Holmium Metal Nanoparticle PbO2 Anode Formed by Electrodeposition for Efficient Removal of Insecticide Acetamiprid and Improved Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
3. Results
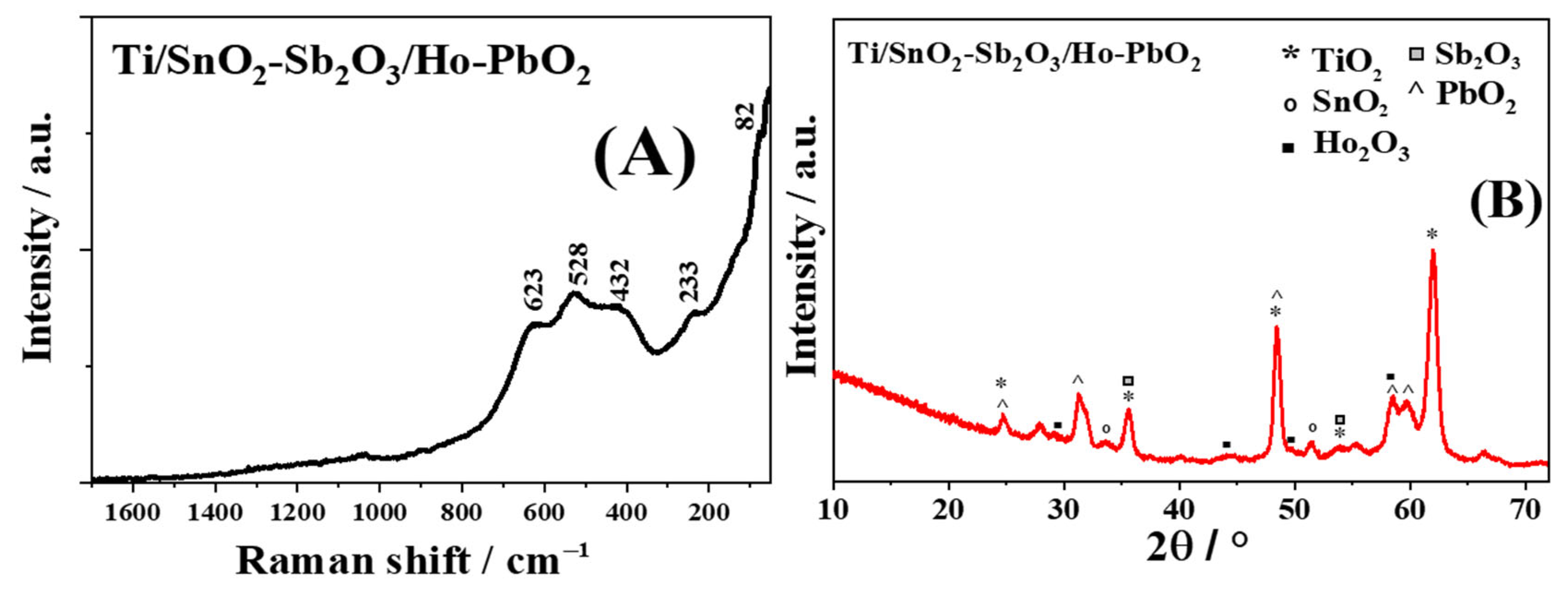
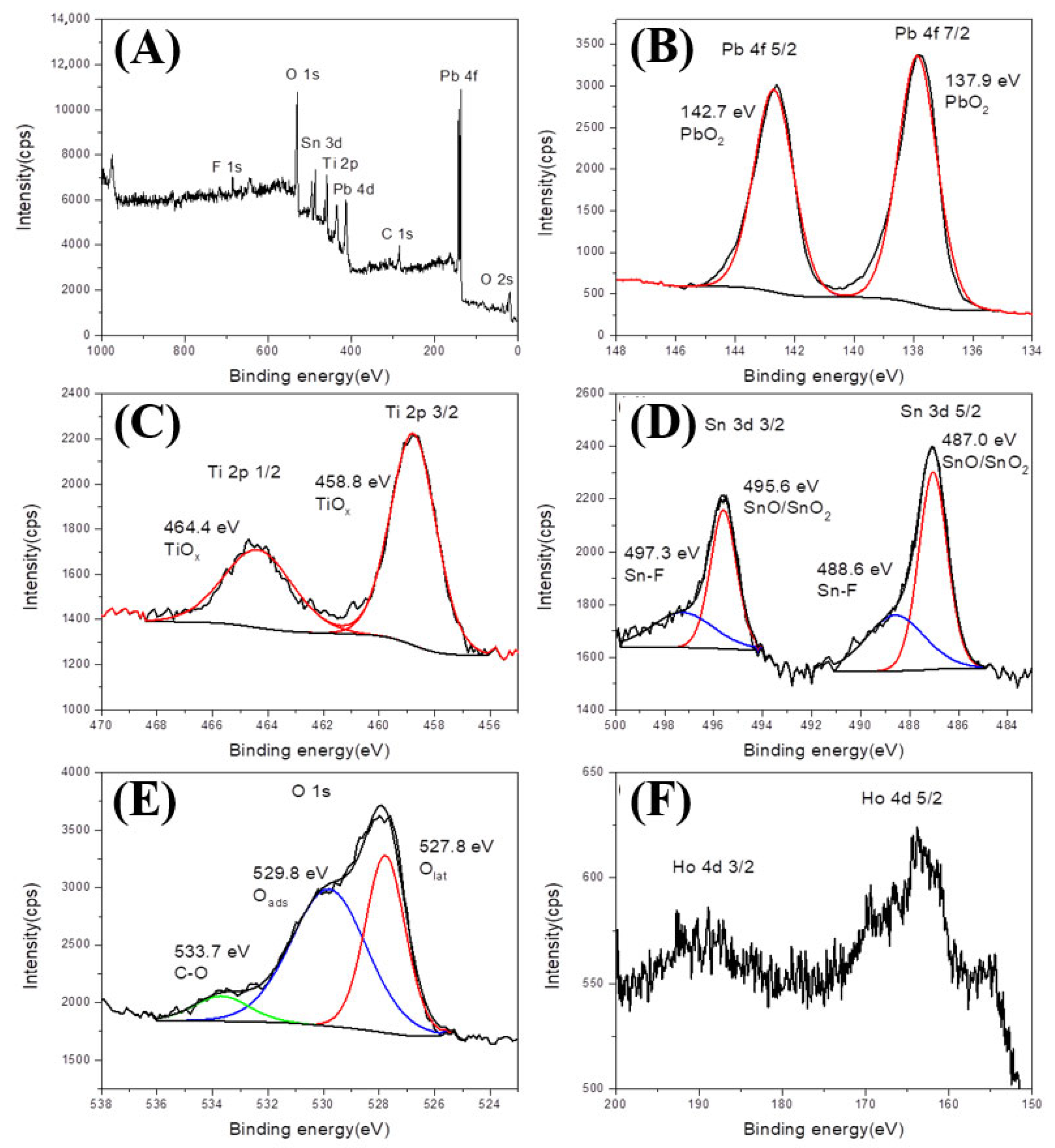
3.1. Characterization of Ti/SnO2-Sb2O3/Ho-PbO2 Electrocatalyst
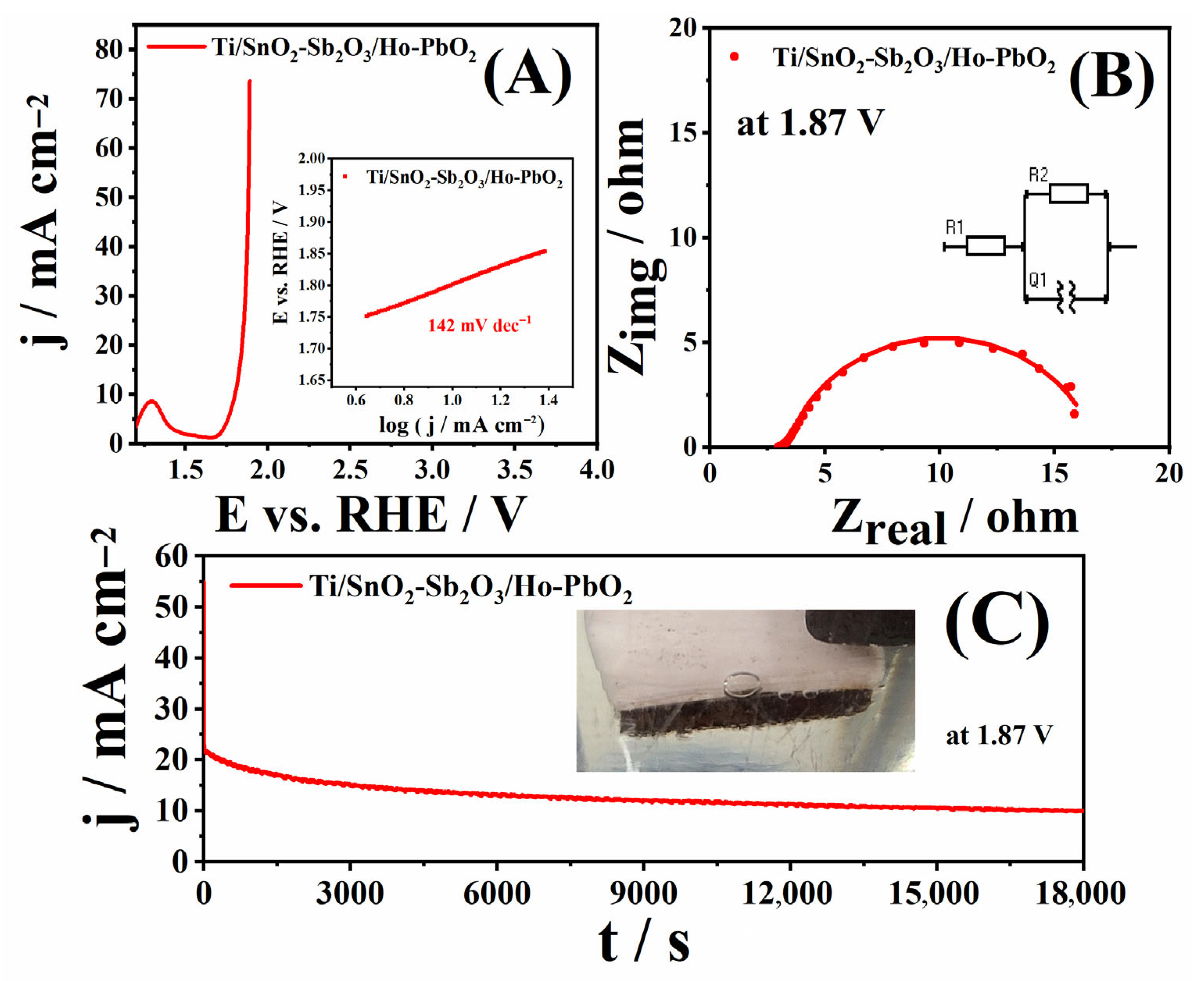
3.2. Oxygen Evolution Reaction Investigation
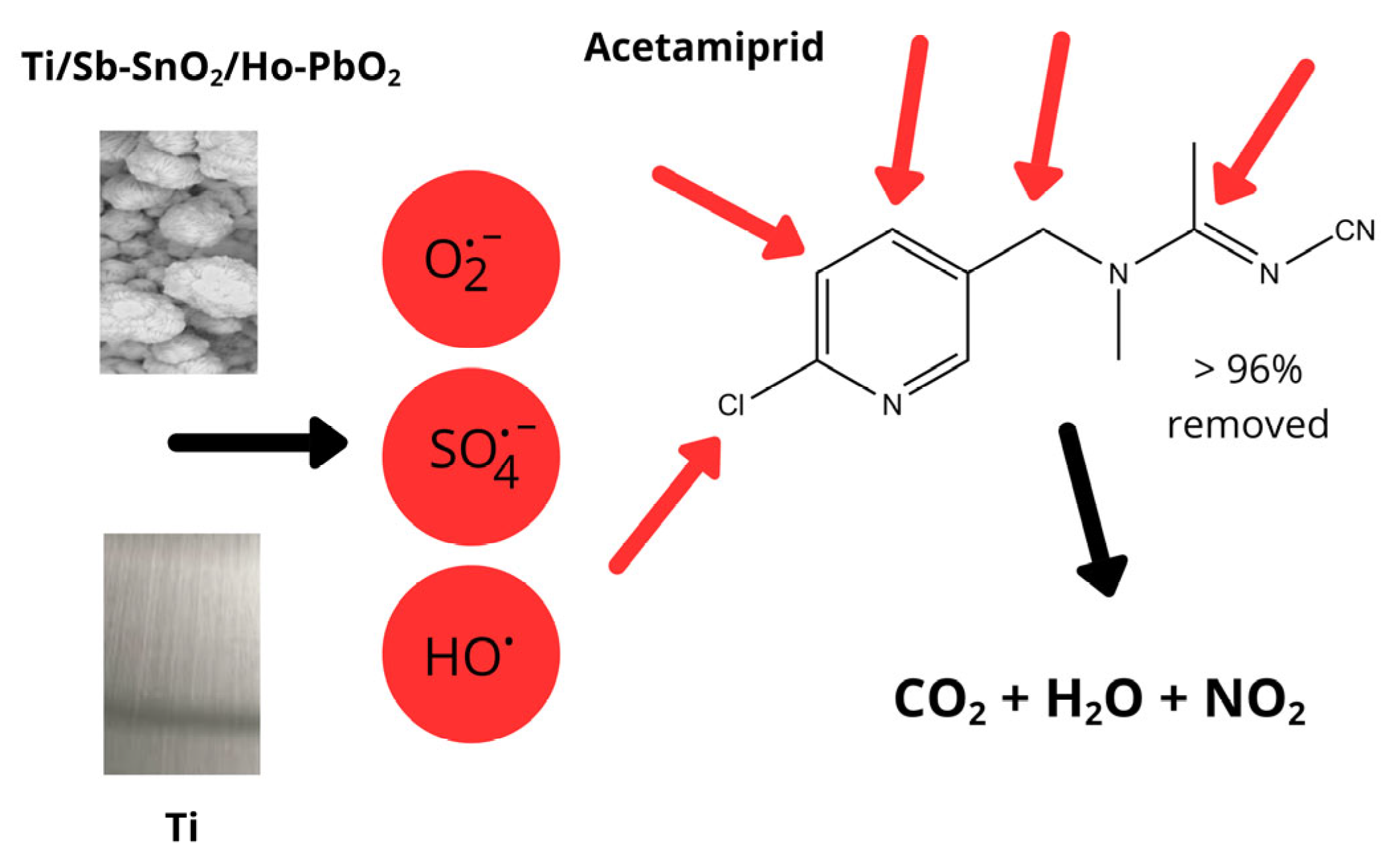
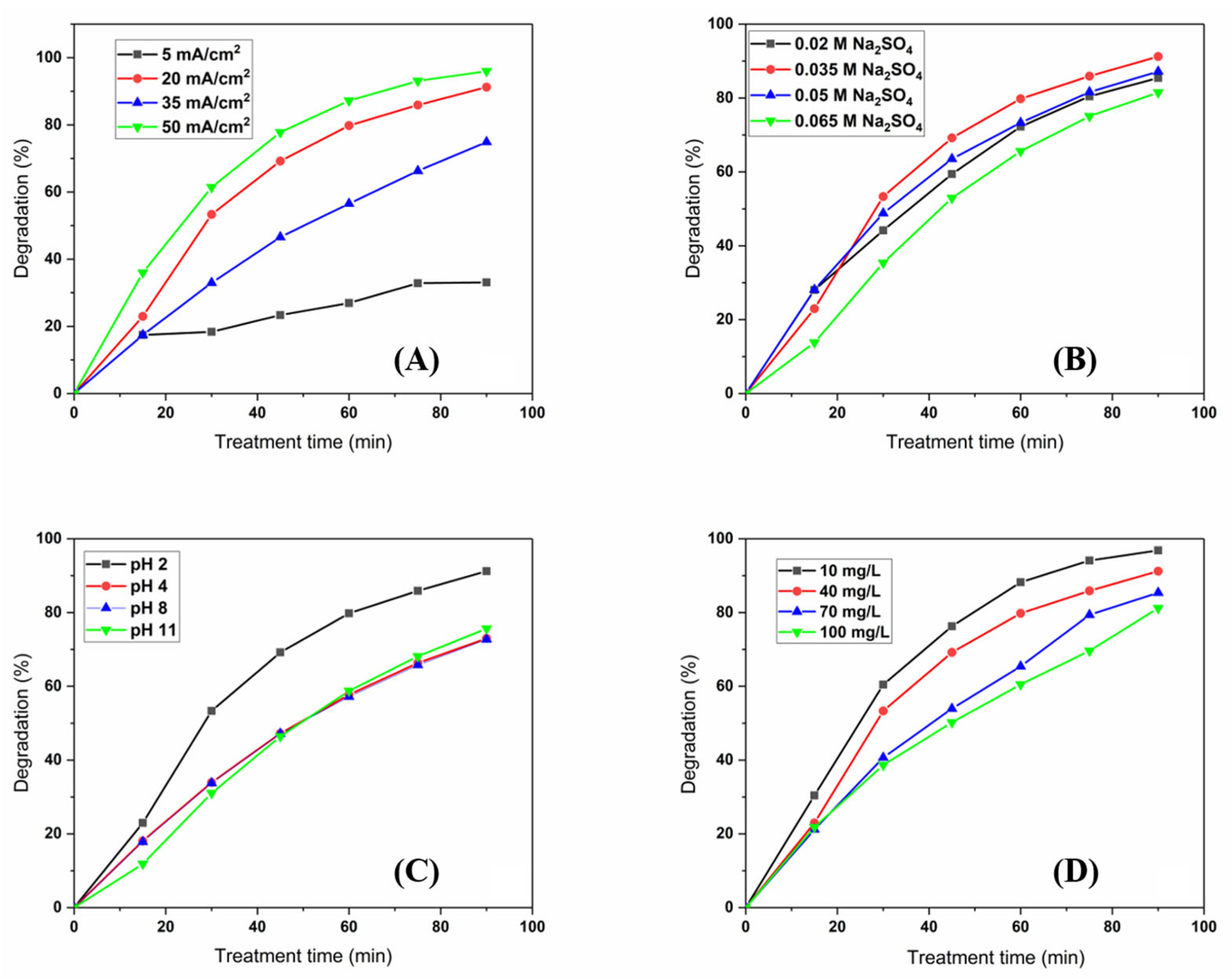
3.3. Acetamiprid Removal Studies
3.4. Comparison of the Degradation Method with Literature Results for the Degradation of Acetamiprid
3.5. Ti/SnO2-Sb2O3/Ho-PbO2 Anode Reuse
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Wang, P.; He, M.; Yuan, X.; Fang, Z.; Li, Z. Rare Earth-Based Nanomaterials in Electrocatalysis. Coord. Chem. Rev. 2023, 489, 215204. [Google Scholar] [CrossRef]
- Shen, W.; Da, P.; Guo, L.; Xi, P.; Yan, C.-H. Rare Earth Interface Structure Materials: Synthesis, Applications, and Mechanisms. Acc. Mater. Res. 2024, 5, 712–725. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Lee, J.-M.; Fu, G. Recent Advances in Rare-Earth-Based Materials for Electrocatalysis. Chem Catal. 2022, 2, 967–1008. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, H.; Liang, Z.; Zhang, Q.; Du, Y. Rare Earth Oxide Based Electrocatalysts: Synthesis, Properties and Applications. Chem. Soc. Rev. 2023, 53, 714–763. [Google Scholar] [CrossRef]
- Kabir, M.H.; Hossain, M.Z.; Jalil, M.A.; Ghosh, S.; Hossain, M.M.; Ali, M.A.; Khandaker, M.U.; Jana, D.; Rahman, M.M.; Hossain, M.K.; et al. The Efficacy of Rare-Earth Doped V2O5 Photocatalyst for Removal of Pollutants from Industrial Wastewater. Opt. Mater. 2024, 147, 114724. [Google Scholar] [CrossRef]
- Yavuz, A.; Aydin, D.; Disli, B.; Ozturk, T.; Gul, B.; Gubbuk, I.H.; Ersoz, M. Enhancing Visible Light Photocatalytic Activity of Holmium Doped G-C3N4 and DFT Theoretical Insights. Environ. Sci. Pollut. Res. 2024, 31, 44828–44847. [Google Scholar] [CrossRef]
- Aslam, A.; Alomayrah, N.; Khan, M.N.; Sohail, M.; Din, M.I.; Warsi, M.F.; Anwar, M. Holmium Doped La2O3 and Its Composite with Graphitic Carbon Nitride for the Removal of Atrazine and Chlorpyrifos from Wastewater. Desalin. Water Treat. 2024, 320, 100813. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ahmed, F.; Alshahrani, T.; Arshi, N. Synergism of Holmium Orthovanadate/Phosphorus-Doped Carbon Nitride Nanocomposite: Nonenzymatic Electrochemical Detection of Hydrogen Peroxide. Inorg. Chem. 2024, 63, 3019–3027. [Google Scholar] [CrossRef]
- Fathi, Z.; Jahani, S.; Foroughi, M.M. Electrode Material Fabricated by Doping Holmium in Nickel Oxide and Its Application in Electrochemical Sensor for Flutamide Determination as a Prostate Cancer Drug. Monatsh. Chem.-Chem. Mon. 2021, 152, 757–766. [Google Scholar] [CrossRef]
- Li, H.; He, M.; Meng, S.; Wang, P.; Yang, C.; Yao, J.; Hu, Z.; Li, Z. Ultrathin Two-Dimensional Mesoporous Holmium Oxide Nanosheet-Stabilized Copper Nanoparticles for Stable and Efficient Electrocatalytic Semi-Hydrogenation of Acetylene. Inorg. Chem. Front. 2025, 12, 2805–2817. [Google Scholar] [CrossRef]
- Tilehkan, A.; Arvand, M. Study on the Electrochemical and Spectroscopic Characteristics of Holmium Ion and Its Interaction with DNA. Sci. Rep. 2024, 14, 20383. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Nastasić, A.; Martins, M.; Sequeira, C.A.C.; Šljukić, B. Air Cathodes and Bifunctional Oxygen Electrocatalysts for Aqueous Metal–Air Batteries. Batteries 2023, 9, 394. [Google Scholar] [CrossRef]
- Milikić, J.; Knežević, S.; Ognjanović, M.; Stanković, D.; Rakočević, L.; Šljukić, B. Template-Based Synthesis of Co3O4 and Co3O4/SnO2 Bifunctional Catalysts with Enhanced Electrocatalytic Properties for Reversible Oxygen Evolution and Reduction Reaction. Int. J. Hydrogen Energy 2023, 48, 27568–27581. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Rakočević, L.; Lazarević, S.; Šljukić, B. Porous Cerium-Zeolite Bifunctional ORR/OER Electrocatalysts in Alkaline Media. J. Electroanal. Chem. 2023, 944, 117668. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient Bifunctional Cerium-Zeolite Electrocatalysts for Oxygen Evolution and Oxygen Reduction Reactions in Alkaline Media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Milikić, J.; Martins, M.; Dobrota, A.S.; Bozkurt, G.; Soylu, G.S.P.; Yurtcan, A.B.; Skorodumova, N.V.; Pašti, I.A.; Šljukić, B.; Santos, D.M.F. A Pt/MnV2O6 Nanocomposite for the Borohydride Oxidation Reaction. J. Energy Chem. 2021, 55, 428–436. [Google Scholar] [CrossRef]
- Bashir, A.; Inayat, A.; Bashir, R.; Jamil, S.; Abbas, S.M.; Sultan, M.; Iqbal, A.; Akhter, Z. Design of Porous Ni and Rare Earth Metal (Ce, Ho, and Eu) Co-Doped TiO2 Nanoarchitectures for Energy Conversion and Storage Applications. New J. Chem. 2023, 47, 3560–3571. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen Evolution Reaction in Alkaline Environment: Material Challenges and Solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen Evolution Reaction (OER) Mechanism under Alkaline and Acidic Conditions. J. Phys. Energy 2021, 3, 026001. [Google Scholar] [CrossRef]
- Rathod, S.; Pataniya, P.; Joshi, K.K.; Shekh, M.; Sumesh, C.K.; Kapatel, S. Synergistic RB5 Dye Degradation and Oxygen Evolution Reaction (OER) Catalysis by WO3 Nano-Pellets: Mechanistic Insights and Water Remediation Applications. Surf. Interfaces 2024, 48, 104216. [Google Scholar] [CrossRef]
- Parchami, K.; Habibzadehnesami, N.; Pirkarami, A.; Haghighat-Nezhad, S.; Gheitasi, N.; Nabavi, M.S.; Ghasemi, E. Enhancing NiAl-LDH@TiO2-Tiv-S Photoelectrocatalyst Performance for Textile Dye Degradation and Oxygen Production: S-Doped Ti Vacancies. Environ. Res. 2025, 279, 121741. [Google Scholar] [CrossRef] [PubMed]
- Mandari, K.K.; Im, Y.; Lee, Y.-A.; Pandey, S.; Kang, M. Ag2S/NiCo2S4@g-C3N4 Heterostructure: A Dual-Functional Catalyst for Enhanced Photocatalytic Degradation and Oxygen Evolution Reactions. Appl. Surf. Sci. 2025, 704, 163452. [Google Scholar] [CrossRef]
- Gyanprakash, D.M.; Gupta, P.K.; Sharma, G.P.; Pala, R.G.S. Surface-Enhanced OER Activity in Co3V2O8 Using Cyclic Charge-Discharge to Balance Electrocatalytic Active Site Generation and Degradation. Electrochim. Acta 2021, 367, 137538. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Wang, J.; Ji, Z.; Lu, P.; Wang, X.; Chen, F. Rational Design of Two Novel Metal–Organic Frameworks as Photocatalysts for Degradation of Organic Dyes and Their Derived Materials as Electrocatalysts for OER. Inorg. Chim. Acta 2021, 523, 120416. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Lu, Y.-X.; Li, B.; Huang, G.-F.; Zeng, F.; Li, Y.-Y.; Pan, A.; Chai, Y.-F.; Huang, W.-Q. Acid-Induced Topological Morphology Modulation of Graphitic Carbon Nitride Homojunctions as Advanced Metal-Free Catalysts for OER and Pollutant Degradation. J. Mater. Sci. Technol. 2021, 86, 210–218. [Google Scholar] [CrossRef]
- Bashir, A.; Munawar, T.; Mukhtar, F.; Nadeem, M.S.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; Iqbal, F. Dual-Functional Fullerene Supported NiO-Based Nanocomposite: Efficient Electrocatalyst for OER and Photocatalyst for MB Dye Degradation. Mater. Chem. Phys. 2023, 293, 126886. [Google Scholar] [CrossRef]
- Chen, L.; Cai, T.; Cheng, C.; Xiong, Z.; Ding, D. Degradation of Acetamiprid in UV/H2O2 and UV/Persulfate Systems: A Comparative Study. Chem. Eng. J. 2018, 351, 1137–1146. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Muhammad, Y.; He, H.; Zhao, Z.; Nie, S.; Zhao, Z. Defect Engineering of NH2-MIL-88B(Fe) Using Different Monodentate Ligands for Enhancement of Photo-Fenton Catalytic Performance of Acetamiprid Degradation. Chem. Eng. J. 2020, 398, 125684. [Google Scholar] [CrossRef]
- Meijide, J.; Rodríguez, S.; Sanromán, M.A.; Pazos, M. Comprehensive Solution for Acetamiprid Degradation: Combined Electro-Fenton and Adsorption Process. J. Electroanal. Chem. 2018, 808, 446–454. [Google Scholar] [CrossRef]
- Marcelo, C.R.; Lopes, R.P.; Cruz, J.C.; Nascimento, M.A.; Silva, A.A.; Lima, C.F. Evaluation of Different Parameters on the Acetamiprid Degradation by Bimetallic Fe/Ni Nanoparticles. Sep. Purif. Technol. 2016, 171, 256–262. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, J.; Jiang, J.; Dai, Y.; Sheng, M. Nitrile Hydratases: From Industrial Application to Acetamiprid and Thiacloprid Degradation. J. Agric. Food Chem. 2021, 69, 10440–10449. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.B.; Raut-Jadhav, S.; Topare, N.S.; Pandit, A.B. Combined Strategy of Hydrodynamic Cavitation and Fenton Chemistry for the Intensified Degradation of Acetamiprid. Sep. Purif. Technol. 2023, 325, 124701. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Huang, C.; Liu, B.; Guo, L. Electrochemical Oxidation of Acetamiprid Using Yb-Doped PbO2 Electrodes: Electrode Characterization, Influencing Factors and Degradation Pathways. Sep. Purif. Technol. 2019, 211, 456–466. [Google Scholar] [CrossRef]
- Zbiljić, J.; Guzsvány, V.; Vajdle, O.; Prlina, B.; Agbaba, J.; Dalmacija, B.; Kónya, Z.; Kalcher, K. Determination of H2O2 by MnO2 Modified Screen Printed Carbon Electrode during Fenton and Visible Light-Assisted Photo-Fenton Based Removal of Acetamiprid from Water. J. Electroanal. Chem. 2015, 755, 77–86. [Google Scholar] [CrossRef]
- Stanković, D.M.; Ognjanović, M.; Espinosa, A.; del Puerto Morales, M.; Bessais, L.; Zehani, K.; Antić, B.; Dojcinović, B. Iron Oxide Nanoflower–Based Screen Print Electrode for Enhancement Removal of Organic Dye Using Electrochemical Approach. Electrocatalysis 2019, 10, 663–671. [Google Scholar] [CrossRef]
- Stanković, D.M.; Kukuruzar, A.; Savić, S.; Ognjanović, M.; Janković-Častvan, I.M.; Roglić, G.; Antić, B.; Manojlović, D.; Dojčinović, B. Sponge-like Europium Oxide from Hollow Carbon Sphere as a Template for an Anode Material for Reactive Blue 52 Electrochemical Degradation. Mater. Chem. Phys. 2021, 273, 125154. [Google Scholar] [CrossRef]
- Jović, M.; Stanković, D.; Manojlović, D.; Anđelković, I.; Milić, A.; Dojčinović, B.; Roglić, G. Study of the Electrochemical Oxidation of Reactive Textile Dyes Using Platinum Electrode. Int. J. Electrochem. Sci. 2013, 8, 16. [Google Scholar] [CrossRef]
- Vlahović, F.; Ognjanović, M.; Djurdjić, S.; Kukuruzar, A.; Antić, B.; Dojčinović, B.; Stanković, D. Design of an Ethidium Bromide Control Circuit Supported by Deep Theoretical Insight. Appl. Catal. B Environ. 2023, 334, 122819. [Google Scholar] [CrossRef]
- Manojlović, D.; Lelek, K.; Roglić, G.; Zherebtsov, D.; Avdin, V.; Buskina, K.; Sakthidharan, C.; Sapozhnikov, S.; Samodurova, M.; Zakirov, R.; et al. Efficiency of Homely Synthesized Magnetite: Carbon Composite Anode toward Decolorization of Reactive Textile Dyes. Int. J. Environ. Sci. Technol. 2020, 17, 2455–2462. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Hong, R.; Yu, C. Electrochemical Desalination of Sea Sand Using Ti/PbO2 Electrodes: Influencing Factors, Parameter Optimization and Removal Mechanism. Desalination 2025, 601, 118576. [Google Scholar] [CrossRef]
- Li, Y.; Sui, X.; Geng, S.; Wang, H.; Duan, X. Anodic Oxidation Using 3D Carbon Felt/PbO2 Anode: A Electron Transfer-Mediated System for Degradation of Rhodamine B. Environ. Technol. 2025, 46, 3047–3064. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Yi, G.; Guo, X.; Yin, Y.; Xu, B.; Zhang, J.; Sun, L.; Zeng, H.; Xing, B.; et al. Carbonized Nitrogen Doped Carbon Dots Functionalized PbO2 Electrode for Efficient Degradation of Organic Pollutants. J. Mol. Struct. 2025, 1319, 139453. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.T.; Feng, Y.; Ai, Q.; Liu, Y.; Yan, Y.; Li, Q.; Lou, J. Hierarchical Porous PbO2 Electrode for Electro-Degradation of Various Contaminants. Small Struct. 2025, 6, 2400389. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, J.; Wu, T.; Duan, X.; Zhao, X.; Ren, X.; Zhou, T. New Ti/CNT/CNT-Ce-PbO2 Anode Synergy Peroxymonosulfate Activation for Efficiently Electrocatalytic Degradation of p-Aminobenzoic Acid. Environ. Res. 2025, 264, 120383. [Google Scholar] [CrossRef]
- Sun, Z.; Gan, L.; Liu, Y.; Liu, Q.; Gao, Q.; Ni, Y. Electrochemical Oxidation of Methylene Blue from High Salinity Dyeing Wastewater by a Novel Reduced Graphene Oxide and La Co-Modified PbO2 Electrode. Desalination 2025, 600, 118464. [Google Scholar] [CrossRef]
- Duan, X.; Ma, F.; Yuan, Z.; Chang, L.; Jin, X. Comparative Studies on the Electro-Catalytic Oxidation Performance of Surfactant–Carbon Nanotube-Modified PbO2 Electrodes. J. Electroanal. Chem. 2012, 677–680, 90–100. [Google Scholar] [CrossRef]
- Kaluđerović, M.; Savić, S.; Bajuk-Bogdanović, D.; Jovanović, A.Z.; Rakočević, L.; Vlahović, F.; Milikić, J.; Stanković, D. Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties. Processes 2025, 13, 1459. [Google Scholar] [CrossRef]
- Costantini, I.; Lottici, P.P.; Bersani, D.; Pontiroli, D.; Casoli, A.; Castro, K.; Madariaga, J.M. Darkening of Lead- and Iron-Based Pigments on Late Gothic Italian Wall Paintings: Energy Dispersive X-Ray Fluorescence, μ-Raman, and Powder X-Ray Diffraction Analyses for Diagnosis: Presence of β-PbO2 (Plattnerite) and α-PbO2 (Scrutinyite). J. Raman Spectrosc. 2020, 51, 680–692. [Google Scholar] [CrossRef]
- Lü, X.; Hao, P.; Xie, G.; Duan, J.; Gao, L.; Liu, B. A Sensor Array Realized by a Single Flexible TiO2/POMs Film to Contactless Detection of Triacetone Triperoxide. Sensors 2019, 19, 915. [Google Scholar] [CrossRef]
- Abdusalyamova, M.N.; Makhmudov, F.A.; Shairmardanov, E.N.; Kovalev, I.D.; Fursikov, P.V.; Khodos, I.I.; Shulga, Y.M. Structural Features of Nanocrystalline Holmium Oxide Prepared by the Thermal Decomposition of Organic Precursors. J. Alloys Compd. 2014, 601, 31–37. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Jovanović, T.; Milikić, J.; Cvjetićanin, N.; Stojadinović, S.; Šljukić, B. Performance of Au/Ti and Au/TiO2 Nanotube Array Electrodes for Borohydride Oxidation and Oxygen Reduction Reaction in Alkaline Media. Electroanalysis 2020, 32, 1867–1874. [Google Scholar] [CrossRef]
- Mo, H.; Chen, Y.; Tang, Y.; Li, T.; Zhuang, S.; Wang, L.; Yang, X.; Wan, P. Direct Determination of Chemical Oxygen Demand by Anodic Oxidative Degradation of Organics at a Composite 3-D Electrode. J. Solid State Electrochem. 2019, 23, 1571–1579. [Google Scholar] [CrossRef]
- Hossain, M.D.; Mustafa, C.M.; Islam, M.M. Effect of Deposition Parameters on the Morphology and Electrochemical Behavior of Lead Dioxide. J. Electrochem. Sci. Technol. 2017, 8, 197–205. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Er, A.O.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and Electronic Properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Tigau, N.; Ciupina, V.; Prodan, G.; Rusu, G.I.; Vasile, E. Structural Characterization of Polycrystalline Sb2O3 Thin Films Prepared by Thermal Vacuum Evaporation Technique. J. Cryst. Growth 2004, 269, 392–400. [Google Scholar] [CrossRef]
- Manjunatha, S.; Sunilkumar, A.; Ravikiran, Y.T.; Machappa, T. Effect of Holmium Oxide on Impedance and Dielectric Behavior of Polyaniline–Holmium Oxide Composites. J. Mater. Sci. Mater. Electron. 2019, 30, 10332–10341. [Google Scholar] [CrossRef]
- Liu, S.; Yue, H.; Ho, S.L.; Kim, S.; Park, J.A.; Tegafaw, T.; Ahmad, M.Y.; Kim, S.; Al Saidi, A.K.A.; Zhao, D.; et al. Polyethylenimine-Coated Ultrasmall Holmium Oxide Nanoparticles: Synthesis, Characterization, Cytotoxicities, and Water Proton Spin Relaxivities. Nanomaterials 2022, 12, 1588. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Cui, D.; Yu, L.; Xue, J.; Yin, X. Enhancing the Stability and Electrocatalytic Activity of Ti-Based PbO2 Anodes by Introduction of an Arc-Sprayed TiN Interlayer. Electrochim. Acta 2021, 399, 139398. [Google Scholar] [CrossRef]
- Lović, J.D.; Eraković Pantović, S.; Rakočević, L.Z.; Ignjatović, N.L.; Dimitrijević, S.B.; Nikolić, N.D. A Novel Two-Step Electrochemical Deposition Method for Sn-Pd Electrocatalyst Synthesis for a Potential Application in Direct Ethanol Fuel Cells. Processes 2023, 11, 120. [Google Scholar] [CrossRef]
- Surya Reddy, D.; Rajagopal Reddy, V.; Janardhanam, V.; Choi, C.-J. Microstructural and Chemical Properties of High-k Holmium Oxide (Ho2O3) and Its Effect on Interface Properties and Current Transport Process of Au/n-GaN/Ti/Al Schottky Contact as an Interlayer. Vacuum 2025, 231, 113780. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Zeng, X.; Wang, W.; Shi, R.; Ma, L. Electro-Catalytic Degradation Pathway and Mechanism of Acetamiprid Using an Er Doped Ti/SnO2-Sb Electrode. RSC Adv. 2015, 5, 68700–68713. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H.; Shi, T.; Tang, T.; Li, J. Preparation of Ag-Doped TiO2 Nanoparticles for Photocatalytic Degradation of Acetamiprid in Water. J. Chem. Technol. Biotechnol. 2008, 83, 546–552. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Hajebrahimi, Z.; Malekahmadi, R.; Ahmadzadeh, S. Electrochemical Oxidation Approach towards the Treatment of Acetamiprid Pesticide from Polluted Water. J. Environ. Health Sustain. Dev. 2022, 7, 1561–1570. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Correia, S. BDD Electrochemical Oxidation of Neonicotinoid Pesticides in Natural Surface Waters. Operational, Kinetic and Energetic Aspects. J. Environ. Manag. 2021, 298, 113538. [Google Scholar] [CrossRef]
- Nunes de Morais, A.M.; Medeiros Araújo, D.; Barbosa Segundo, I.D.; Vieira dos Santos, E.; Leal de Castro, S.S.; Martínez-Huitle, C.A.; Fernandes Alves, J.J. A Sustainable Electrochemical-Based Solution for Removing Acetamiprid from Water. Appl. Sci. 2023, 13, 10963. [Google Scholar] [CrossRef]
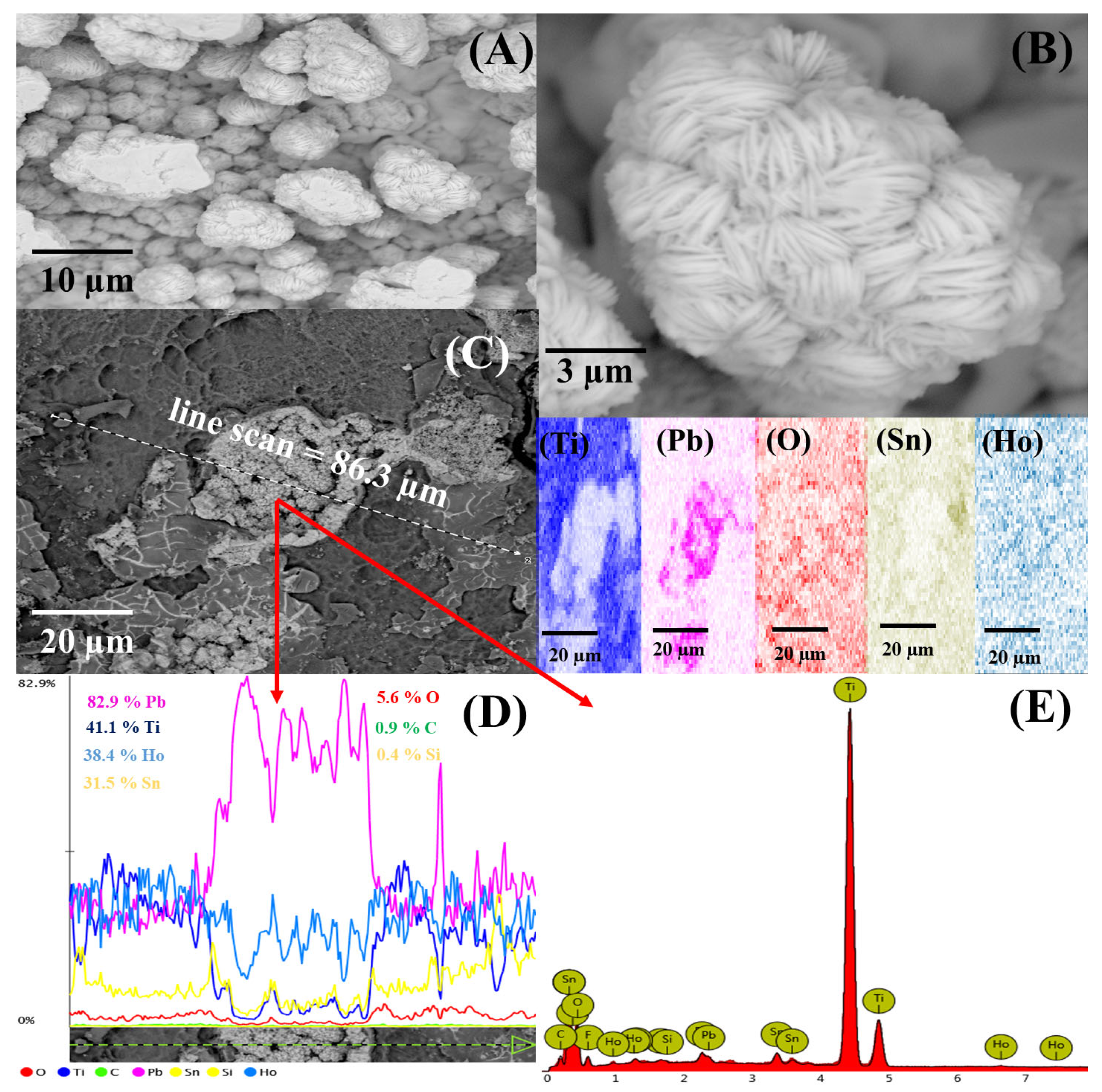

| Element | Atomic Concentration (%) | Weight Concentration (wt %) | Weight Concentration (wt %) (ICP-OES) |
|---|---|---|---|
| Pb | 0.80 | 5.21 | 77.04 |
| O | 45.49 | 22.93 | / |
| C | 6.69 | 2.53 | / |
| Sn | 0.97 | 3.62 | 3.98 |
| Sb | / | / | 1.13 |
| Ti | 39.89 | 60.16 | / |
| F | 5.62 | 3.37 | 1.12 |
| Ho | 0.40 | 2.05 | 2.09 |
| Element | At % | Wt % |
|---|---|---|
| Pb | 12.5 | 62.2 |
| Ti | 2.4 | 2.8 |
| Sn | 1.5 | 4.3 |
| O | 62.0 | 23.8 |
| C | 17.7 | 5.1 |
| F | 3.9 | 1.8 |
| Ho | <0.1 | <0.1 |
| OER Electrocatalysts | Electrolyte | Eonset/V | ηonset/mV | b/mV dec−1 | j at 2 V/mA cm−2 | Ref. |
|---|---|---|---|---|---|---|
| Ti/SnO2-Sb2O3/Ho-PbO2 | 1 M KOH (pH = 14) | 1.61 | 410 | 142 | 73.9 | This work. |
| Ti/SnO2-Sb2O3/PbO2 | 1 M KOH (pH = 14) | 1.83 | 630 | 389 | 67.1 | [48] |
| Ti/SnO2-Sb2O3/Sm-PbO2 | 1 M KOH (pH = 14) | 1.80 | 600 | 489 | 168.4 | [48] |
| Ce-Cli | 1 M KOH (pH = 14) | 1.73 | 530 | 220 | 1.54 | [15] |
| Ce-β | 1 M KOH (pH = 14) | 1.72 | 520 | 312 | 2.6 | [14] |
| TiO2 | 0.1 M NaOH (pH = 13) | ~1.85 | ~650 | 151 | / | [17] |
| Ni-5 | 0.1 M NaOH (pH = 13) | ~1.65 | ~450 | 143 | / | [17] |
| Ni-Eu-3 | 0.1 M NaOH (pH = 13) | ~1.65 | ~450 | 134 | / | [17] |
| Ni-Ce-3 | 0.1 M NaOH (pH = 13) | ~1.65 | ~450 | 141 | / | [17] |
| Ni-Ho-3 | 0.1 M NaOH (pH = 13) | ~1.65 | ~450 | 137 | / | [17] |
| Electrocatalyst | R1 (Ω) | R2 (Ω) | Q1 (mF) | n |
|---|---|---|---|---|
| Ti/SnO2-Sb2O3/Ho-PbO2 | 3.4 | 13.5 | 2.4 × 10−2 | 0.834 |
| Material | Current Density (mA cm−2) | Acetamiprid Concentration (mg L−1) | Supporting Electrolyte Concentration (mol dm−3) | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Yb-doped PbO2 | 150 | 40 | 0.05 Na2SO4 | 98.96 | [34] |
| Er-doped Ti/SnO2–Sb | 10 | 50 | 0.5 M Na2SO4 | 87.45 | [63] |
| Iron | 12 | 3.5 | 0.05 Na2SO4 | 99.02 | [65] |
| Boron-doped diamond | 34.14 | 0.23 | 0.01 Na2SO4 | 87.8 | [66] |
| TiO2-RuO2-IrO2 | 90 | 30 | 0.5 M Na2SO4 | / | [67] |
| Ti/SnO2-Sb2O3/Ho-PbO2 | 20 | 10 | 0.035 | 96.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaludjerović, M.; Savić, S.; Bajuk-Bogdanović, D.; Jovanović, A.; Rakočević, L.; Roglić, G.; Milikić, J.; Stanković, D. Holmium Metal Nanoparticle PbO2 Anode Formed by Electrodeposition for Efficient Removal of Insecticide Acetamiprid and Improved Oxygen Evolution Reaction. Micromachines 2025, 16, 960. https://doi.org/10.3390/mi16080960
Kaludjerović M, Savić S, Bajuk-Bogdanović D, Jovanović A, Rakočević L, Roglić G, Milikić J, Stanković D. Holmium Metal Nanoparticle PbO2 Anode Formed by Electrodeposition for Efficient Removal of Insecticide Acetamiprid and Improved Oxygen Evolution Reaction. Micromachines. 2025; 16(8):960. https://doi.org/10.3390/mi16080960
Chicago/Turabian StyleKaludjerović, Milica, Sladjana Savić, Danica Bajuk-Bogdanović, Aleksandar Jovanović, Lazar Rakočević, Goran Roglić, Jadranka Milikić, and Dalibor Stanković. 2025. "Holmium Metal Nanoparticle PbO2 Anode Formed by Electrodeposition for Efficient Removal of Insecticide Acetamiprid and Improved Oxygen Evolution Reaction" Micromachines 16, no. 8: 960. https://doi.org/10.3390/mi16080960
APA StyleKaludjerović, M., Savić, S., Bajuk-Bogdanović, D., Jovanović, A., Rakočević, L., Roglić, G., Milikić, J., & Stanković, D. (2025). Holmium Metal Nanoparticle PbO2 Anode Formed by Electrodeposition for Efficient Removal of Insecticide Acetamiprid and Improved Oxygen Evolution Reaction. Micromachines, 16(8), 960. https://doi.org/10.3390/mi16080960








