Design of Hydrogel Microneedle Arrays for Physiology Monitoring of Farm Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Dextran-Methacrylate (Dex-MA) Synthesis
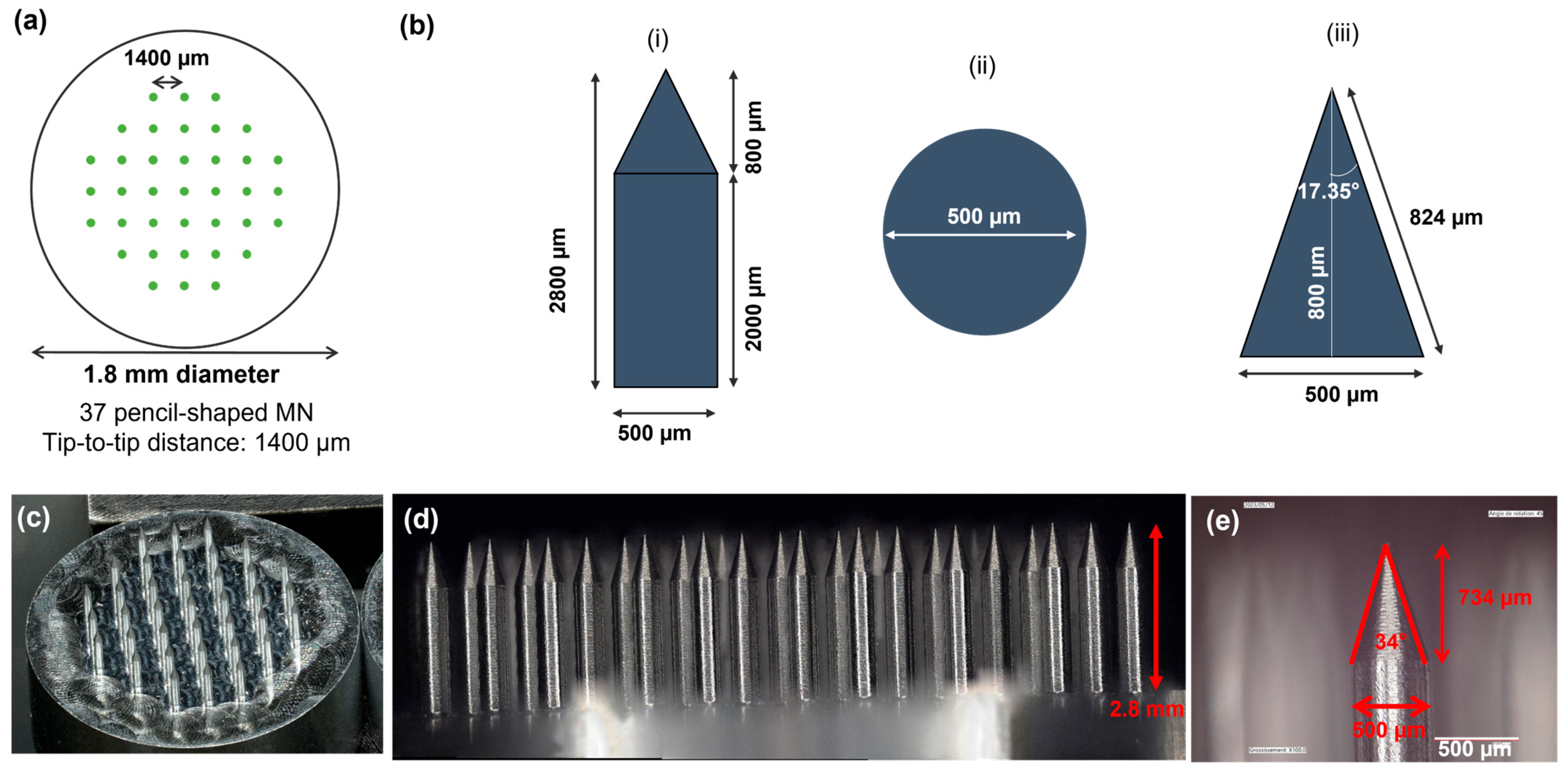
2.2. Microneedle Array Fabrication
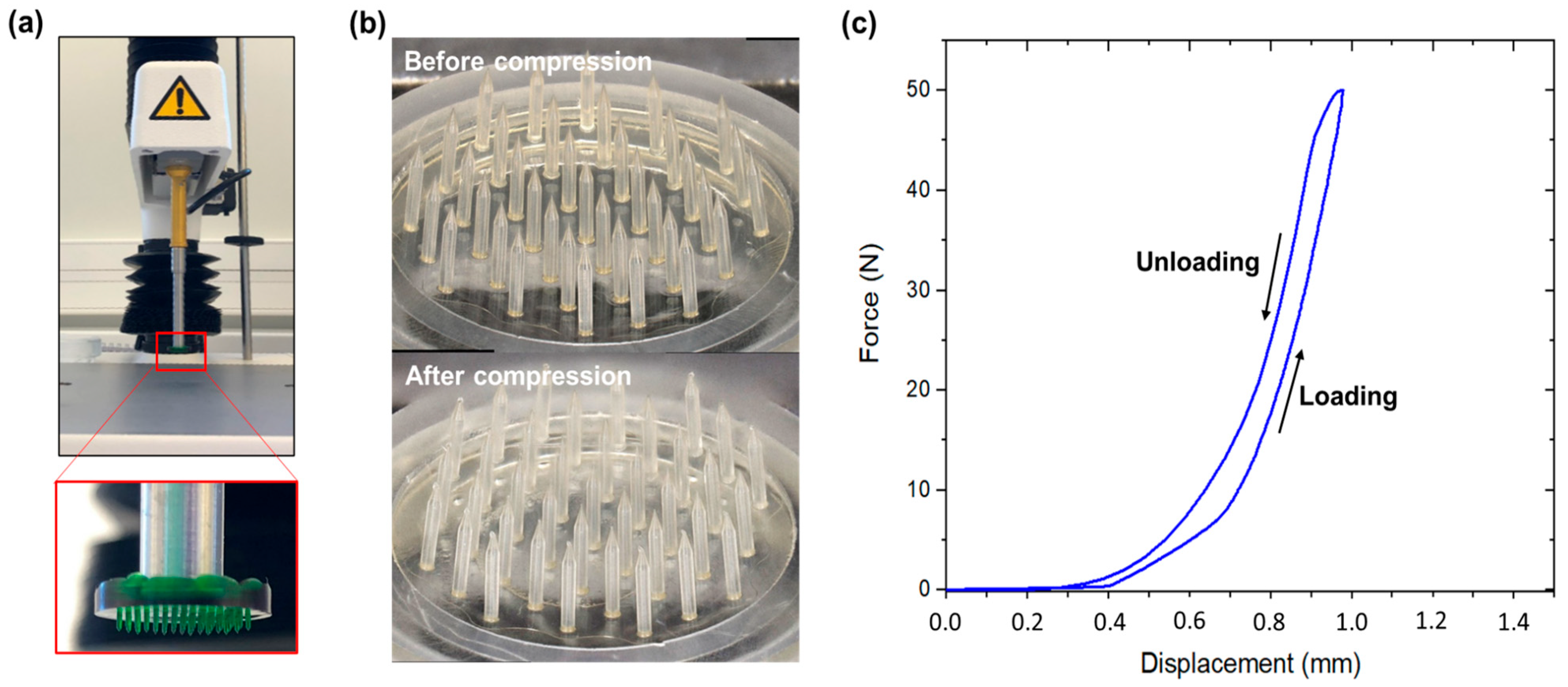
2.3. Mechanical Characterization of the Microneedle Arrays
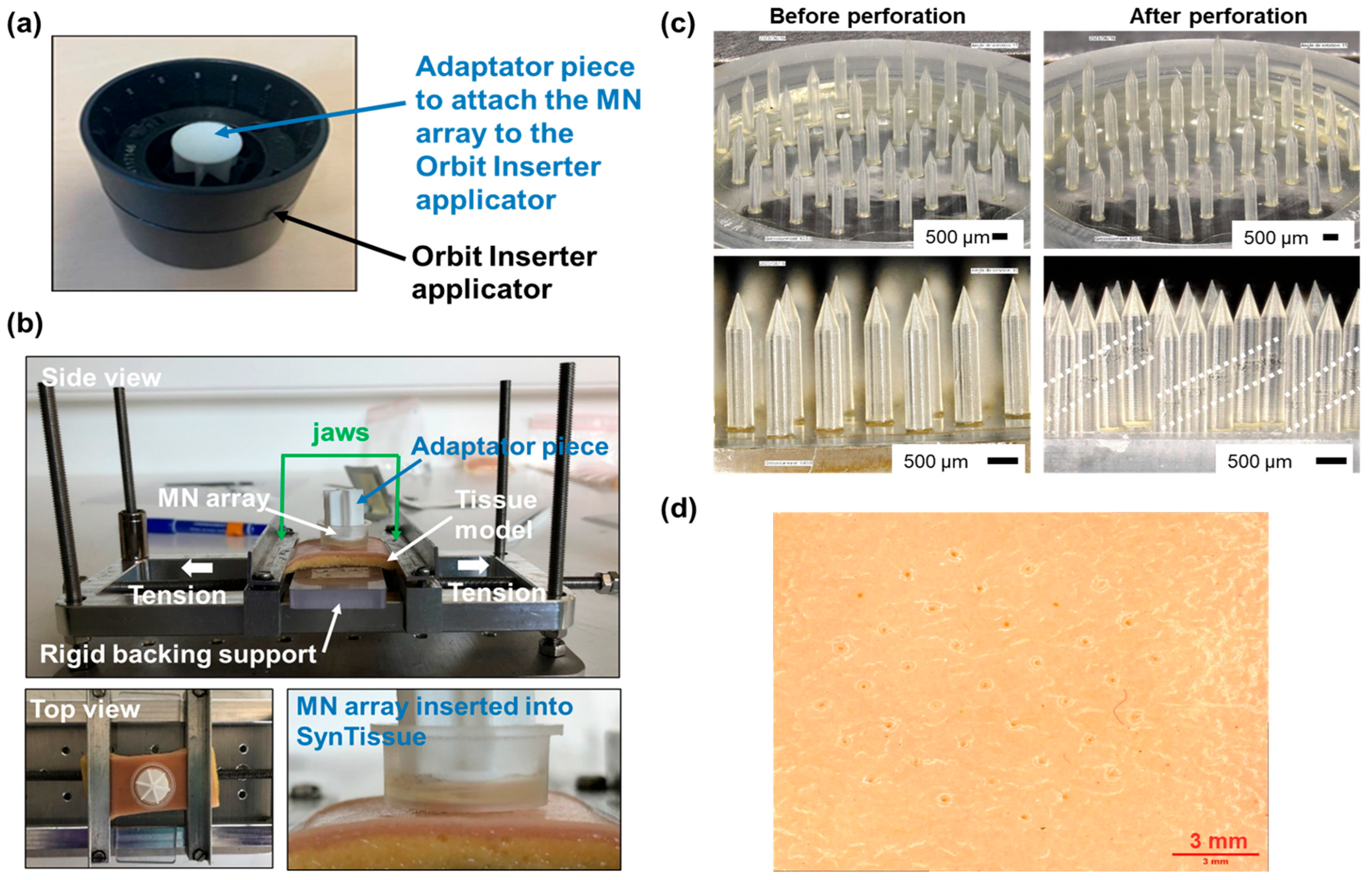
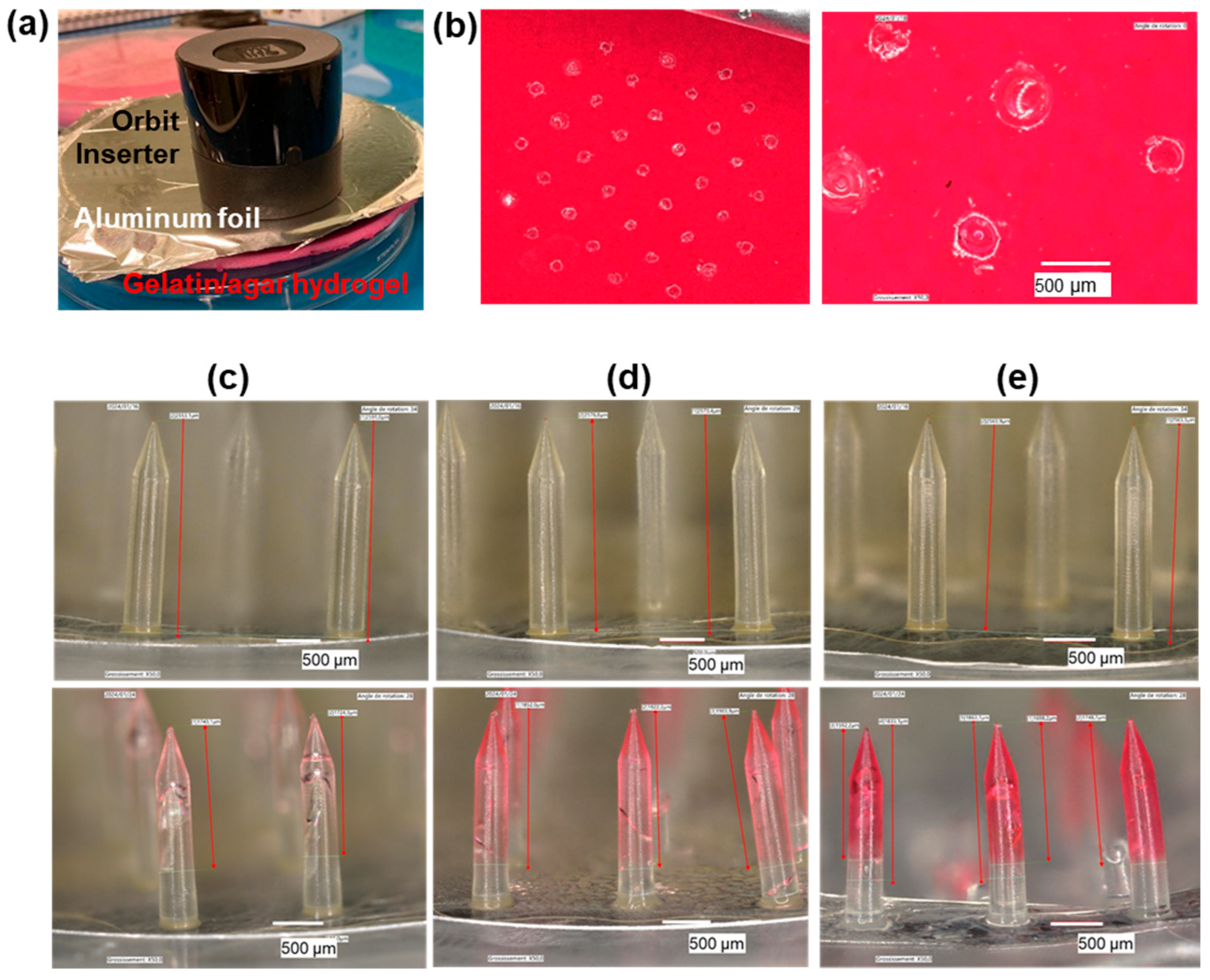
2.4. Model Skin Perforation Experiments
2.5. Model Skin Fluid Uptake Experiments
2.6. Ex Vivo Animal Skin Perforation Experiments
3. Results
3.1. Microneedle Arrays (MNA) Design and Fabrication
3.2. MNA Mechanical Characterization
3.3. Development of Perforation Protocol on Human Skin Model
3.4. Fluid Uptake Experiments on Model Skin
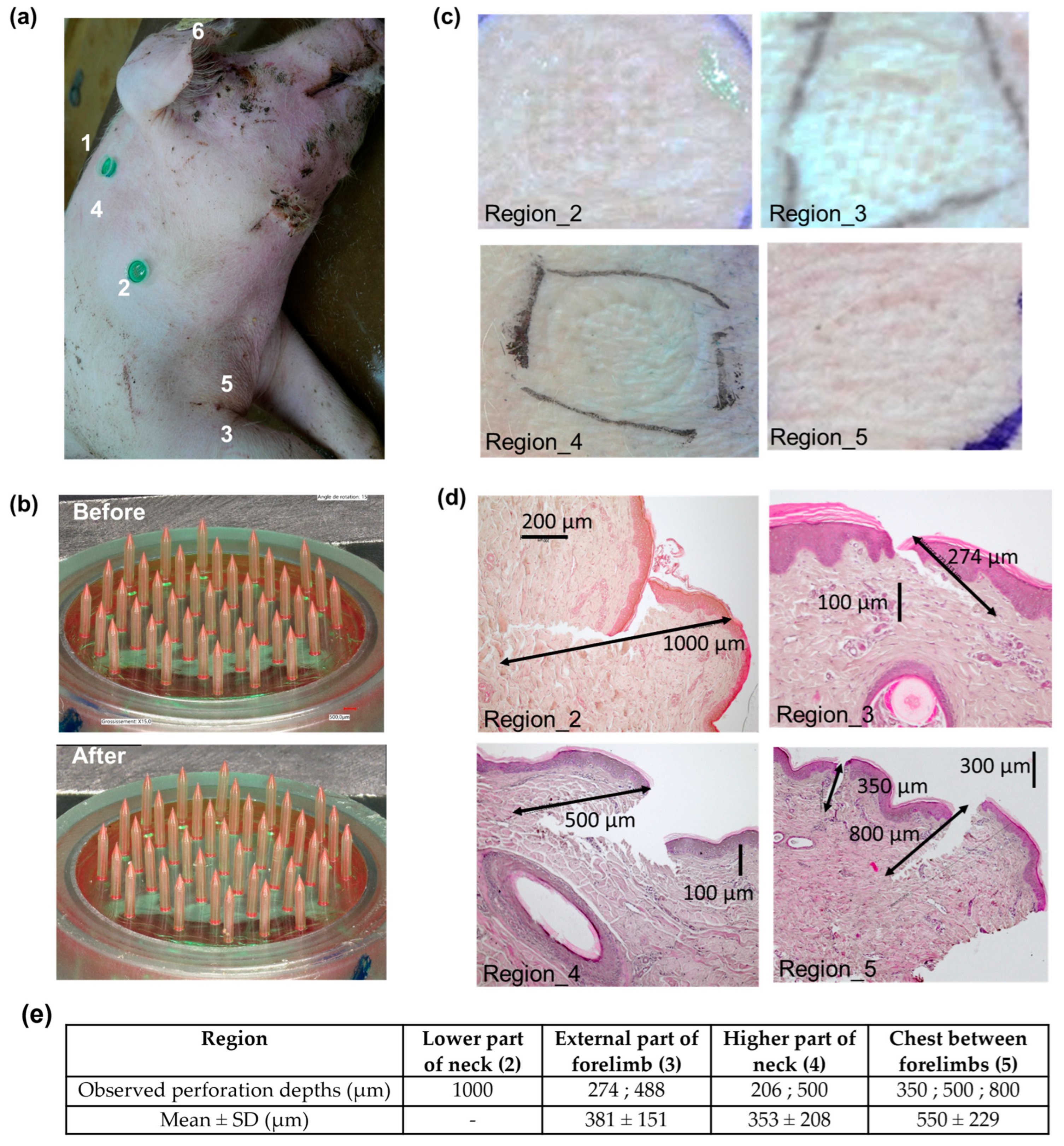
3.5. Porcine Skin Perforation Experiments
3.6. Cow Skin Perforation Experiments
4. Discussion
4.1. Hydrogel-Based MNA for ISF Uptake
4.2. MNA Design
4.3. MNA Fabrication
4.4. MNA Skin Perforation
4.5. MNA Fluid Uptake
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez, S.; Calvo, J.H.; Calvete, C.; Joy, M.; Lobón, S. Mitigation and Animal Response to Water Stress in Small Ruminants. Anim. Front. 2023, 13, 81–88. [Google Scholar] [CrossRef]
- Sorrentino, I.; Verplanck, C.; Thomas, Y.R.J. Electrochemical Sensors for Animal Welfare. Proceedings 2024, 97, 45. [Google Scholar] [CrossRef]
- Babington, S.; Tilbrook, A.J.; Maloney, S.K.; Fernandes, J.N.; Crowley, T.M.; Ding, L.; Fox, A.H.; Zhang, S.; Kho, E.A.; Cozzolino, D.; et al. Finding Biomarkers of Experience in Animals. J. Anim. Sci. Biotechnol. 2024, 15, 28. [Google Scholar] [CrossRef]
- Marco-Ramell, A.; De Almeida, A.M.; Cristobal, S.; Rodrigues, P.; Roncada, P.; Bassols, A. Proteomics and the Search for Welfare and Stress Biomarkers in Animal Production in the One-Health Context. Mol. Biosyst. 2016, 12, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.; Tönges, S.; Böhl, F.; Lyko, F. Epigenetic Biomarkers for Animal Welfare Monitoring. Front. Vet. Sci. 2023, 9, 1107843. [Google Scholar] [CrossRef]
- Zachut, M.; Šperanda, M.; De Almeida, A.M.; Gabai, G.; Mobasheri, A.; Hernández-Castellano, L.E. Biomarkers of Fitness and Welfare in Dairy Cattle: Healthy Productivity. J. Dairy Res. 2020, 87, 4–13. [Google Scholar] [CrossRef]
- Madden, J.; O’Mahony, C.; Thompson, M.; O’Riordan, A.; Galvin, P. Biosensing in Dermal Interstitial Fluid Using Microneedle Based Electrochemical Devices. Sens. Bio-Sens. Res. 2020, 29, 100348. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Munaz, A.; Moghadas, H.; Yadav, S.; Umer, M.; Nguyen, N.-T. Microneedle Arrays for Sampling and Sensing Skin Interstitial Fluid. Chemosensors 2021, 9, 83. [Google Scholar] [CrossRef]
- Lu, H.; Zada, S.; Yang, L.; Dong, H. Microneedle-Based Device for Biological Analysis. Front. Bioeng. Biotechnol. 2022, 10, 851134. [Google Scholar] [CrossRef]
- Liu, G.-S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.-R.; Wu, M.X. Microneedles for Transdermal Diagnostics: Recent Advances and New Horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Wu, Z.; Qiao, Z.; Chen, S.; Fan, S.; Liu, Y.; Qi, J.; Lim, C.T. Interstitial Fluid-Based Wearable Biosensors for Minimally Invasive Healthcare and Biomedical Applications. Commun. Mater. 2024, 5, 33. [Google Scholar] [CrossRef]
- Oharazawa, A.; Maimaituxun, G.; Watanabe, K.; Nishiyasu, T.; Fujii, N. Metabolome Analyses of Skin Dialysate: Insights into Skin Interstitial Fluid Biomarkers. J. Dermatol. Sci. 2024, 114, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and Challenges in the Diagnostic Utility of Dermal Interstitial Fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Himawan, A.; Vora, L.K.; Permana, A.D.; Sudir, S.; Nurdin, A.R.; Nislawati, R.; Hasyim, R.; Scott, C.J.; Donnelly, R.F. Where Microneedle Meets Biomarkers: Futuristic Application for Diagnosing and Monitoring Localized External Organ Diseases. Adv. Healthc. Mater. 2023, 12, 2202066. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Muñoz, K.; Midkiff, K.; Woessner, A.; Afshar-Mohajer, M.; Zou, M.; Pollock, E.; Gonzalez-Nino, D.; Prinz, G.; Hutchinson, L.; Li, R.; et al. A Multicomponent Microneedle Patch for the Delivery of Meloxicam for Veterinary Applications. ACS Nano 2024, 18, 25716–25739. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, X.; Chen, Z.; Lin, X.; Hu, Y.; Zeng, Q.; Wang, D.; Guo, P.; Yin, G.; Wang, L. Dissolving Cinnamaldehyde HA/PVA Microneedles for the Treatment of Skin Diseases Caused by Microsporum Canis. J. Drug Deliv. Sci. Technol. 2025, 107, 106841. [Google Scholar] [CrossRef]
- Vreman, S.; Stockhofe-Zurwieden, N.; Popma-de Graaf, D.J.; Savelkoul, H.F.J.; Barnier-Quer, C.; Collin, N.; Collins, D.; McDaid, D.; Moore, A.C.; Rebel, J.M.J. Immune Responses Induced by Inactivated Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccine in Neonatal Pigs Using Different Adjuvants. Vet. Immunol. Immunopathol. 2021, 232, 110170. [Google Scholar] [CrossRef]
- Choi, I.-J.; Na, W.; Kang, A.; Ahn, M.-H.; Yeom, M.; Kim, H.-O.; Lim, J.-W.; Choi, S.-O.; Baek, S.-K.; Song, D.; et al. Patchless Administration of Canine Influenza Vaccine on Dog’s Ear Using Insertion-Responsive Microneedles (IRMN) without Removal of Hair and Its in Vivo Efficacy Evaluation. Eur. J. Pharm. Biopharm. 2020, 153, 150–157. [Google Scholar] [CrossRef]
- Steinbach, S.; Jalili-Firoozinezhad, S.; Srinivasan, S.; Melo, M.B.; Middleton, S.; Konold, T.; Coad, M.; Hammond, P.T.; Irvine, D.J.; Vordermeier, M.; et al. Temporal Dynamics of Intradermal Cytokine Response to Tuberculin in Mycobacterium Bovis BCG-Vaccinated Cattle Using Sampling Microneedles. Sci. Rep. 2021, 11, 7074. [Google Scholar] [CrossRef]
- Taylor, R.M.; Miller, P.R.; Ebrahimi, P.; Polsky, R.; Baca, J.T. Minimally-Invasive, Microneedle-Array Extraction of Interstitial Fluid for Comprehensive Biomedical Applications: Transcriptomics, Proteomics, Metabolomics, Exosome Research, and Biomarker Identification. Lab. Anim. 2018, 52, 526–530. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Freeman, D.M.E.; Rawson, T.M.; Rogers, M.L.; Wilson, R.C.; Holmes, A.H.; Cass, A.E.; O’Hare, D. Development of a Minimally Invasive Microneedle-Based Sensor for Continuous Monitoring of β-Lactam Antibiotic Concentrations in Vivo. ACS Sens. 2019, 4, 1072–1080. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Gao, B. A Review of Recent Advances in Microneedle-Based Sensing within the Dermal ISF That Could Transform Medical Testing. ACS Sens. 2024, 9, 1149–1161. [Google Scholar] [CrossRef]
- Wilkirson, E.C.; Li, D.; Lillehoj, P.B. Lateral Flow-Based Skin Patch for Rapid Detection of Protein Biomarkers in Human Dermal Interstitial Fluid. ACS Sens. 2024, 9, 5792–5801. [Google Scholar] [CrossRef] [PubMed]
- Aroche, A.F.; Nissan, H.E.; Daniele, M.A. Hydrogel-Forming Microneedles and Applications in Interstitial Fluid Diagnostic Devices. Adv. Healthc. Mater. 2025, 14, 2401782. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, E.; De Crescenzo, G.; Nieto-Argüello, A.; Banquy, X.; Brambilla, D. Superswelling Microneedle Arrays for Dermal Interstitial Fluid (Prote) Omics. Adv. Funct. Mater. 2021, 31, 2106061. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Brady, A.J.; Eltayib, E.; Meng, T.; Alonso-Vicente, A.; Gonzalez-Vazquez, P.; Torrisi, B.M.; Vicente-Perez, E.M.; Mooney, K.; Jones, D.S.; et al. Hydrogel-Forming Microneedle Arrays Allow Detection of Drugs and Glucose In Vivo: Potential for Use in Diagnosis and Therapeutic Drug Monitoring. PLoS ONE 2015, 10, e0145644. [Google Scholar] [CrossRef]
- Al Sulaiman, D.; Chang, J.Y.H.; Bennett, N.R.; Topouzi, H.; Higgins, C.A.; Irvine, D.J.; Ladame, S. Hydrogel-Coated Microneedle Arrays for Minimally Invasive Sampling and Sensing of Specific Circulating Nucleic Acids from Skin Interstitial Fluid. ACS Nano 2019, 13, 9620–9628. [Google Scholar] [CrossRef]
- Ranamukhaarachchi, S.A.; Lehnert, S.; Ranamukhaarachchi, S.L.; Sprenger, L.; Schneider, T.; Mansoor, I.; Rai, K.; Häfeli, U.O.; Stoeber, B. A Micromechanical Comparison of Human and Porcine Skin before and after Preservation by Freezing for Medical Device Development. Sci. Rep. 2016, 6, 32074. [Google Scholar] [CrossRef]
- Suurs, P.; Van Den Brand, H.; Ten Have, R.; Daamen, W.F.; Barbut, S. Evaluation of Cattle Skin Collagen for Producing Co-Extrusion Sausage Casing. Food Hydrocoll. 2023, 140, 108595. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal Models for Percutaneous Absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef]
- Todo, H. Transdermal Permeation of Drugs in Various Animal Species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Renaudeau, D.; Leclercq-Smekens, M.; Herin, M. Differences in Skin Characteristics in European (Large White) and Caribbean (Creole) Growing Pigs with Reference to Thermoregulation. Anim. Res. 2006, 55, 209–217. [Google Scholar] [CrossRef]
- Simon, J.; Mailley, P.; Pin, D.; Mailley, S.; Alava, T.; Ferlay, A.; Blanc, F. Determination of an Implantation Area for Interstitial Fluid Extraction in Cows and Feasibility of Adapted Microneedles. Biosyst. Eng. 2022, 222, 62–70. [Google Scholar] [CrossRef]
- Hamid, M.A.; Husain, S.M.I.; Khan, M.K.I.; Islam, M.N.; Biswas, M.A.A. Skin Thickness in Relation to Milk Production of Crossbred Cows. Pak. J. Biol. Sci. 2000, 3, 1525–1529. [Google Scholar] [CrossRef]
- Dowling, D. The Thickness of Cattle Skin. Aust. J. Agric. Res. 1955, 6, 776–785. [Google Scholar] [CrossRef]
- Darmau, B.; Hoang, A.; Gross, A.J.; Texier, I. Water-Based Synthesis of Dextran-Methacrylate and Its Use to Design Hydrogels for Biomedical Applications. Eur. Polym. J. 2024, 221, 113515. [Google Scholar] [CrossRef]
- Kozlowska, J.; Pauter, K.; Sionkowska, A. Carrageenan-Based Hydrogels: Effect of Sorbitol and Glycerin on the Stability, Swelling and Mechanical Properties. Polym. Test. 2018, 67, 7–11. [Google Scholar] [CrossRef]
- Palencia, M.S.; Mora, M.A.; Palencia, S.L. Biodegradable Polymer Hydrogels Based in Sorbitol and Citric Acid for Controlled Release of Bioactive Substances from Plants (Polyphenols). Curr. Chem. Biol. 2017, 11, 36–43. [Google Scholar] [CrossRef]
- Sabri, A.H.B.; Anjani, Q.K.; Donnelly, R.F. Synthesis and Characterization of Sorbitol Laced Hydrogel-Forming Microneedles for Therapeutic Drug Monitoring. Int. J. Pharm. 2021, 607, 121049. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Weng, Z.; Sun, H.; Nistala, R.; Zhang, Y. Microneedle-Based Potentiometric Sensing System for Continuous Monitoring of Multiple Electrolytes in Skin Interstitial Fluids. ACS Sens. 2021, 6, 2181–2190. [Google Scholar] [CrossRef]
- Darmau, B.; Sacchi, M.; Texier, I.; Gross, A.J. Self-Extracting Dextran-Based Hydrogel Microneedle Arrays with an Interpenetrating Bioelectroenzymatic Sensor for Transdermal Monitoring with Matrix Protection. Adv. Healthc. Mater. 2025, 14, 2403209. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of Microneedles into Skin: Measurement and Prediction of Insertion Force and Needle Fracture Force. J. Biomech. 2003, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A Proposed Model Membrane and Test Method for Microneedle Insertion Studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Romgens, A.M.; Bader, D.L.; Bouwstra, J.A.; Baajiens, F.P.T.; Oomens, C.W.J. Monitoring the Penetration Process of Single Microneedles with Varying Tip Diameters. J. Mech. Behav. Biomed. Mater. 2014, 40, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Ingrole, R.S.J.; Azizoglu, E.; Dul, M.; Birchall, J.C.; Gill, H.S.; Prausnitz, M.R. Trends of Microneedle Technology in the Scientific Literature, Patents, Clinical Trials and Internet Activity. Biomaterials 2021, 267, 120491. [Google Scholar] [CrossRef]
- Chua, B.; Desai, S.P.; Tierney, M.J.; Tamada, J.A.; Jina, A.N. Effect of Microneedles Shape on Skin Penetration and Minimally Invasive Continuous Glucose Monitoring in Vivo. Sens. Actuators Phys. 2013, 203, 373–381. [Google Scholar] [CrossRef]
- Wei, J.C.J.; Cartmill, I.D.; Kendall, M.A.F.; Crichton, M.L. In Vivo, in Situ and Ex Vivo Comparison of Porcine Skin for Microprojection Array Penetration Depth, Delivery Efficiency and Elastic Modulus Assessment. J. Mech. Behav. Biomed. Mater. 2022, 130, 105187. [Google Scholar] [CrossRef]
- Knox, F.S.; Wachtel, T.L.; McCahan, G.R.; Knapp, S.C. Thermal Properties Calculated from Measured Water Content as a Function of Depth in Porcine Skin. Burns 1986, 12, 556–562. [Google Scholar] [CrossRef]



| Duration of MNA Insertion | Amount of Up Taken Fluid (mg) | Length of MN Tip into Which the Liquid Has Diffused (µm) (% of the MN Total Height) |
|---|---|---|
| 15 min | ND 1 | 1665 (65%) |
| 3 h | 10.7 | 1908 (75%) |
| 24 h | 31.4 | 1579 (62%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautier, L.; Wiart-Letort, S.; Massé, A.; Xavier, C.; Novais-Gameiro, L.; Hoang, A.; Escudé, M.; Sorrentino, I.; Bonnet, M.; Gondret, F.; et al. Design of Hydrogel Microneedle Arrays for Physiology Monitoring of Farm Animals. Micromachines 2025, 16, 1015. https://doi.org/10.3390/mi16091015
Gautier L, Wiart-Letort S, Massé A, Xavier C, Novais-Gameiro L, Hoang A, Escudé M, Sorrentino I, Bonnet M, Gondret F, et al. Design of Hydrogel Microneedle Arrays for Physiology Monitoring of Farm Animals. Micromachines. 2025; 16(9):1015. https://doi.org/10.3390/mi16091015
Chicago/Turabian StyleGautier, Laurabelle, Sandra Wiart-Letort, Alexandra Massé, Caroline Xavier, Lorraine Novais-Gameiro, Antoine Hoang, Marie Escudé, Ilaria Sorrentino, Muriel Bonnet, Florence Gondret, and et al. 2025. "Design of Hydrogel Microneedle Arrays for Physiology Monitoring of Farm Animals" Micromachines 16, no. 9: 1015. https://doi.org/10.3390/mi16091015
APA StyleGautier, L., Wiart-Letort, S., Massé, A., Xavier, C., Novais-Gameiro, L., Hoang, A., Escudé, M., Sorrentino, I., Bonnet, M., Gondret, F., Verplanck, C., & Texier, I. (2025). Design of Hydrogel Microneedle Arrays for Physiology Monitoring of Farm Animals. Micromachines, 16(9), 1015. https://doi.org/10.3390/mi16091015






