Technology for 3D System Integration for Flexible Wireless Biomedical Applications
Abstract
1. Introduction
2. Design
2.1. Theory of Induction Coils
2.2. Dummy Chip Experiment
2.3. 3D Packaging Process Design
3. Fabrication
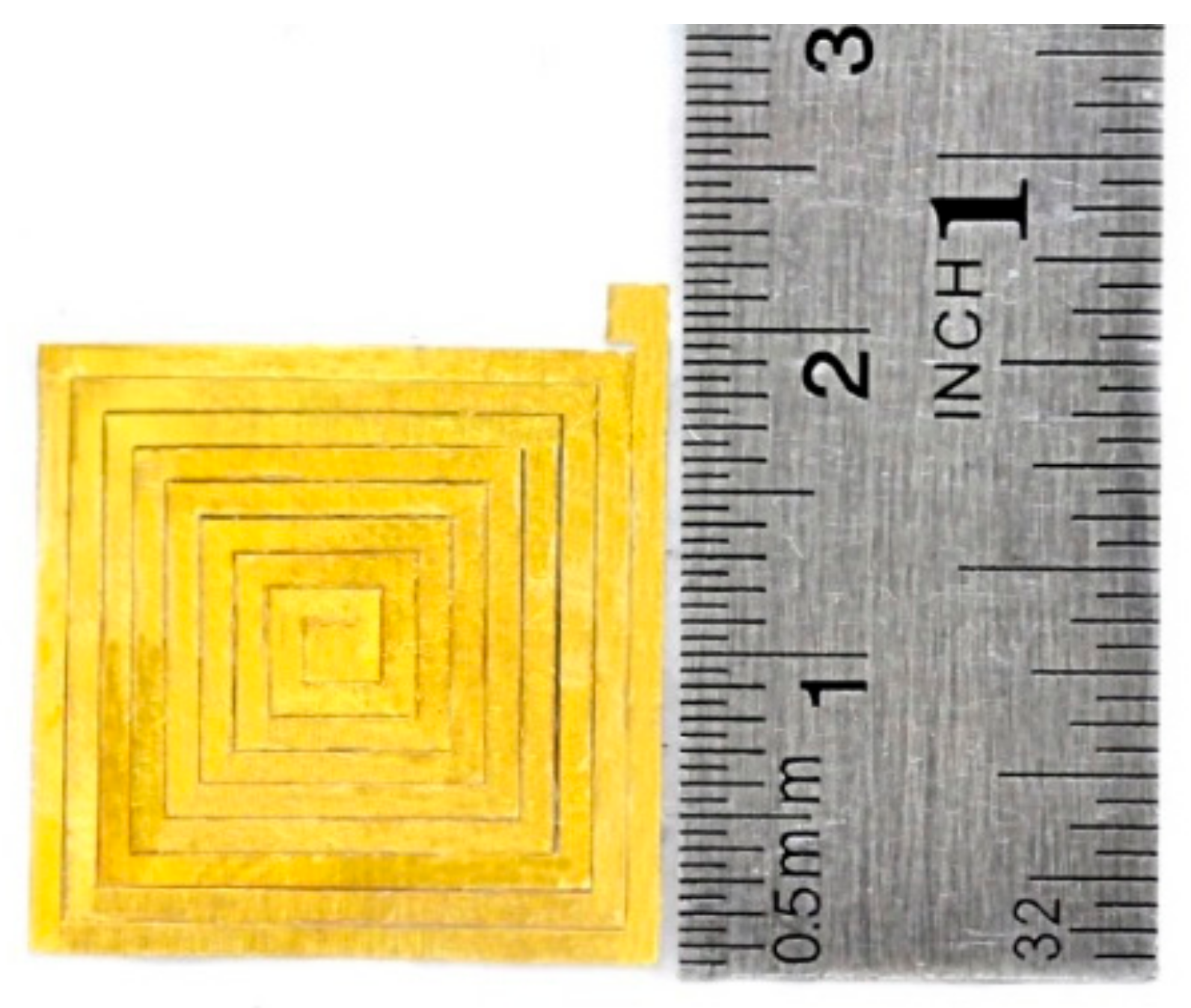
3.1. Induction Coil Fabrication
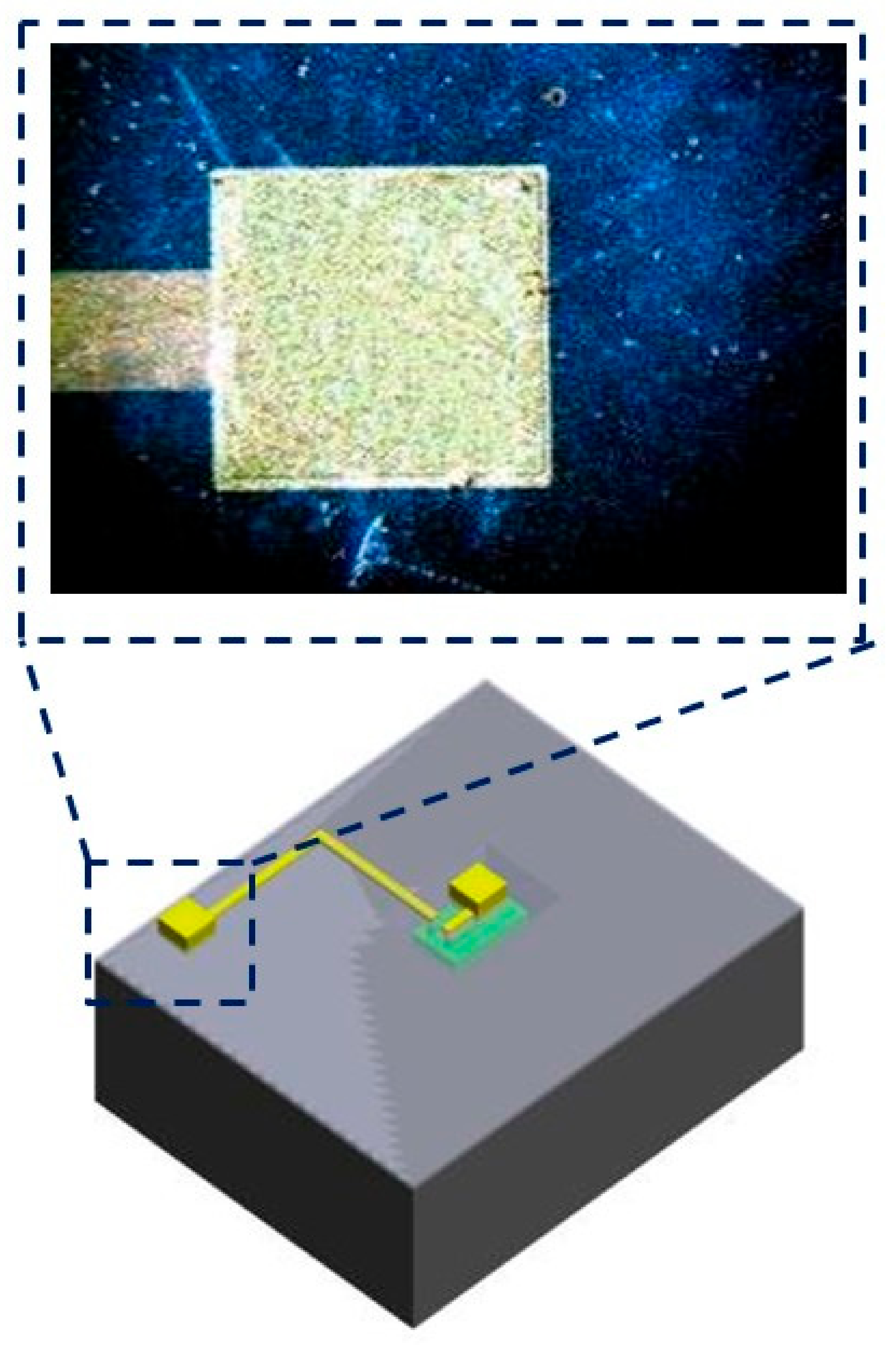
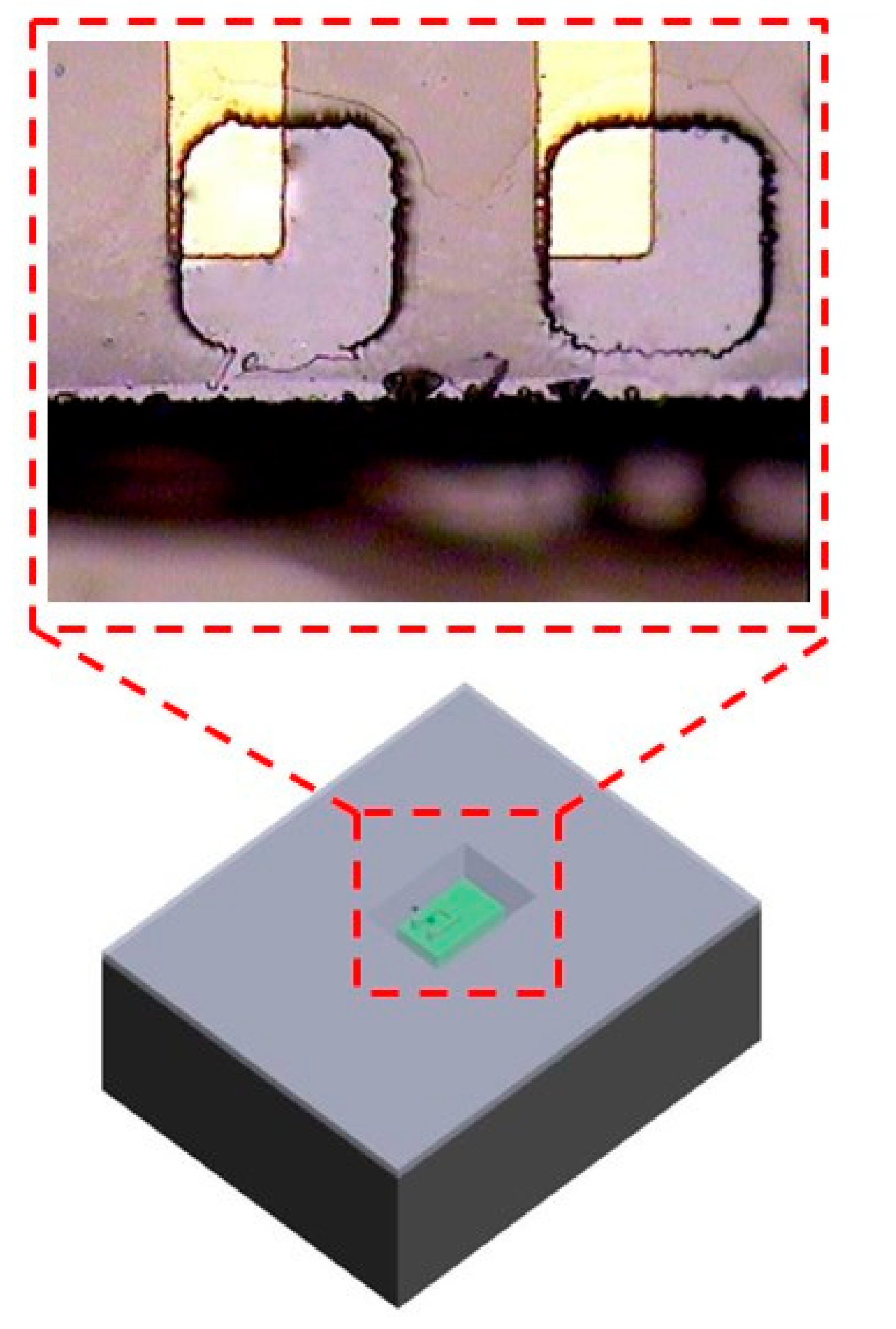
3.2. Induction Coil and Chip Integration
- Step a:
- ICP etching
- Step b:
- Parylene coating
- Step c:
- Chip embedding
- Step d:
- First parylene package
- Step e:
- Photoresist patterning
- Step f:
- First parylene etched using RIE
- Step g:
- First gold deposition
- Step h:
- Photoresist patterning
- Step i:
- First gold etching
- Step j:
- Second parylene package
- Step k:
- Photoresist patterning
- Step l:
- Second parylene etched by RIE
- Step m:
- Second gold deposition
- Step n:
- Photoresist patterning
- Step o:
- Second gold etching
- Step p:
- Gold foil pasting
- Step q:
- Third parylene package
- Step r:
- Wafer removal
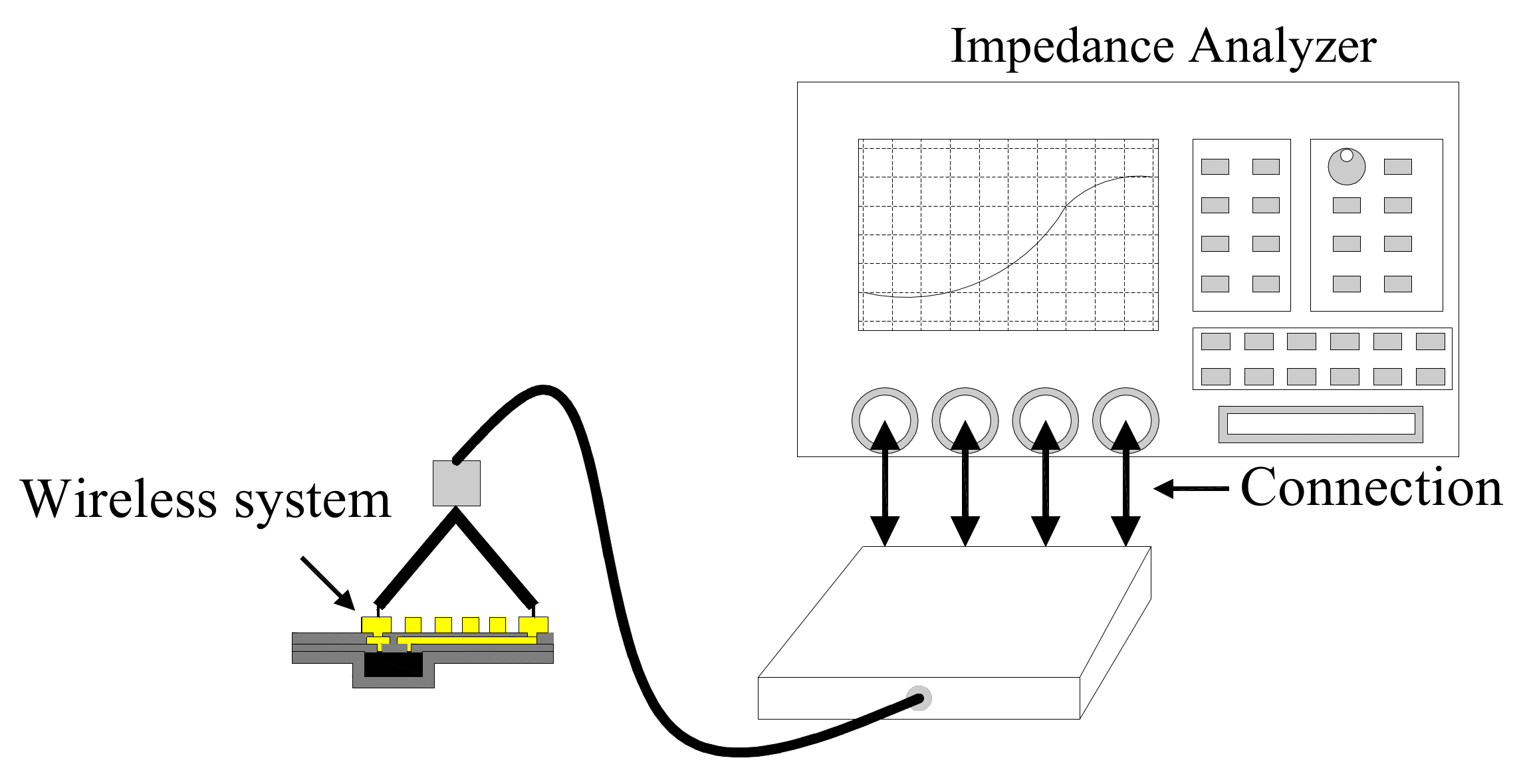
4. Measurement Setup
5. Results and Discussion
5.1. Patterned Coil
5.2. Wireless Microsystem
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schnakenberg, U.; Krüger, C.; Pfeffer, J.G.; Mokwa, W.; vom Bögel, G.; Günther, R.; Schmitz-Rode, T. Intravascular pressure monitoring system. Sens. Actuators A 2004, 100, 61–67. [Google Scholar] [CrossRef]
- Coosemans, J.; Puers, R. An autonomous bladder pressure monitoring system. Sens. Actuators A 2005, 123–124, 155–161. [Google Scholar] [CrossRef]
- Li, W.; Rodger, D.C.; Weiland, J.D.; Humayun, M.S.; Tai, Y.C. Integrated flexible ocular coil for power and data transfer in retinal prostheses. In Proceedings of the 27th Annual Conference of the IEEE Engineering in Medicine and Biology, Shanghai, China, 1–4 September 2005; pp. 1028–1031. [Google Scholar]
- Tummala, R.R.; Swaminathan, M. Introduction to System-on-Package (SOP); McGraw-Hill: New York, NY, USA, 2008; pp. 3–23. [Google Scholar]
- Pavlidis, V.F.; Friedman, E.G. Three-Dimensional Integrated Circuit Design; Elsevier Inc.: New York, NY, USA, 2009; pp. 17–36. [Google Scholar]
- Yang, Y.J.; Huang, Y.J.; Liao, H.H.; Wang, T.; Huang, P.L.; Lin, C.W.; Wang, Y.H.; Lu, S.S. A release-on-demand wireless CMOS drug delivery SoC based on electrothermal activation technique. In Proceedings of the IEEE International Solid-State Circuits Conference, Taipei, Taiwan, 10 February 2009; pp. 288–290. [Google Scholar]
- Chiu, H.W.; Lin, M.L.; Lin, C.W.; Ho, I.H.; Lin, W.T.; Fang, P.H.; Lee, Y.C.; Wen, Y.R.; Lu, S.S. Pain Control On Demand Based on Pulsed Radio-Frequency Stimulation of the Dorsal Root Ganglion Using a Batteryless Implantable CMOS SoC. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.C.; Liu, Y.; Kang, D.; Monge, M.; Zhao, Y.; Yu, C.C.; Emami-Neyestanak, A.; Weiland, J.; Yun, M.H.; Tai, Y.C. Packaging Study for a 512-Channel Intraocular Epiretinal Implant. In Proceedings of the IEEE MEMS Conference, Taipei, Taiwan, 20–24 January 2013; pp. 1046–1048. [Google Scholar]
- Yole Development. 3DIC & TSV Report: Cost, Technologies & Markets; Yole Development: Lyon, France, 2007. [Google Scholar]
- Karnezos, M. 3-D Packaging: Where All Technologies Come Together. In Proceedings of the IEEE/SEMI International Electronics Manufacturing Technology Symposium, San Jose, CA, USA, 14–16 July 2004; pp. 64–67. [Google Scholar]
- Miettinen, J.; Mantysalo, M.; Kaija, K.; Ristolainen, E.O. System Design Issues for 3D System-in-Package (SiP). In Proceedings of the IEEE International Electronic Components and Technology Conference, Las Vegas, NV, USA, 4 June 2004; pp. 610–615. [Google Scholar]
- Faure, C.; Val, A.; Couderc, P.; Chandler, N.; Preziosi, E.; Ousten, Y. 3D system-inpackage: Technology improvements for volume manufacturing. In Proceedings of the IMAPS MicroTech, Cambridge, UK, 7–8 March 2006. [Google Scholar]
- Eloy, J.C. Market Trends & Cost Analysis for 3D ICs; Yole Development: Lyon, France, 2007. [Google Scholar]
- Berenyi, R. Prototyping of a reliable 3D flexible IC cube package by laser micromachining. Microelectron. Reliab. 2009, 49, 800–805. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Rodger, D.C.; Weiland, J.D.; Humayunb, M.S.; Tai, Y.C. Scalable high lead-count parylene package for retinal prostheses. Sens. Actuators B 2006, 117, 107–114. [Google Scholar] [CrossRef]
- Yue, C.P.; Wong, S.S. On-Chip Spiral Inductors with Patterned Ground Shields for Si-Based RF IC’s. Solid-State Circuits 1998, 33, 743–752. [Google Scholar] [CrossRef]
- O’Driscoll, S.; Poon, A.S.Y.; Meng, T.H. A mm-Sized Implantable Power Receiver with Adaptive Link Compensation. In Proceedings of the IEEE International Solid-State Circuits Conference—Diqest of Technical Papers 2009 (ISSCC 2009), San Francisco, CA, USA, 8–12 February 2009; pp. 294–295. [Google Scholar]
- Sauer, C.; Stanaćević, M.; Cauwenberghs, G.; Thakor, N. Power Harvesting and Telemetry in CMOS for Implanted Devices. IEEE Trans. Circuits Syst. I Regul. Pap. 2005, 52, 2605–2613. [Google Scholar] [CrossRef]
- Vaillancourt, P.; Djemouai, A.; Harvey, J.F.; Sawan, M. EM Radiation Behavior upon Biological Tissues in a Radio-Frequency Power Transfer Link for a Cortical Visual Implant. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 30 October–2 November 1997; Volume 6, pp. 2499–2502. [Google Scholar]
- Li, W.; Rodger, D.C.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Flexible parylene packaged intraocular coil for retinal prostheses. In Proceedings of the IEEE Microtechnologies in Medicine and Biology Conference, Okinawa, Japan, 9–12 May 2006; pp. 105–108. [Google Scholar]
- Kuo, W.-C.; Hsu, C.-Y.; Yang, Y.-J. New Fabrication Method for High-Q MEMS Inductors. In Proceedings of the ASME 2012 International Mechanical Engineering Congress & Exposition, Houston, TX, USA, 9–15 November 2012; pp. 1–5. [Google Scholar]
- Li, W.; Rodger, D.C.; Pinto, A.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Parylene-based integrated wireless single-channel neurostimulator. Sens. Actuators A 2011, 166, 193–200. [Google Scholar] [CrossRef]
- Rais-Zadeh, M.; Ayazi, F. Characterization of high-Q spiral inductors on thick insulator-on-silicon. J. Micromech. Microeng. 2005, 15, 2105–2112. [Google Scholar] [CrossRef][Green Version]
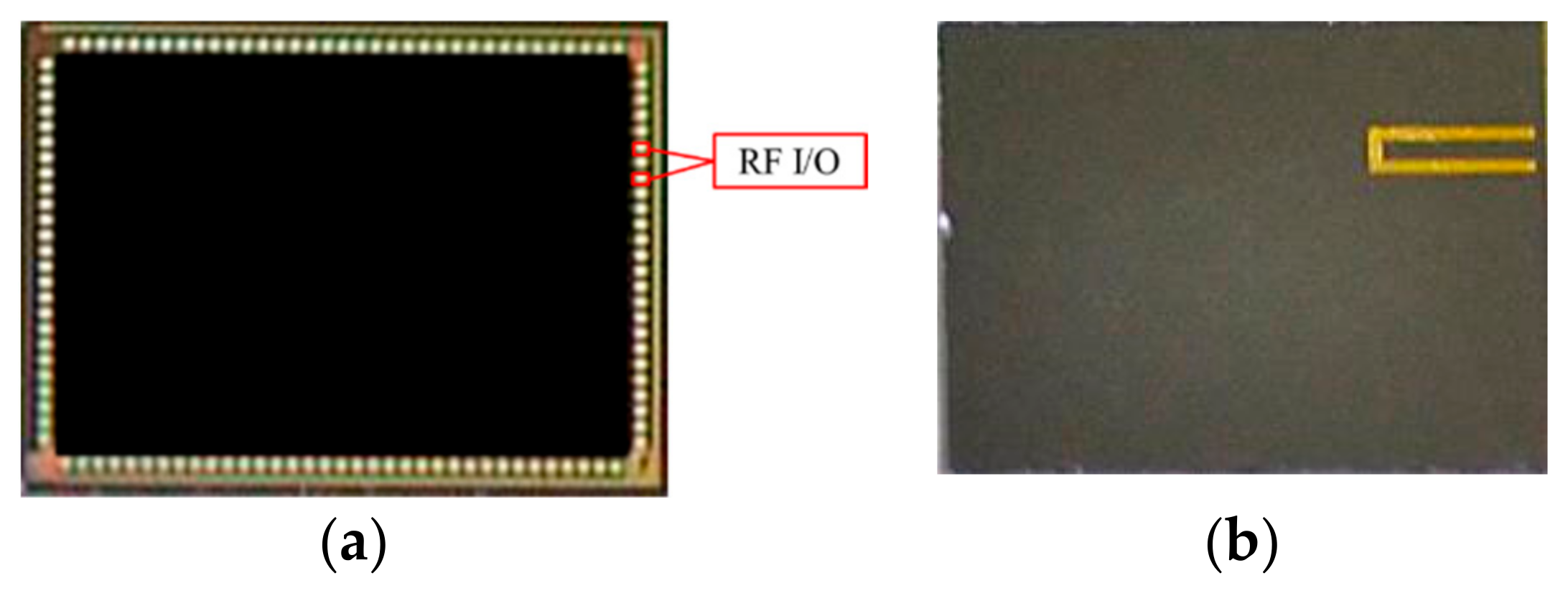
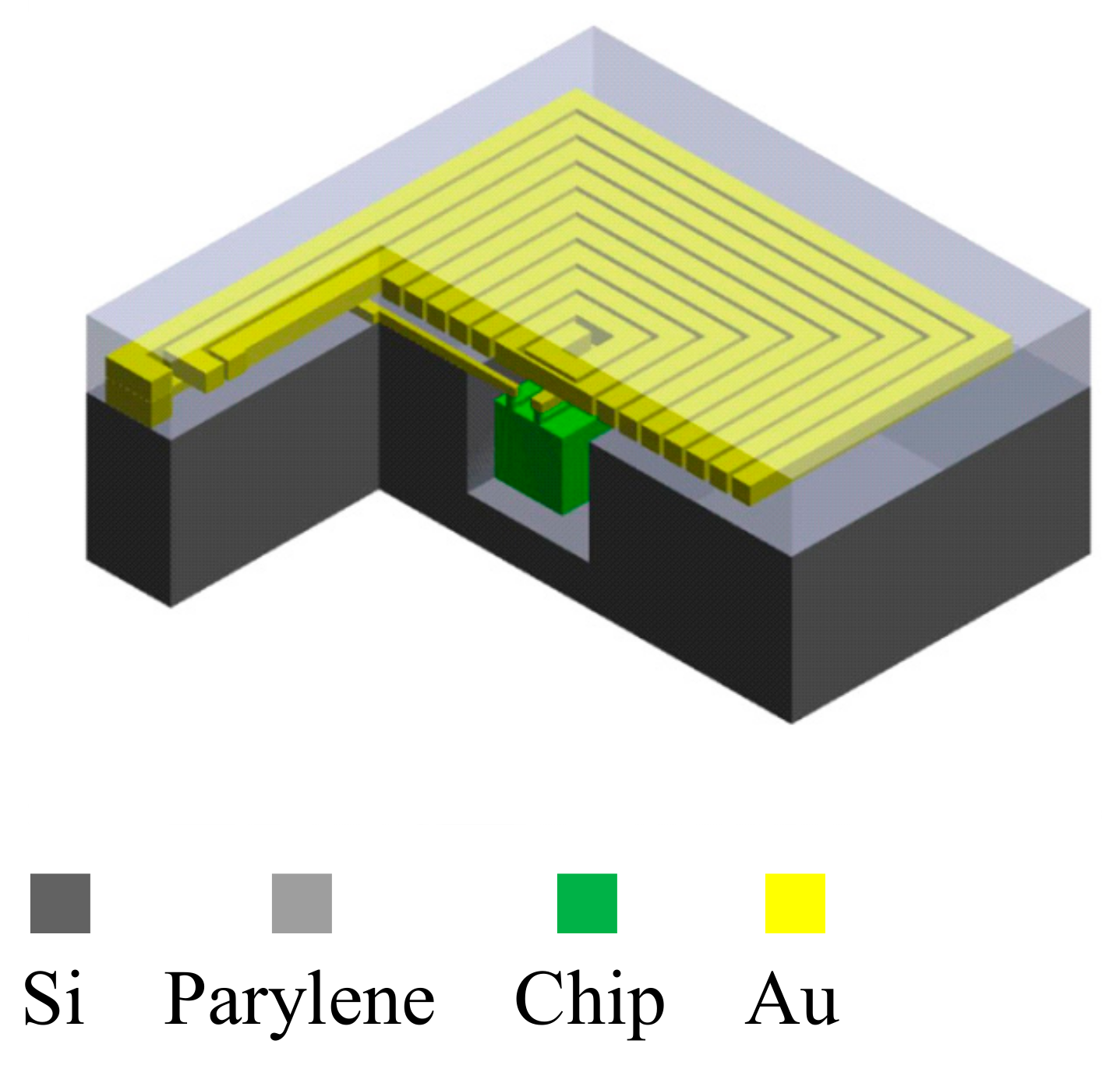
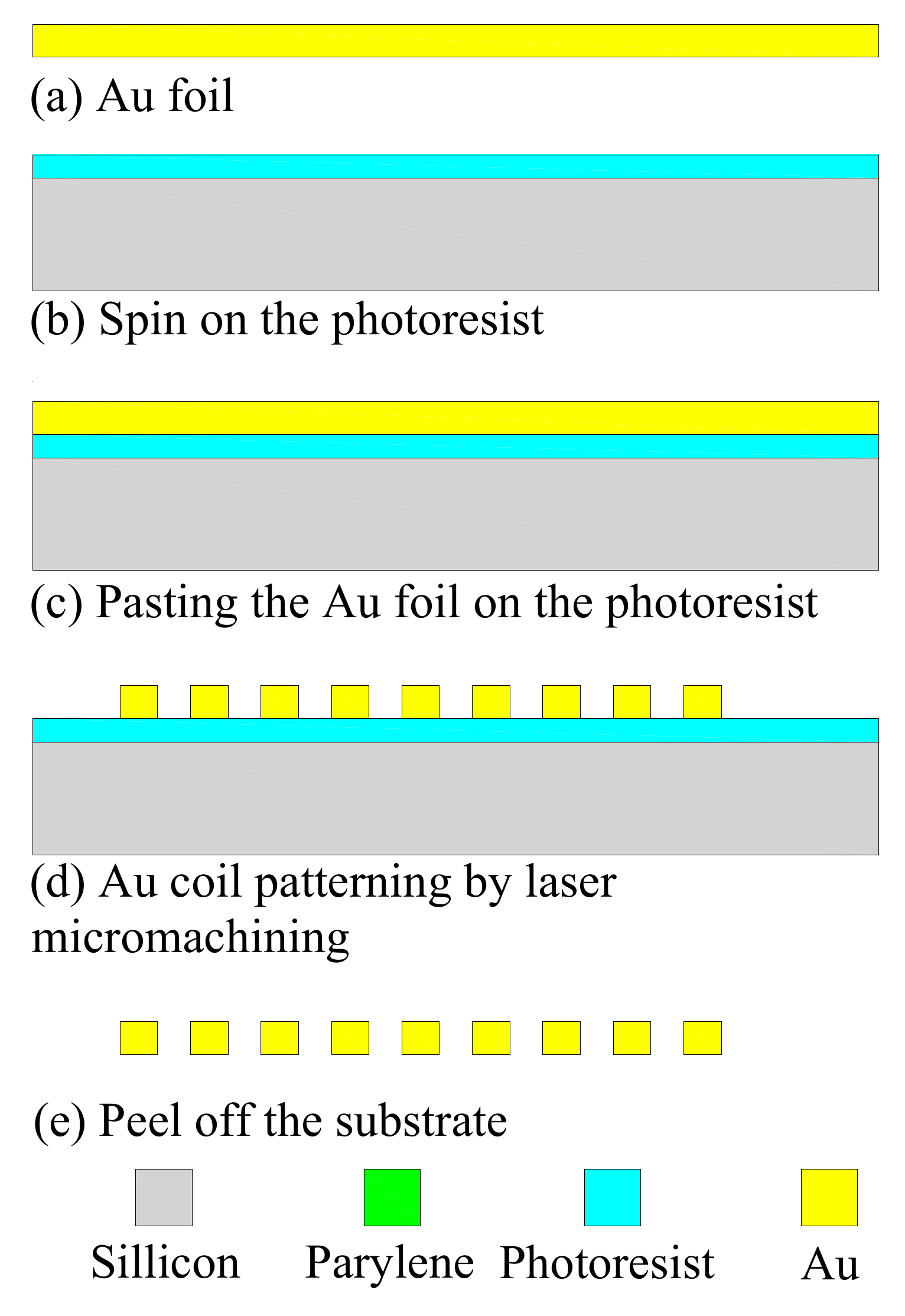

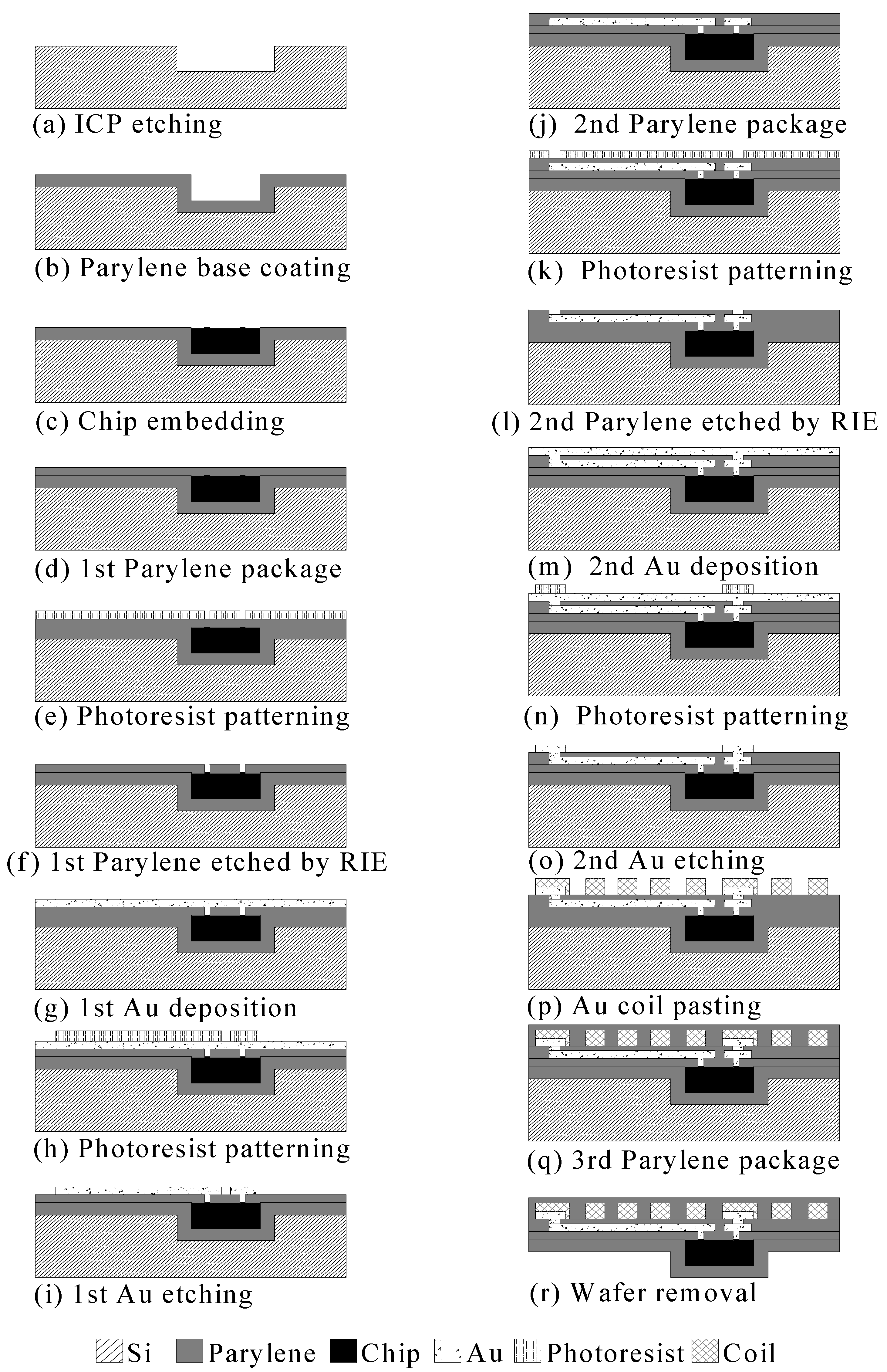
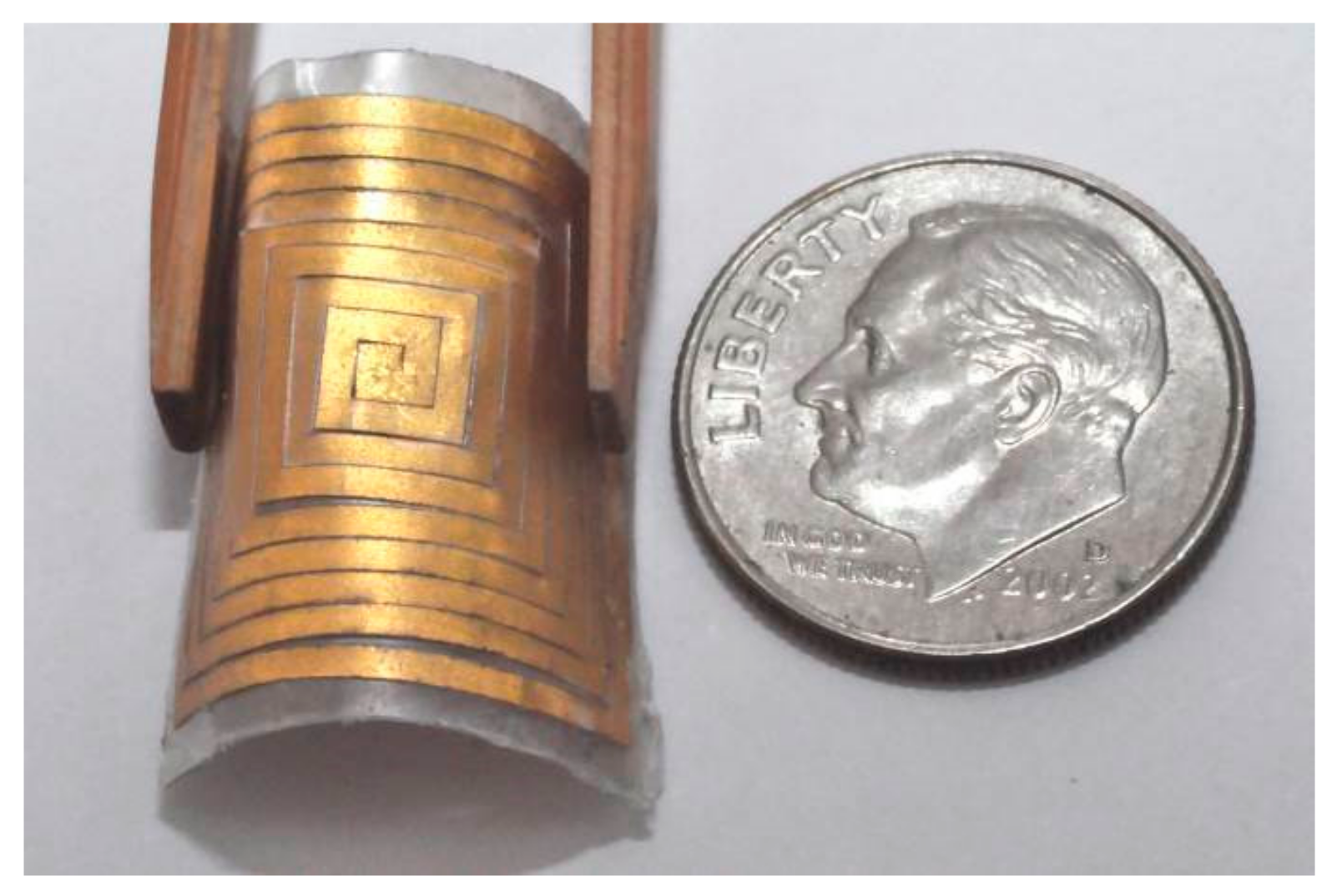

| Item | Specification |
|---|---|
| Material | Gold |
| Width of strip | 1000 µm |
| Outer diameter | 20 mm |
| The distance between strips of coil | ~60 µm |
| Thickness | 50 µm |
| Number of turns | 8.5 |
| Item | Inductance (nH) | Resistance (mΩ) | Q-Factor |
|---|---|---|---|
| Induction coil | 600 | 570.24 | 6.61 |
| Induction coil with system | 557 | 819.15 | 4.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, W.-C.; Huang, C.-W. Technology for 3D System Integration for Flexible Wireless Biomedical Applications. Micromachines 2018, 9, 213. https://doi.org/10.3390/mi9050213
Kuo W-C, Huang C-W. Technology for 3D System Integration for Flexible Wireless Biomedical Applications. Micromachines. 2018; 9(5):213. https://doi.org/10.3390/mi9050213
Chicago/Turabian StyleKuo, Wen-Cheng, and Chih-Wei Huang. 2018. "Technology for 3D System Integration for Flexible Wireless Biomedical Applications" Micromachines 9, no. 5: 213. https://doi.org/10.3390/mi9050213
APA StyleKuo, W.-C., & Huang, C.-W. (2018). Technology for 3D System Integration for Flexible Wireless Biomedical Applications. Micromachines, 9(5), 213. https://doi.org/10.3390/mi9050213





