The Role of Merlin/NF2 Loss in Meningioma Biology
Abstract
1. Introduction
2. Evidence Linking NF2 and Meningiomas—Inherited Disorders
2.1. Neurofibromatosis Type 2 (NF2)
2.2. Schwannomatosis
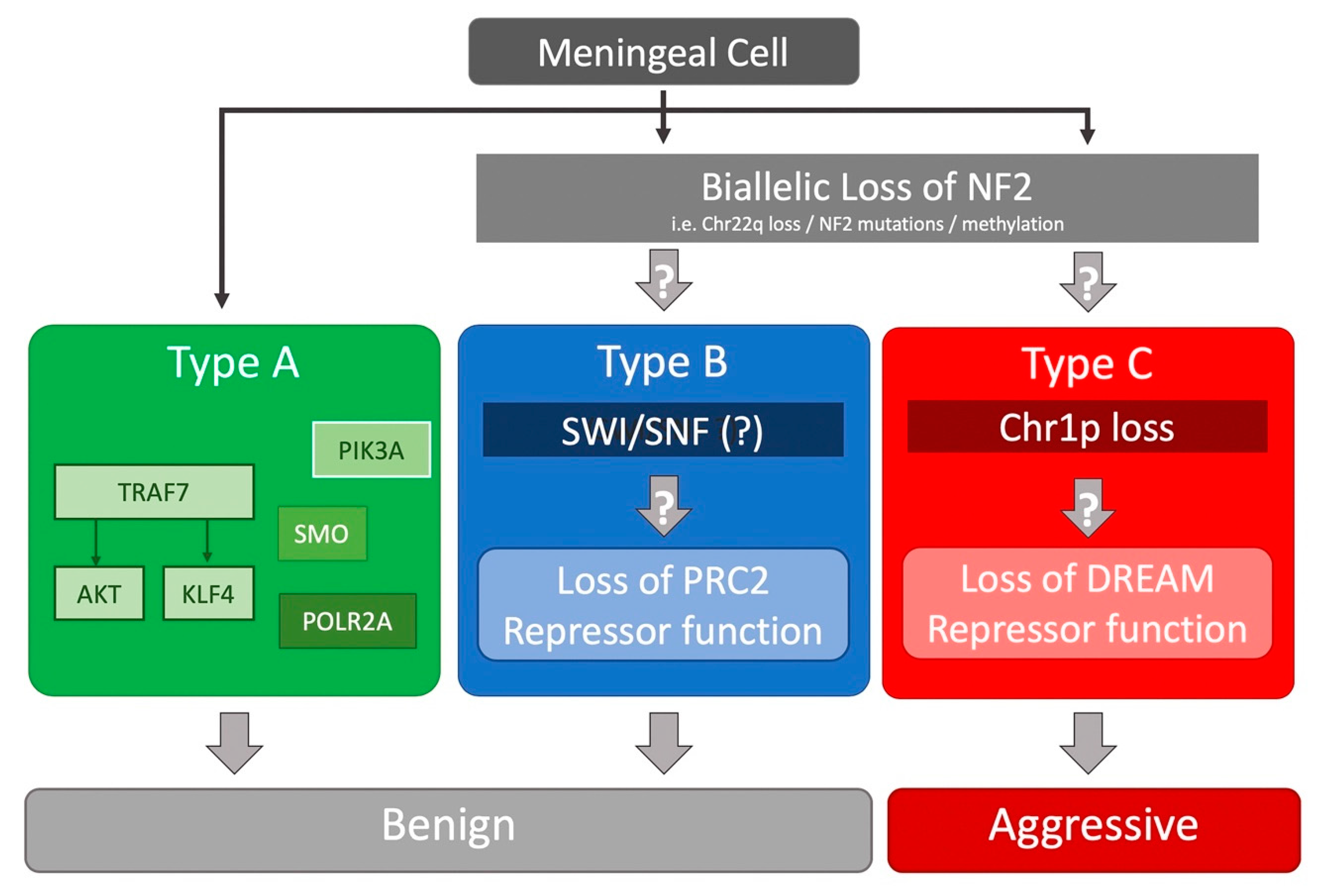
3. Insights from NGS Studies
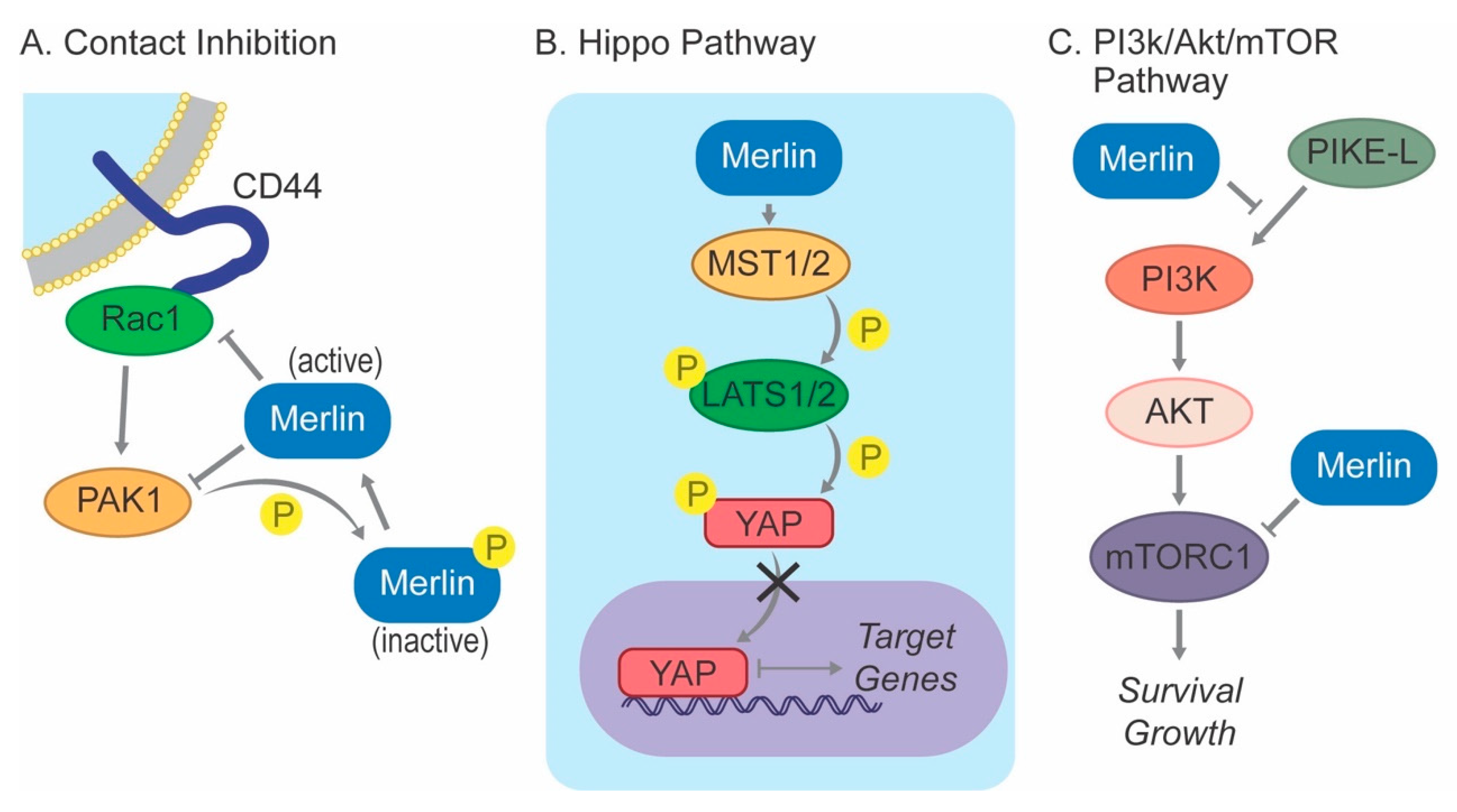
4. Merlin Signaling
4.1. Molecular Conformation
4.2. Contact Inhibition
4.3. Hippo Pathway
4.4. PI3K/AKT/mTOR Pathway
5. Animal Models
5.1. Xenograft Models
5.2. Genetically Engineered Mouse Models (GEMM)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asthagiri, A.R.; Parry, D.M.; Butman, J.A.; Kim, H.J.; Tsilou, E.T.; Zhuang, Z.; Lonser, R.R. Neurofibromatosis type 2. Lancet Lond. Engl. 2009, 373, 1974–1986. [Google Scholar] [CrossRef]
- Stamenkovic, I.; Yu, Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr. Protein Pept. Sci. 2010, 11, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.M.; Fernández-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Trofatter, J.A.; MacCollin, M.M.; Rutter, J.L.; Murrell, J.R.; Duyao, M.P.; Parry, D.M.; Eldridge, R.; Kley, N.; Menon, A.G.; Pulaski, K. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993, 72, 791–800. [Google Scholar] [CrossRef]
- Rouleau, G.A.; Merel, P.; Lutchman, M.; Sanson, M.; Zucman, J.; Marineau, C.; Hoang-Xuan, K.; Demczuk, S.; Desmaze, C.; Plougastel, B. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993, 363, 515–521. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. A 2010, 152A, 327–332. [Google Scholar] [CrossRef]
- Baser, M.E.; Friedman, J.M.; Wallace, A.J.; Ramsden, R.T.; Joe, H.; Evans, D.G.R. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology 2002, 59, 1759–1765. [Google Scholar] [CrossRef]
- Evans, D.G.; Huson, S.M.; Donnai, D.; Neary, W.; Blair, V.; Newton, V.; Harris, R. A clinical study of type 2 neurofibromatosis. Q. J. Med. 1992, 84, 603–618. [Google Scholar]
- Ahronowitz, I.; Xin, W.; Kiely, R.; Sims, K.; MacCollin, M.; Nunes, F.P. Mutational spectrum of the NF2 gene: A meta-analysis of 12 years of research and diagnostic laboratory findings. Hum. Mutat. 2007, 28, 1–12. [Google Scholar] [CrossRef]
- Parry, D.M.; Eldridge, R.; Kaiser-Kupfer, M.I.; Bouzas, E.A.; Pikus, A.; Patronas, N. Neurofibromatosis 2 (NF2): Clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am. J. Med. Genet. 1994, 52, 450–461. [Google Scholar] [CrossRef]
- Kluwe, L.; Mautner, V.F. A missense mutation in the NF2 gene results in moderate and mild clinical phenotypes of neurofibromatosis type 2. Hum. Genet. 1996, 97, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Andermann, A.A.; Phelan, C.M.; Claudio, J.O.; Han, F.Y.; Chretien, N.; Rangaratnam, S.; MacCollin, M.; Short, P.; Parry, D.; et al. Type of mutation in the neurofibromatosis type 2 gene (NF2) frequently determines severity of disease. Am. J. Hum. Genet. 1996, 59, 331–342. [Google Scholar] [PubMed]
- Kluwe, L.; MacCollin, M.; Tatagiba, M.; Thomas, S.; Hazim, W.; Haase, W.; Mautner, V.F. Phenotypic variability associated with 14 splice-site mutations in the NF2 gene. Am. J. Med. Genet. 1998, 77, 228–233. [Google Scholar] [CrossRef]
- Baser, M.E.; Kuramoto, L.; Joe, H.; Friedman, J.M.; Wallace, A.J.; Gillespie, J.E.; Ramsden, R.T.; Evans, D.G.R. Genotype-phenotype correlations for nervous system tumors in neurofibromatosis 2: A population-based study. Am. J. Hum. Genet. 2004, 75, 231–239. [Google Scholar] [CrossRef]
- Evans, D.G.; Wallace, A.J.; Wu, C.L.; Trueman, L.; Ramsden, R.T.; Strachan, T. Somatic mosaicism: A common cause of classic disease in tumor-prone syndromes? Lessons from type 2 neurofibromatosis. Am. J. Hum. Genet. 1998, 63, 727–736. [Google Scholar]
- Kluwe, L.; Mautner, V.; Heinrich, B.; Dezube, R.; Jacoby, L.B.; Friedrich, R.E.; MacCollin, M. Molecular study of frequency of mosaicism in neurofibromatosis 2 patients with bilateral vestibular schwannomas. J. Med. Genet. 2003, 40, 109–114. [Google Scholar] [CrossRef]
- Moyhuddin, A.; Baser, M.E.; Watson, C.; Purcell, S.; Ramsden, R.T.; Heiberg, A.; Wallace, A.J.; Evans, D.G.R. Somatic mosaicism in neurofibromatosis 2: Prevalence and risk of disease transmission to offspring. J. Med. Genet. 2003, 40, 459–463. [Google Scholar] [CrossRef]
- Mautner, V.F.; Lindenau, M.; Baser, M.E.; Hazim, W.; Tatagiba, M.; Haase, W.; Samii, M.; Wais, R.; Pulst, S.M. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery 1996, 38, 880–885; discussion 885–886. [Google Scholar] [CrossRef]
- Otsuka, G.; Saito, K.; Nagatani, T.; Yoshida, J. Age at symptom onset and long-term survival in patients with neurofibromatosis Type 2. J. Neurosurg. 2003, 99, 480–483. [Google Scholar] [CrossRef]
- Mautner, V.F.; Tatagiba, M.; Lindenau, M.; Fünsterer, C.; Pulst, S.M.; Baser, M.E.; Kluwe, L.; Zanella, F.E. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am. J. Roentgenol. 1995, 165, 951–955. [Google Scholar] [CrossRef]
- Dirks, M.S.; Butman, J.A.; Kim, H.J.; Wu, T.; Morgan, K.; Tran, A.P.; Lonser, R.R.; Asthagiri, A.R. Long-term natural history of neurofibromatosis Type 2–associated intracranial tumors. J. Neurosurg. 2012, 117, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, S.; Bah, A.B.; Henin, D.; Parfait, B.; Grayeli, A.B.; Sterkers, O.; Kalamarides, M. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: Clinical, radiological, and molecular features. Neuro-oncology 2012, 14, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat. Genet. 1994, 6, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; Giordano, M.J.; Fishback, A.S.; Guha, A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology 1997, 49, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Lomas, J.; Bello, M.J.; Arjona, D.; Alonso, M.E.; Martinez-Glez, V.; Lopez-Marin, I.; Amiñoso, C.; de Campos, J.M.; Isla, A.; Vaquero, J.; et al. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas: NF2 in Meningiomas. Genes. Chromosomes Cancer 2005, 42, 314–319. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef]
- MacCollin, M.; Chiocca, E.A.; Evans, D.G.; Friedman, J.M.; Horvitz, R.; Jaramillo, D.; Lev, M.; Mautner, V.F.; Niimura, M.; Plotkin, S.R.; et al. Diagnostic criteria for schwannomatosis. Neurology 2005, 64, 1838–1845. [Google Scholar] [CrossRef]
- Koontz, N.A.; Wiens, A.L.; Agarwal, A.; Hingtgen, C.M.; Emerson, R.E.; Mosier, K.M. Schwannomatosis: The overlooked neurofibromatosis? AJR Am. J. Roentgenol. 2013, 200, W646–W653. [Google Scholar] [CrossRef]
- Hulsebos, T.J.M.; Plomp, A.S.; Wolterman, R.A.; Robanus-Maandag, E.C.; Baas, F.; Wesseling, P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am. J. Hum. Genet. 2007, 80, 805–810. [Google Scholar] [CrossRef]
- Piotrowski, A.; Xie, J.; Liu, Y.F.; Poplawski, A.B.; Gomes, A.R.; Madanecki, P.; Fu, C.; Crowley, M.R.; Crossman, D.K.; Armstrong, L.; et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat. Genet. 2014, 46, 182–187. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Farschtschi, S.; Mautner, V.-F.; Cooper, D.N. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum. Genet. 2017, 136, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Merker, V.L.; Esparza, S.; Smith, M.J.; Stemmer-Rachamimov, A.; Plotkin, S.R. Clinical features of schwannomatosis: A retrospective analysis of 87 patients. Oncologist 2012, 17, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Bacci, C.; Sestini, R.; Provenzano, A.; Paganini, I.; Mancini, I.; Porfirio, B.; Vivarelli, R.; Genuardi, M.; Papi, L. Schwannomatosis associated with multiple meningiomas due to a familial SMARCB1 mutation. Neurogenetics 2010, 11, 73–80. [Google Scholar] [CrossRef]
- Melean, G.; Velasco, A.; Hernández-Imaz, E.; Rodríguez-Álvarez, F.J.; Martín, Y.; Valero, A.; Hernández-Chico, C. RNA-based analysis of two SMARCB1 mutations associated with familial schwannomatosis with meningiomas. Neurogenetics 2012, 13, 267–274. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, P.; Christiaans, I.; Kenter, S.B.; Baas, F.; Hulsebos, T.J.M. Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 2012, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Reuss, D.E.; Piro, R.M.; Jones, D.T.W.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F.; Pusch, S.; Meyer, J.; et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013, 125, 351–358. [Google Scholar] [CrossRef]
- Clark, V.E.; Harmancı, A.S.; Bai, H.; Youngblood, M.W.; Lee, T.I.; Baranoski, J.F.; Ercan-Sencicek, A.G.; Abraham, B.J.; Weintraub, A.S.; Hnisz, D.; et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat. Genet. 2016, 48, 1253–1259. [Google Scholar] [CrossRef]
- Agnihotri, S.; Suppiah, S.; Tonge, P.D.; Jalali, S.; Danesh, A.; Bruce, J.P.; Mamatjan, Y.; Klironomos, G.; Gonen, L.; Au, K.; et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nat. Commun. 2017, 8, 186. [Google Scholar] [CrossRef]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat. Commun. 2017, 8, 14433. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Wan, Y.-W.; Al-Ouran, R.; Revelli, J.-P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef] [PubMed]
- Modha, A.; Gutin, P.H. Diagnosis and treatment of atypical and anaplastic meningiomas: A review. Neurosurgery 2005, 57, 538–550; discussion 538–550. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.E.; Markert, J.M.; Fisher, W.S.; Guthrie, B.L.; Fiveash, J.B.; Palmer, C.A.; Riley, K. Hitting a moving target: Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg. Focus 2008, 24, E3. [Google Scholar] [CrossRef] [PubMed]
- Aghi, M.K.; Carter, B.S.; Cosgrove, G.R.; Ojemann, R.G.; Amin-Hanjani, S.; Martuza, R.L.; Curry, W.T.; Barker, F.G. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009, 64, 56–60; discussion 60. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Braunstein, S.E.; Phillips, J.J.; Pekmezci, M.; Tomlin, B.A.; Wu, A.; Reis, G.F.; Magill, S.T.; Zhang, J.; Feng, F.Y.; et al. Comprehensive Molecular Profiling Identifies FOXM1 as a Key Transcription Factor for Meningioma Proliferation. Cell Rep. 2018, 22, 3672–3683. [Google Scholar] [CrossRef]
- Kia, S.K.; Gorski, M.M.; Giannakopoulos, S.; Verrijzer, C.P. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol. Cell. Biol. 2008, 28, 3457–3464. [Google Scholar] [CrossRef]
- Kadoch, C.; Copeland, R.A.; Keilhack, H. PRC2 and SWI/SNF Chromatin Remodeling Complexes in Health and Disease. Biochemistry 2016, 55, 1600–1614. [Google Scholar] [CrossRef]
- Sadasivam, S.; DeCaprio, J.A. The DREAM complex: Master coordinator of cell cycle-dependent gene expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef]
- Dewan, R.; Pemov, A.; Dutra, A.S.; Pak, E.D.; Edwards, N.A.; Ray-Chaudhury, A.; Hansen, N.F.; Chandrasekharappa, S.C.; Mullikin, J.C.; Asthagiri, A.R.; et al. First insight into the somatic mutation burden of neurofibromatosis type 2-associated grade I and grade II meningiomas: A case report comprehensive genomic study of two cranial meningiomas with vastly different clinical presentation. BMC Cancer 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.; Bretscher, A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 1995, 6, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.A.; Reczek, D.; Bretscher, A.; Karplus, P.A. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000, 101, 259–270. [Google Scholar] [CrossRef]
- Shaw, R.J.; Paez, J.G.; Curto, M.; Yaktine, A.; Pruitt, W.M.; Saotome, I.; O’Bryan, J.P.; Gupta, V.; Ratner, N.; Der, C.J.; et al. The Nf2 Tumor Suppressor, Merlin, Functions in Rac-Dependent Signaling. Dev. Cell 2001, 1, 63–72. [Google Scholar] [CrossRef]
- Okada, T.; Lopez-Lago, M.; Giancotti, F.G. Merlin/ NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J. Cell Biol. 2005, 171, 361–371. [Google Scholar] [CrossRef]
- Jin, H.; Sperka, T.; Herrlich, P.; Morrison, H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 2006, 442, 576–579. [Google Scholar] [CrossRef]
- Hennigan, R.F.; Foster, L.A.; Chaiken, M.F.; Mani, T.; Gomes, M.M.; Herr, A.B.; Ip, W. Fluorescence Resonance Energy Transfer Analysis of Merlin Conformational Changes. Mol. Cell. Biol. 2010, 30, 54–67. [Google Scholar] [CrossRef]
- Ali Khajeh, J.; Ju, J.H.; Atchiba, M.; Allaire, M.; Stanley, C.; Heller, W.T.; Callaway, D.J.E.; Bu, Z. Molecular Conformation of the Full-Length Tumor Suppressor NF2/Merlin—A Small-Angle Neutron Scattering Study. J. Mol. Biol. 2014, 426, 2755–2768. [Google Scholar] [CrossRef]
- Chinthalapudi, K.; Mandati, V.; Zheng, J.; Sharff, A.J.; Bricogne, G.; Griffin, P.R.; Kissil, J.; Izard, T. Lipid binding promotes the open conformation and tumor-suppressive activity of neurofibromin 2. Nat. Commun. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Morrison, H. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001, 15, 968–980. [Google Scholar] [CrossRef]
- Ahmad, Z.; Brown, C.M.; Patel, A.K.; Ryan, A.F.; Ongkeko, R.; Doherty, J.K. Merlin knockdown in human Schwann cells: Clues to vestibular schwannoma tumorigenesis. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2010, 31, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-Y.; Dong, B.; Duron, S.G.; Campbell, D.A.; Ong, C.C.; Hoeflich, K.P.; Chang, L.-S.; Welling, D.B.; Yang, Z.; Chernoff, J. Group I Paks as therapeutic targets in NF2-deficient meningioma. Oncotarget 2015, 6, 1981. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.I.; Harvey, K.F.; Gregorevic, P. Regulation of Tissue Growth by the Mammalian Hippo Signaling Pathway. Front. Physiol. 2017, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Striedinger, K.; VandenBerg, S.R.; Baia, G.S.; McDermott, M.W.; Gutmann, D.H.; Lal, A. The Neurofibromatosis 2 Tumor Suppressor Gene Product, Merlin, Regulates Human Meningioma Cell Growth by Signaling through YAP. Neoplasia 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, K.; Natsume, A.; Ohka, F.; Motomura, K.; Alim, A.; Tanaka, I.; Senga, T.; Harada, I.; Fukuyama, R.; Sumiyoshi, N.; et al. Activation of Yes-Associated Protein in Low-Grade Meningiomas Is Regulated by Merlin, Cell Density, and Extracellular Matrix Stiffness. J. Neuropathol. Exp. Neurol. 2015, 74, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Noorolyai, S.; Shajari, N.; Baghbani, E.; Sadreddini, S.; Baradaran, B. The relation between PI3K/AKT signalling pathway and cancer. Gene 2019, 698, 120–128. [Google Scholar] [CrossRef]
- Rong, R.; Tang, X.; Gutmann, D.H.; Ye, K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc. Natl. Acad. Sci. USA 2004, 101, 18200–18205. [Google Scholar] [CrossRef]
- Mawrin, C. Different Activation of Mitogen-Activated Protein Kinase and Akt Signaling Is Associated with Aggressive Phenotype of Human Meningiomas. Clin. Cancer Res. 2005, 11, 4074–4082. [Google Scholar] [CrossRef]
- Bush, M.L.; Oblinger, J.; Brendel, V.; Santarelli, G.; Huang, J.; Akhmametyeva, E.M.; Burns, S.S.; Wheeler, J.; Davis, J.; Yates, C.W.; et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-oncology 2011, 13, 983–999. [Google Scholar] [CrossRef]
- Weller, M.; Roth, P.; Sahm, F.; Burghardt, I.; Schuknecht, B.; Rushing, E.J.; Regli, L.; Lindemann, J.P.; von Deimling, A. Durable Control of Metastatic AKT1-Mutant WHO Grade 1 Meningothelial Meningioma by the AKT Inhibitor, AZD5363. J. Natl. Cancer Inst. 2017, 109, 1–4. [Google Scholar] [CrossRef]
- James, M.F.; Lelke, J.M.; Maccollin, M.; Plotkin, S.R.; Stemmer-Rachamimov, A.O.; Ramesh, V.; Gusella, J.F. Modeling NF2 with human arachnoidal and meningioma cell culture systems: NF2 silencing reflects the benign character of tumor growth. Neurobiol. Dis. 2008, 29, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, D.J. Tuberous sclerosis: From tubers to mTOR. Ann. Hum. Genet. 2003, 67, 87–96. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/Merlin Is a Novel Negative Regulator of mTOR Complex 1, and Activation of mTORC1 Is Associated with Meningioma and Schwannoma Growth. Mol. Cell. Biol. 2009, 29, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. mTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B.; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A.; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neurooncol. 2016, 129, 281–288. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Friend, K.E.; Gerdes, T.M.; Zhang, B.-M.; Wildrick, D.M.; Fuller, G.N. Intracranial injection of human meningioma cells in athymic mice: An orthotopic model for meningioma growth. J. Neurosurg. 2000, 92, 306–314. [Google Scholar] [CrossRef]
- Püttmann, S.; Senner, V.; Braune, S.; Hillmann, B.; Exeler, R.; Rickert, C.H.; Paulus, W. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab. Investig. J. Tech. Methods Pathol. 2005, 85, 1163–1171. [Google Scholar] [CrossRef]
- Akat, K.; Mennel, H.-D.; Kremer, P.; Gassler, N.; Bleck, C.K.E.; Kartenbeck, J. Molecular characterization of desmosomes in meningiomas and arachnoidal tissue. Acta Neuropathol. (Berl.) 2003, 106, 337–347. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Greenwald, N.F.; Agar, N.Y.; Beroukhim, R.; Dunn, G.P.; Dunn, I.F. Genomic profile of human meningioma cell lines. PLoS ONE 2017, 12, e0178322. [Google Scholar] [CrossRef]
- Akat, K.; Bleck, C.K.E.; Lee, Y.-M.A.; Haselmann-Weiss, U.; Kartenbeck, J. Characterization of a novel type of adherens junction in meningiomas and the derived cell line HBL-52. Cell Tissue Res. 2008, 331, 401–412. [Google Scholar] [CrossRef]
- McClatchey, A.I.; Saotome, I.; Mercer, K.; Crowley, D.; Gusella, J.F.; Bronson, R.T.; Jacks, T. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 1998, 12, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Kalamarides, M. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 2002, 16, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef]
- Kalamarides, M.; Stemmer-Rachamimov, A.O.; Niwa-Kawakita, M.; Chareyre, F.; Taranchon, E.; Han, Z.-Y.; Martinelli, C.; Lusis, E.A.; Hegedus, B.; Gutmann, D.H.; et al. Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene 2011, 30, 2333–2344. [Google Scholar] [CrossRef]
| Diagnostic Criteria | Additional Findings Needed |
|---|---|
| Bilateral vestibular schwannomas | None. |
| Family history of NF2 | Unilateral vestibular schwannoma, OR at least two of: meningioma, schwannoma, glioma, neurofibroma, cataract. |
| Unilateral vestibular schwannoma | At least two of: meningioma, schwannoma, glioma, neurofibroma, cataract. |
| Multiple meningiomas | Unilateral vestibular schwannoma, OR at least two of: schwannoma, glioma neurofibroma, cataract. |
| Study | Tumor Type (n) | Genetic Alterations | Key Findings |
|---|---|---|---|
| Clark et al. (2013) [36] | WHO I/II (243/57) | NF2/ch22q loss TRAF7/KLF4 TRAF7/AKT1 SMO | Mutually exclusive non-NF2 driver mutations. NF2 tumors are more aggressive. Non-NF2 tumors are benign and localize to medial skull base. |
| Brastianos et al. (2013) [37] | WHO I/II/III (47/15/3) | NF2/ch22q loss SMO AKT1 | As above. |
| Reuss et al. (2013) [38] | Secretory (30) | TRAF7/KLF4 | All secretory meningiomas carried the KLF4 K409Q mutation. |
| Clark et al. (2016) [39] | WHO I/II/III/? (552/214/7/2) | POLR2A SMARCB1 | Identification of POLR2A driver mutation. SMARCB1 and NF2 mutations co-occur. |
| Agnihotri et al. (2017) [40] | Radiation-induced (31) | NF2/ch22q loss | NF2 gene rearrangements common in radiation-induced tumors. Non-NF2 driver mutations not observed. |
| Bi et al. (2017) [41] | WHO I/II/III (75/113/21) | NF2/ch22q loss Genomic instability | NF2/ch22q loss and genomic disruptions occur early in progression and remain consistent over time. |
| Harmanci et al. (2017) [42] | WHO I/II/III/? (548/211/7/9) | NF2/genomic instability NF2/SMARCB1 | NF2 is the sole driver mutation in atypical meningiomas and occurs in conjunction with genomic instability or SMARCB1 mutations. |
| Patel et al. (2019) [43] | WHO I/II/III (119/33/5) | Loss of PRC2 or DREAM complex repression | Transcriptional signatures identified a sole subgroup with recurring tumors, characterized by DREAM target genes activation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Karas, P.J.; Hadley, C.C.; Bayley V, J.C.; Khan, A.B.; Jalali, A.; Sweeney, A.D.; Klisch, T.J.; Patel, A.J. The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers 2019, 11, 1633. https://doi.org/10.3390/cancers11111633
Lee S, Karas PJ, Hadley CC, Bayley V JC, Khan AB, Jalali A, Sweeney AD, Klisch TJ, Patel AJ. The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers. 2019; 11(11):1633. https://doi.org/10.3390/cancers11111633
Chicago/Turabian StyleLee, Sungho, Patrick J. Karas, Caroline C. Hadley, James C. Bayley V, A. Basit Khan, Ali Jalali, Alex D. Sweeney, Tiemo J. Klisch, and Akash J. Patel. 2019. "The Role of Merlin/NF2 Loss in Meningioma Biology" Cancers 11, no. 11: 1633. https://doi.org/10.3390/cancers11111633
APA StyleLee, S., Karas, P. J., Hadley, C. C., Bayley V, J. C., Khan, A. B., Jalali, A., Sweeney, A. D., Klisch, T. J., & Patel, A. J. (2019). The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers, 11(11), 1633. https://doi.org/10.3390/cancers11111633






