 2–4 mM for 24 and 48 h | MLT demonstrated anti-OC activity through LSD1 down-regulation | [87] |
| HSC-3 and OECM-1 OC cell lines | 1 mM for 24 h | MLT inhibited migration of tumor cells through down-regulation of MMP-9 expression and activity by decreasing CREBBP/EP300-dependent H3 and H4 histone acetylation on MMP-9 promoter | [90] | |
| Promoter activity | HONE-1, NPC-39 and NPC-BM nasopharyngeal carcinoma cell lines. | 0.5–1 mM | MLT reduced MMP-9 promoter activity through inhibition of SP-1 transcription factor expression | [91] |
| SCC9, SCC25 and CAL27 OSCC cell lines. | 10 μg/mL for 72 h | MLT reduced miR-155 and increased miR-21. | [102] | |
| Non-coding micro-RNAs | 121 OC specimens and 66 normal counterparts for the study of miR-24 expression HCT 116 and MCF-7 cells. | 1 μM for 72 h | MLT decreased miR-24 expression, which pairs with the regulation of cell proliferation, DNA damage and oncogenic transformation genes. | [100] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capote-Moreno, A.; Ramos, E.; Egea, J.; López-Muñoz, F.; Gil-Martín, E.; Romero, A. Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics. Cancers 2019, 11, 1712. https://doi.org/10.3390/cancers11111712
Capote-Moreno A, Ramos E, Egea J, López-Muñoz F, Gil-Martín E, Romero A. Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics. Cancers. 2019; 11(11):1712. https://doi.org/10.3390/cancers11111712
Chicago/Turabian StyleCapote-Moreno, Ana, Eva Ramos, Javier Egea, Francisco López-Muñoz, Emilio Gil-Martín, and Alejandro Romero. 2019. "Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics" Cancers 11, no. 11: 1712. https://doi.org/10.3390/cancers11111712
APA StyleCapote-Moreno, A., Ramos, E., Egea, J., López-Muñoz, F., Gil-Martín, E., & Romero, A. (2019). Potential of Melatonin as Adjuvant Therapy of Oral Cancer in the Era of Epigenomics. Cancers, 11(11), 1712. https://doi.org/10.3390/cancers11111712








