Bright Blue Light Emission of ZnCl2-Doped CsPbCl1Br2 Perovskite Nanocrystals with High Photoluminescence Quantum Yield
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Cs-Oleate Precursor
2.3. Synthesis of the CsPbBr3 NCs
2.4. Synthesis of the CsPbClxBr3−x NCs
2.5. Synthesis of the ZnCl2-Doped CsPbCl1Br2 NCs
2.6. Post-Treatment
2.7. Measurement and Characterization
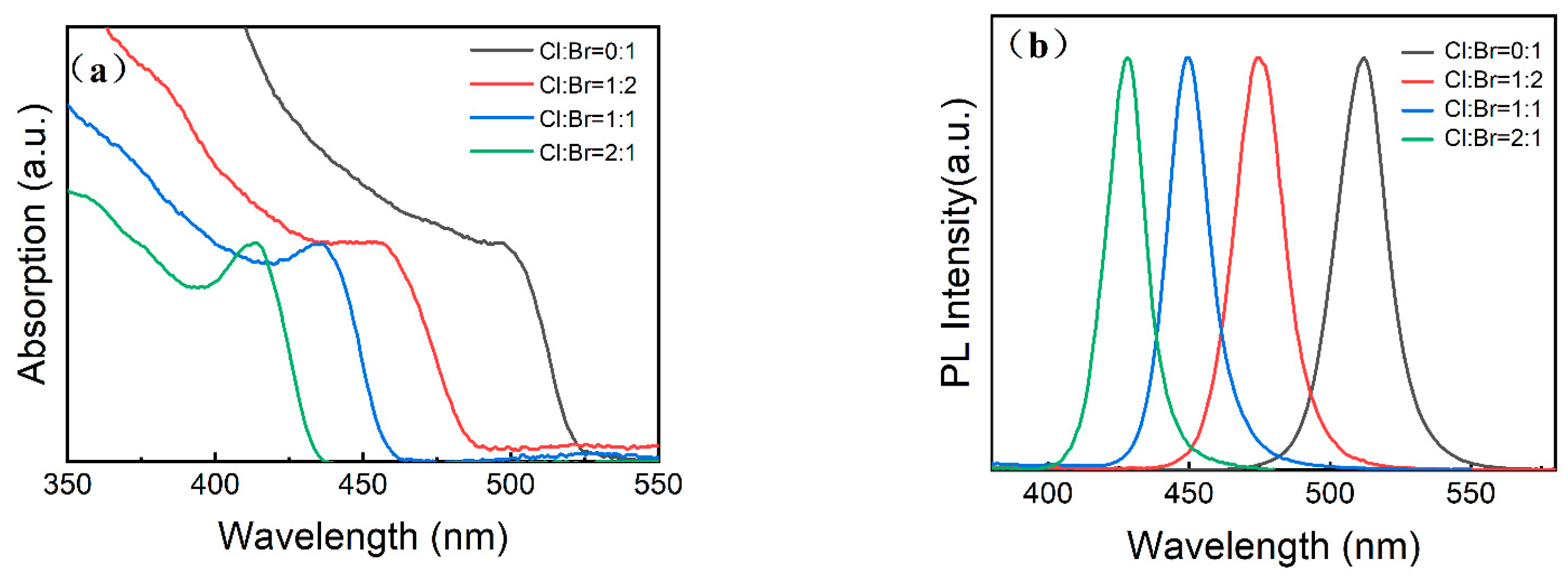
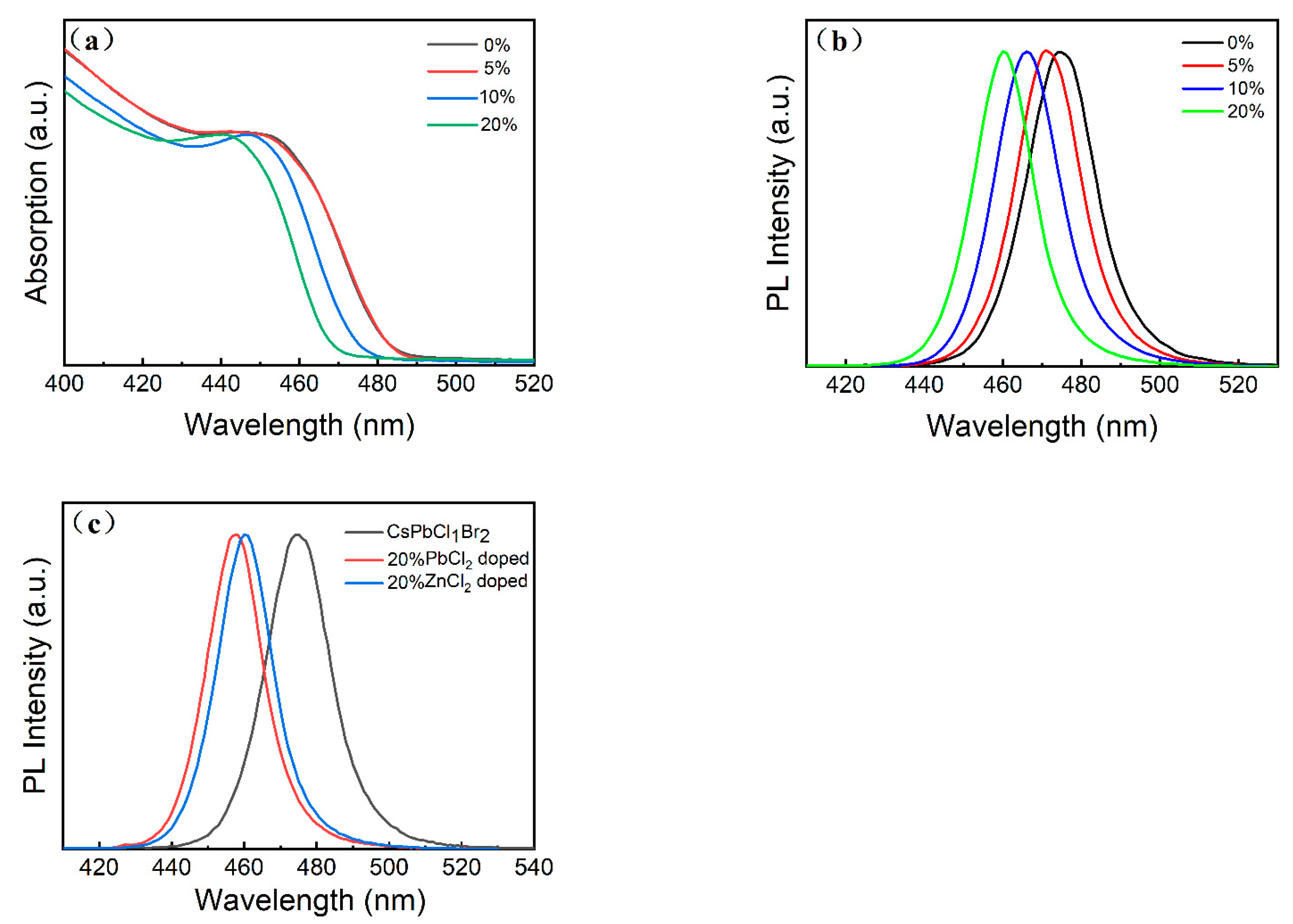
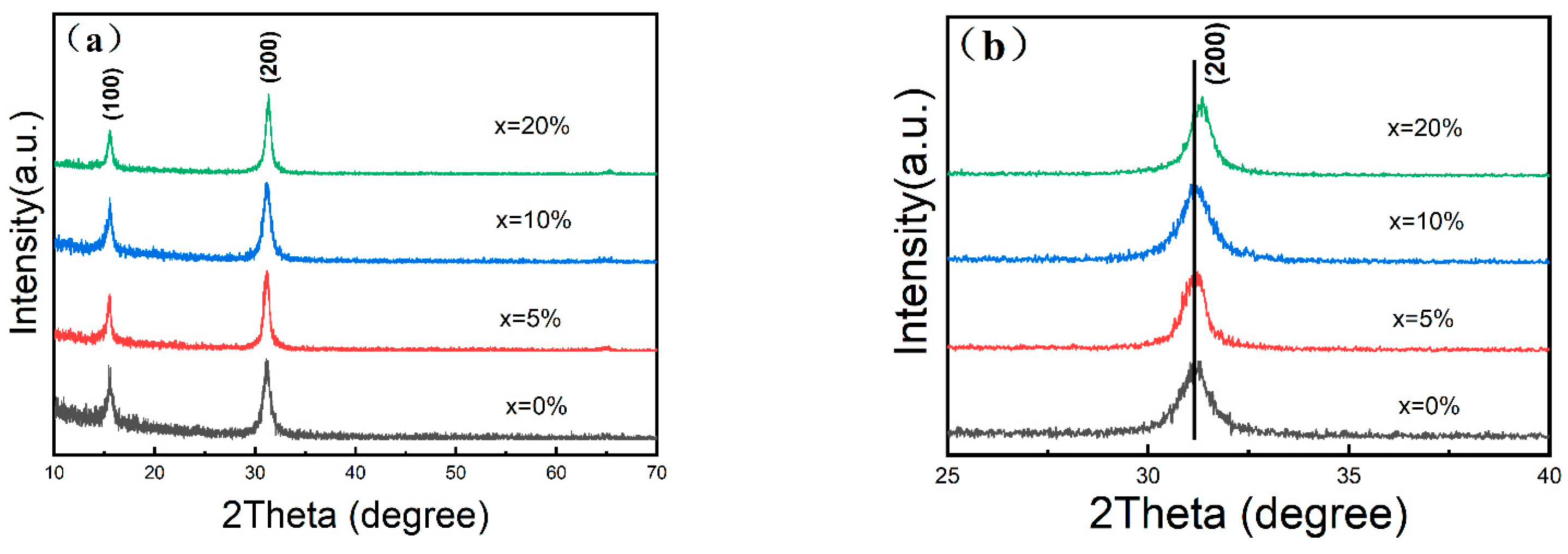
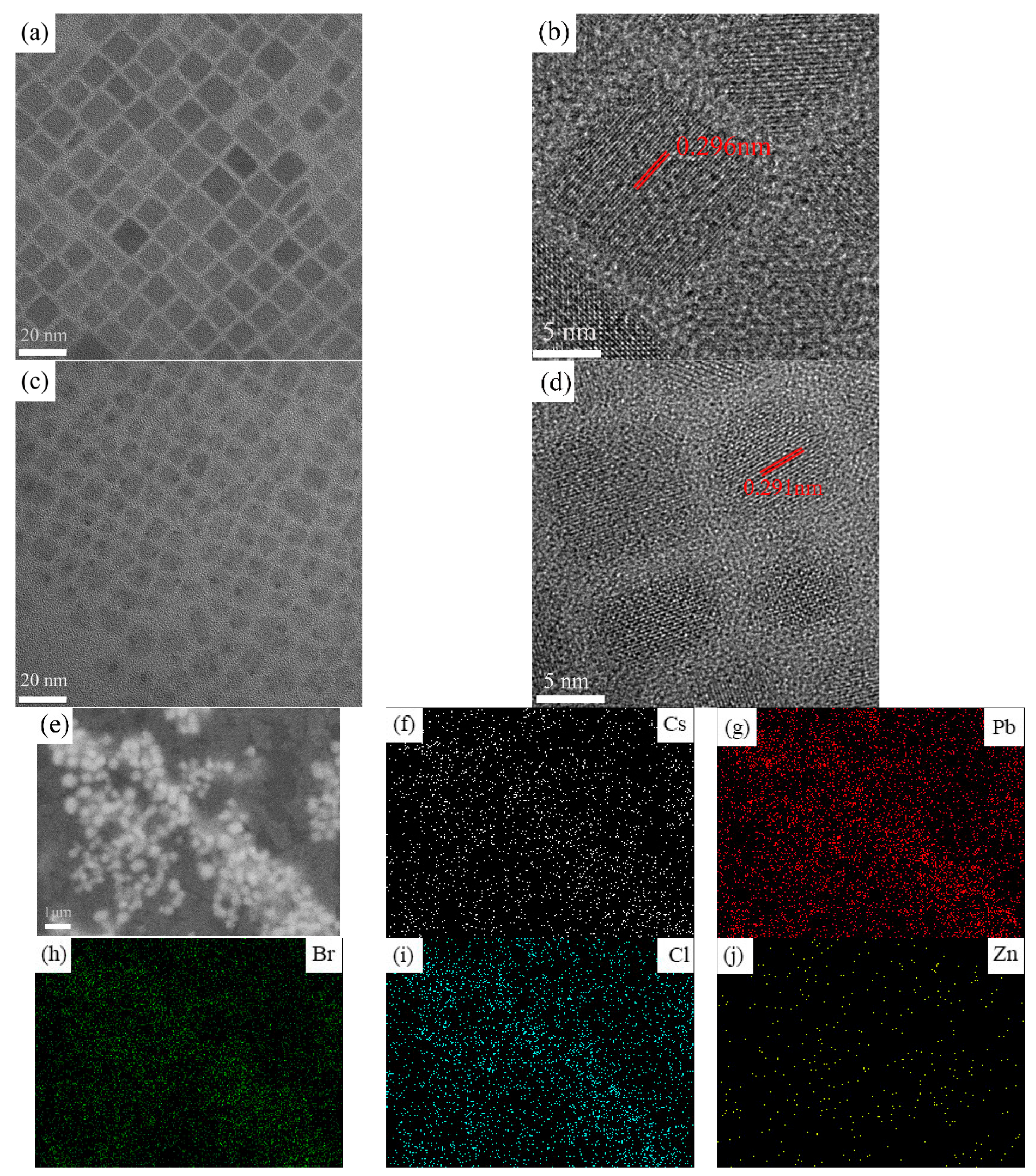
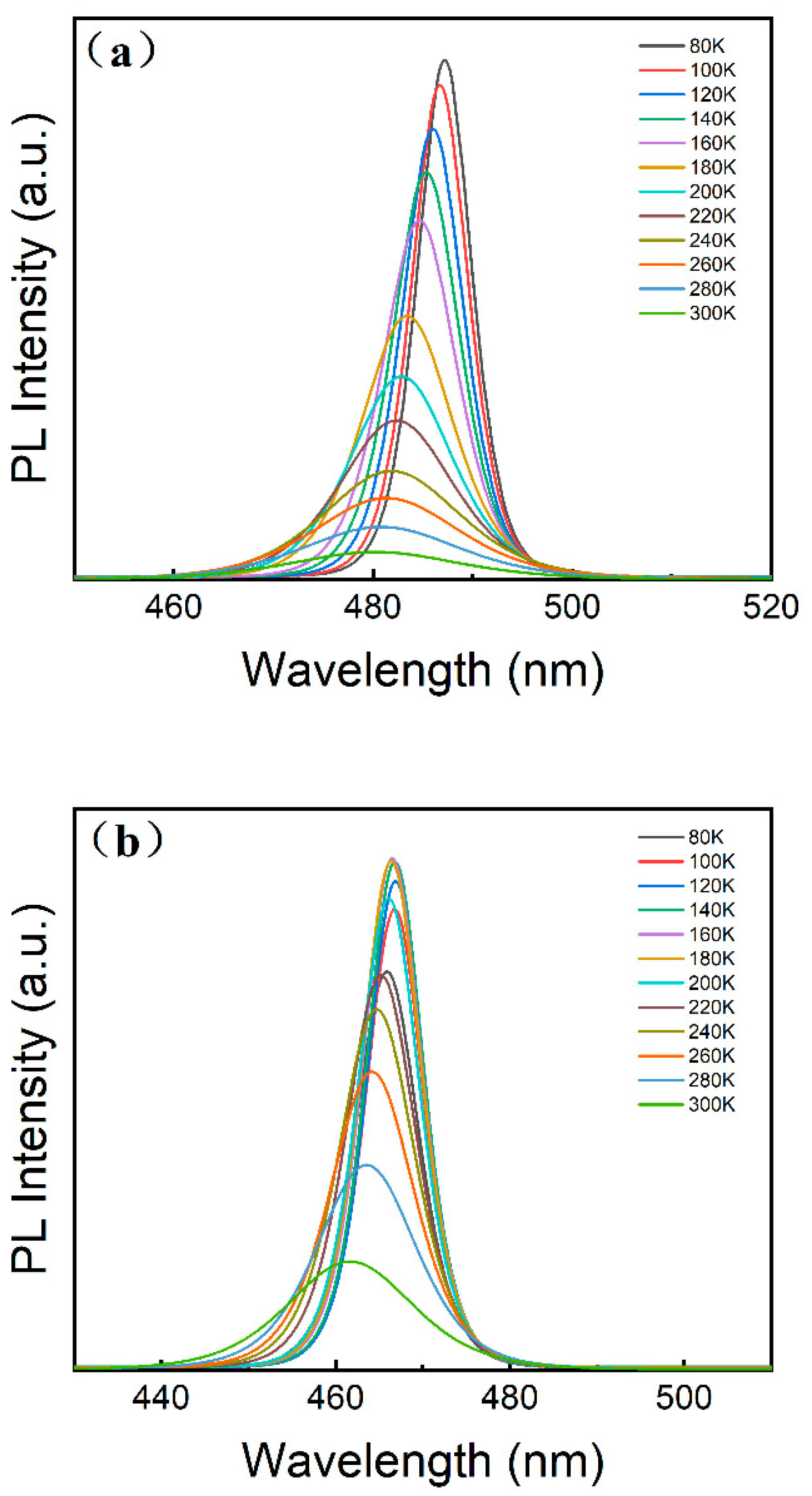
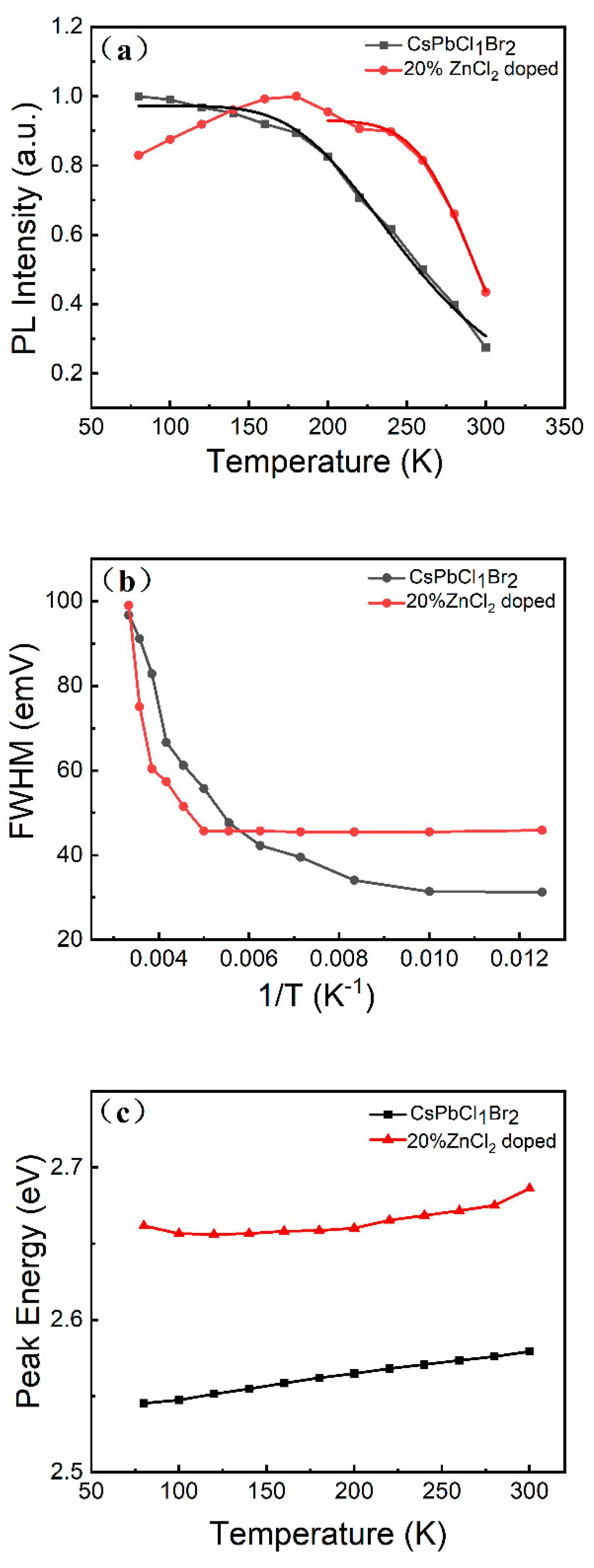
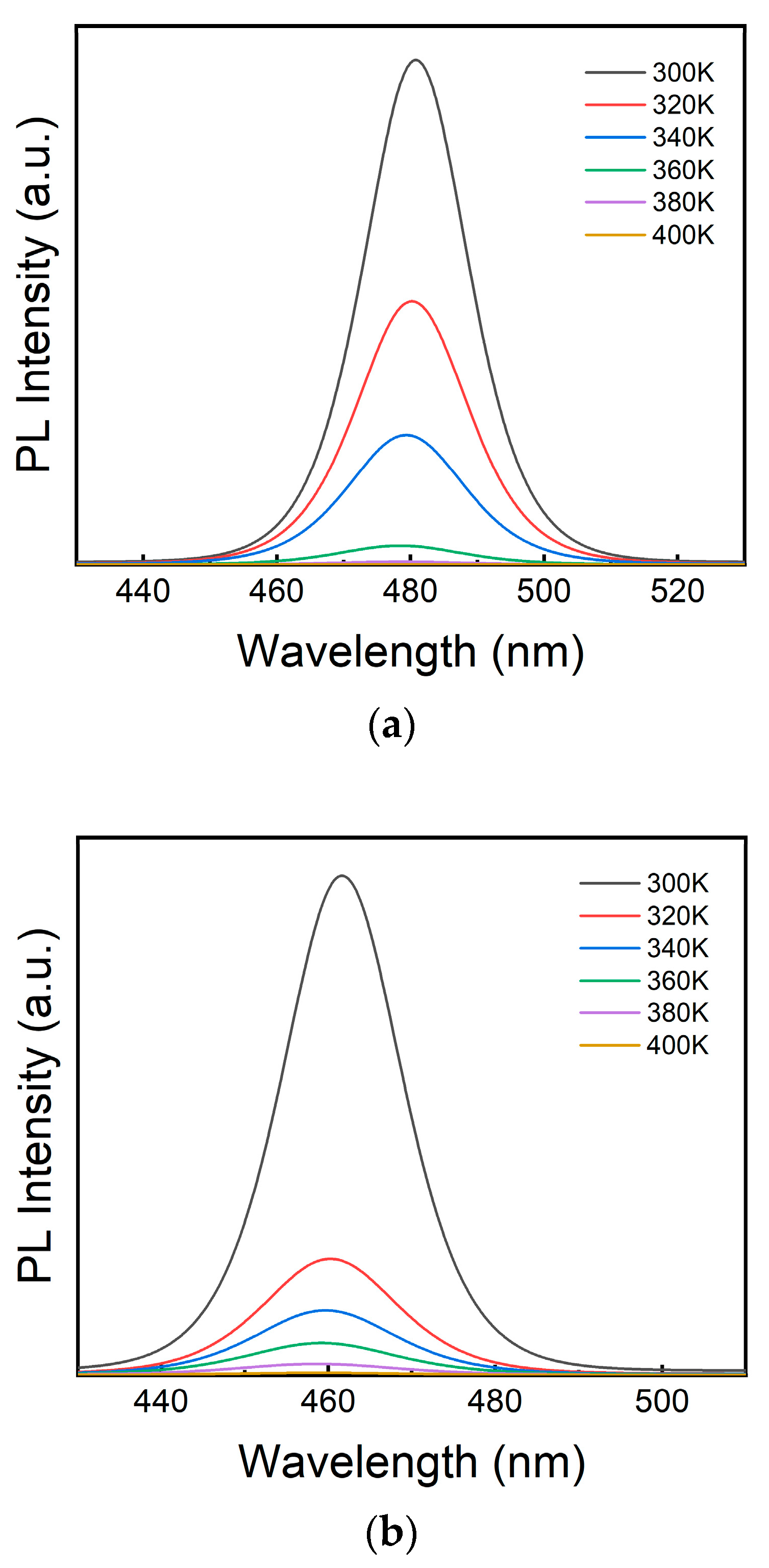
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, L.; Li, X.; Li, X.; Song, J.; Nong, Y.; Huang, J.; Wei, C.; Zhang, W.-H.; Xu, B. Eliminating Chlorine Vacancies of Perovskite Nanocrystals Using Hydrazine Cations Enables Efficient Pure Blue Light-Emitting Diodes. ACS Energy Lett. 2024, 9, 1210–1218. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Ma, J.; Lu, C.; Yu, Z.; Tu, G.; Zhang, J. Yttrium Cation Doping and Phenylphosphonic Acid Passivation for Pure-Red Perovskite Light-Emitting Diodes. ACS Energy Lett. 2024, 9, 4699–4707. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Gan, X.; Yu, L.; Yuan, H.; Shang, M.; Lu, C.; Hou, D.; Hu, Z.; Zhu, Y.; et al. Pb-Reduced CsPb0.9Zn0.1I2Br Thin Films for Efficient Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900896. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Qin, J.; Chen, G.; Liu, Y.; Fan, S.; Ma, C.; Yao, F.; Cui, H.; Zhou, S.; et al. Buried interface modification and light outcoupling strategy for efficient blue perovskite light-emitting diodes. Sci. Bull. 2024, 69, 2231–2240. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Chen, P.; Hou, J.; Sun, Y.; Zhao, G.; Zhang, N.; Zou, J.; Xu, J.-Y.; Fang, Y.; et al. Excitonic Luminescence Engineering in Tervalent-Europium-Doped Cesium Lead Halide Perovskite Nanocrystals and Their Temperature-Dependent Energy Transfer Emission Properties. J. Phys. Chem. C 2018, 122, 29044–29050. [Google Scholar] [CrossRef]
- Ni, J.; Ji, S.; Wang, Z.; Liu, S.; Hu, Y.; Chen, Y.; Li, J.; Li, X.; Chu, J.; Wu, D.; et al. Unidirectional unpolarized luminescence emission via vortex excitation. Nat. Photonics 2023, 17, 601–606. [Google Scholar] [CrossRef]
- Ščajev, P.; Litvinas, D.; Kreiza, G.; Stanionytė, S.; Malinauskas, T.; Tomašiūnas, R.; Juršėnas, S. Highly efficient nanocrystalline CsxMA1−xPbBrx perovskite layers for white light generation. Nanotechnology 2019, 30, 345702. [Google Scholar] [CrossRef] [PubMed]
- Ščajev, P.; Litvinas, D.; Soriūtė, V.; Kreiza, G.; Stanionytė, S.; Juršėnas, S. Crystal Structure Ideality Impact to Bimolecular, Auger and Diffusion Coefficients in Mixed Cation CsxMA1−xPbBr3 and CsxFA1−xPbBr3 Perovskites. J. Phys. Chem. 2019, 123, 23838–23844. [Google Scholar] [CrossRef]
- Peng, H.; Shipei, S.; Hairui, L.; Yongyou, Z.; Haiyan, Q.; Haizheng, Z. Nonlocal Interaction Enhanced Biexciton Emission in Large CsPbBr3 Nanocrystals. eLight 2023, 3, 10. [Google Scholar]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X. = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, G.H.; Hemming, S.; Baeza, E.; Evelien, A.J.; van Thoor, E.A.J.; Albert, P.H.J.; Debije, S.M.G. Advanced Optical Materials for Sunlight Control in Greenhouses. Adv. Opt. Mater. 2020, 8, 2000738. [Google Scholar] [CrossRef]
- Gencai, P.; Xue, B.; Wen, X.; Xu, C.; Yue, Z.; Jinyang, Z.; He, S.; Nan, D.; Lin, X.; Biao, D.; et al. Bright Blue Light Emission of Ni 2+ Ion-Doped CsPbClxBr3−x Perovskite Quantum Dots Enabling Efficient Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 14195–14202. [Google Scholar]
- Qiqi, Z.; Feitong, C.; Qi, H.; Kaiyu, W.; Changqian, L.; Rui, W.; Chenyang, L.; Wenli, X.; Rui, L.; Huiling, Z.; et al. A review of metal–organic frameworks for flexible optoelectronic applications. J. Mater. Chem. C 2024, 12, 4234. [Google Scholar]
- Wu, Y.; Liu, Y.; Fiuza-Maneiro, N.; Gómez-Graña, S.; Imran, M.; Polavarapu, L.; Rogach, A.L.; Li, X.; Zeng, H. Amine-Free CsPbBr 3 Perovskite Nanoplatelets Produced with Monolayer-Precision Thickness Control. ACS Mater. Lett. 2024, 6, 2425–2433. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, J.; Qin, Z.; Wang, J.; Sun, J.; Chan, C.C.S.; Ding, S.; Wang, K.; Chen, R.; Wong, K.S.; et al. High-Performance Blue Perovskite Light-Emitting Diodes Enabled by Efficient Energy Transfer between Coupled Quasi-2D Perovskite Layers. Adv. Mater. 2021, 33, e2005570. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Cui, Y.; Li, Y.; Vigil, J.A.; Jiang, Z.; Nandi, P.; Colby, R.; Zhang, C.; Karunadasa, H.I.; Lindenberg, A.M. Phase segregation dynamics in mixed-halide perovskites revealed by plunge-freeze cryo-electron microscopy. Cell Rep. Phys. Sci. 2025, 6, 102653. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Sokolova, A.V.; Tatarinov, D.A.; Zhang, X.; Zheng, W.; Litvin, A.P.; Rogach, A.L. Trap-Mediated Sensitization Governs Near-Infrared Emission from Yb3+-Doped Mixed-Halide CsPbClxBr3−x Perovskite Nanocrystals. Nano Lett. 2024, 24, 3347–3354. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Fei, Z.; Xinzhen, J.; Wenqing, L.; Ying, L.; Zhuangzhuang, M.; Meng, W.; Yue, W.; Di, W.; Xu, C.; Dongwen, Y.; et al. Two-Dimensional Semiconductor Nanoplatelets for Ultraviolet Photodetection. Mater. Horiz. 2021, 8, 3432. [Google Scholar]
- Liu, J.; Lu, R.; Yu, A. Origin of the low-energy tail in the photoluminescence spectrum of CsPbBr3 nanoplatelets: A femtosecond transient absorption spectroscopic study. Phys. Chem. Chem. Phys. 2024, 26, 12179. [Google Scholar] [CrossRef] [PubMed]
- Varnakavi, N.; Velpugonda, J.L.; Lee, N.; Nah, S.; Lin, L.Y. In Situ Synthesis of Br-Rich CsPbBr3 Nanoplatelets: Enhanced Stability and High PLQY for Wide Color Gamut Displays. Adv. Funct. Mater. 2025, 35, 2413320. [Google Scholar] [CrossRef]
- Huang, Q.; Yin, W.; Gao, B.; Zeng, Q.; Yao, D.; Zhang, H.; Zhao, Y.; Zheng, W.; Zhang, J.; Yang, X.; et al. Enhancing crystal integrity and structural rigidity of CsPbBr3 nanoplatelets to achieve a narrow color-saturated blue emission. Light Sci. Appl. 2024, 13, 111. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, F.; Wang, J.; Yang, K.; Li, Y.; Bai, G. Enhancing Luminescence and Thermal Stability of CsPbBr3 Quantum Dot Glasses through Doping with Eu3+ Ions. ACS Appl. Nano Mater. 2025, 8, 3608–3616. [Google Scholar] [CrossRef]
- Wang, D.; Hong, Z.; Sun, J.; Wang, Y. High-Performance In3+-Doped CsPbBr3 Quantum Dots for Wide Color Gamut Backlighting. ACS Appl. Nano Mater. 2024, 7, 323–330. [Google Scholar] [CrossRef]
- Jiang, X.; Geng, C.; Yu, X.; Pan, J.; Zheng, H.; Liang, C.; Li, B.; Long, F.; Han, L.; Cheng, Y.-B.; et al. Doping with KBr to Achieve High-Performance CsPbBr3 Semitransparent Perovskite Solar Cells. ACS Appl. Mater. 2024, 16, 19039. [Google Scholar] [CrossRef]
- Yong, Z.J.; Guo, S.Q.; Ma, J.P.; Zhang, J.Y.; Li, Z.Y.; Chen, Y.M.; Zhang, B.B.; Zhou, Y.; Shu, J.; Gu, J.L.; et al. Doping-enhanced short-range order of perovskite nanocrystals for near-unity violet luminescence quantum yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef] [PubMed]
- Makula, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Yan, F.; Demir, H.V. Mixed Lead–Tin Halide Perovskites for Efficient and Wavelength-Tunable Near-Infrared Light-Emitting Diodes. Nanoscale 2019, 11, 11402. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-alloyed CsPbI3 nanocrystals for highly efficient perovskite light-emitting devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Y.; Wang, J.; Ma, J.; Wang, L.; Jiang, W. Synergistic Effects of Surface Ligand Engineering and Double Matrices Protecting Enable Ultrastable and Highly Emissive Blue/Cyan Perovskite Nanocrystal Films for Multifunctional Applications. Laser Photonics Rev. 2023, 17, 2200651. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef]
- van der Stam, W.; Geuchies, J.J.; Altantzis, T.; van den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; de Mello Donega, C. Highly emissive divalent-ion-doped colloidal CsPb1−xMxBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef]
- Zeng, Y.T.; Li, Z.R.; ChangS, P.; Ansay, A.; Wang, Z.H.; Huang, C.Y. Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping. Nanomaterials 2022, 12, 759. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I.J. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, D.; Pan, G.; Chen, X.; Li, D.; Xu, W.; Bai, X.; Song, H. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Downconverter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149. [Google Scholar] [CrossRef]
- Eames, C.; Frost, J.M.; Barnes, P.R.F.; O’rEgan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef]
- Nenon, D.P.; Pressler, K.; Kang, J.; Koscher, B.A.; Olshansky, J.H.; Osowiecki, W.T.; Koc, M.A.; Wang, L.-W.; Alivisatos, A.P. Design Principles for Trap-Free CsPbX3 Nanocrystals: Enumerating and Eliminating Surface Halide Vacancies with Softer Lewis Bases. J. Am. Chem. Soc. 2018, 140, 17760. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hou, Y.; Sun, H.T.; Mohammed, O.F.; Sargent, E.H.; Bakr, O.M. Reducing Defects in Halide Perovskite Nanocrystals for Light-Emitting Applications. J. Phys. Chem. Lett. 2019, 10, 2629. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.Y.; Kim, Y.; Bae, J.; Kim, T.G.; Kim, J.W.; Lee, D.C.; Jeong, S. Highly stable cesium lead halide perovskite nanocrystals through in situ lead halide inorganic passivation. Chem. Mater. 2017, 29, 7088–7092. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. A Density Functional Theory Study of the Mechanism of the Mo-Cofactor-Catalyzed Reduction of Dinitrogen. J. Chin. Chem. Soc. 2003, 50, 1093. [Google Scholar] [CrossRef]
- Dang, Z.; Shamsi, J.; Palazon, F.; Imran, M.; Akkerman, Q.A.; Park, S.; Bertoni, G.; Prato, M.; Brescia, R.; Manna, L. In Situ Transmission Electron Microscopy Study of Electron Beam-Induced Transformations in Colloidal Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 2124. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-Doped Cesium Lead Bromide Perovskite Nanocrystals with Stable Blue Photoluminescence Used for Display Backlight. Adv. Sci. 2017, 4, 1700335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zheng, J.; Zeng, R.; Jing, P.; Ji, W.; Zhao, J.; Yang, W.; Li, H. Thermal stability of Mn2+ ion luminescence in Mn-doped core-shell quantum dots. Nanoscale 2014, 6, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, M.; Yang, S.; Wu, R.; Gong, S.; Han, Q.; Wu, W. Spectral and dynamic analysis of CsPbBr3 perovskite nanocrystals with enhanced water stability using sodium passivation. J. Alloys Compd. 2021, 889, 161721. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, B.; Xie, L.; Li, X.; Lu, B.; Wang, M.; Wu, Y.; Jiang, T.; Zhang, F.; Li, X.; et al. Vacancy-Ordered Double Perovskite Rb2ZrCl6−xBrx: Facile Synthesis and Insight into Efficient Intrinsic Self-Trapped Emission. Adv. Opt. Mater. 2022, 10, 2101661. [Google Scholar] [CrossRef]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal Halide Perovskite for next-generation optoelectronics: Progresses and prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
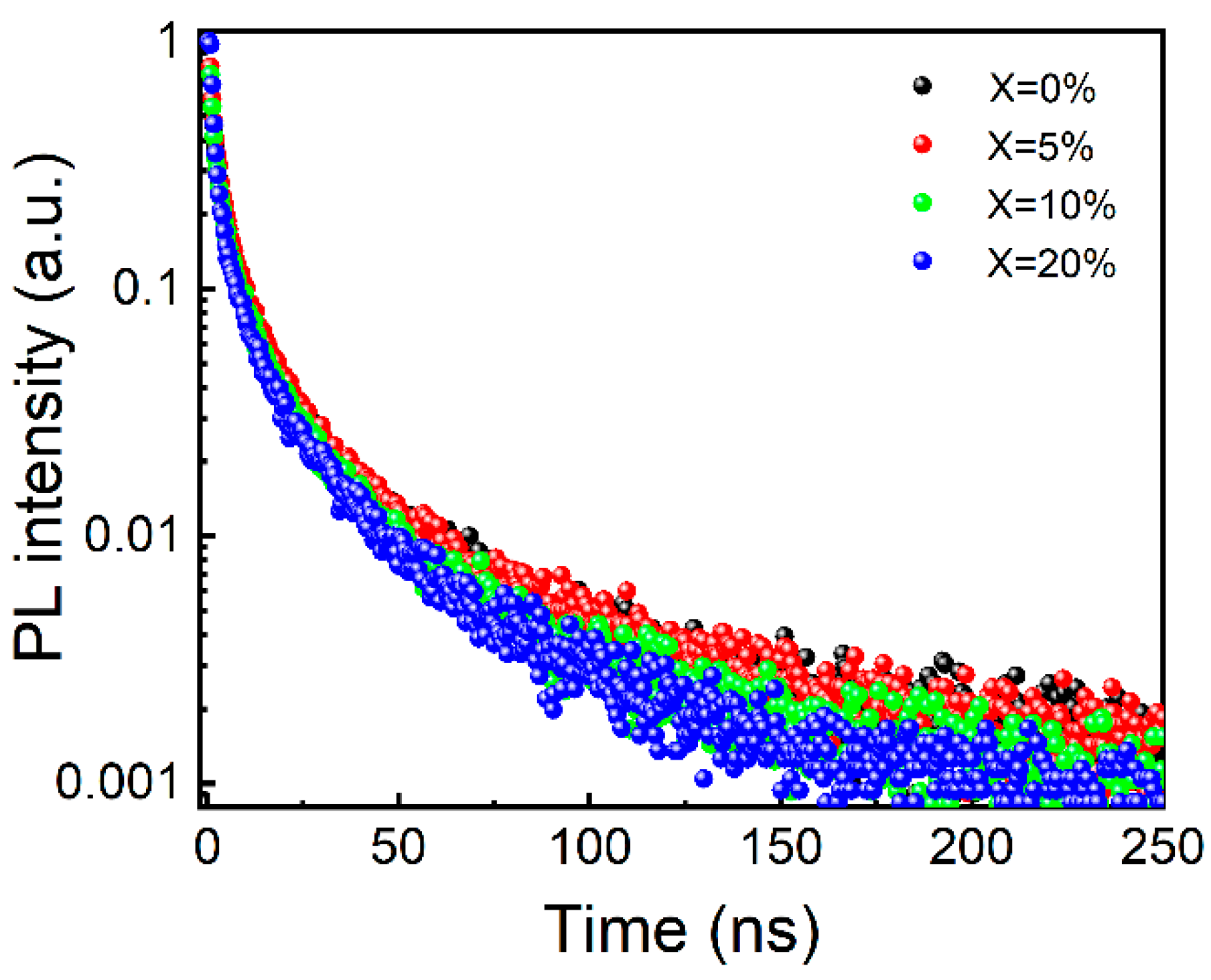
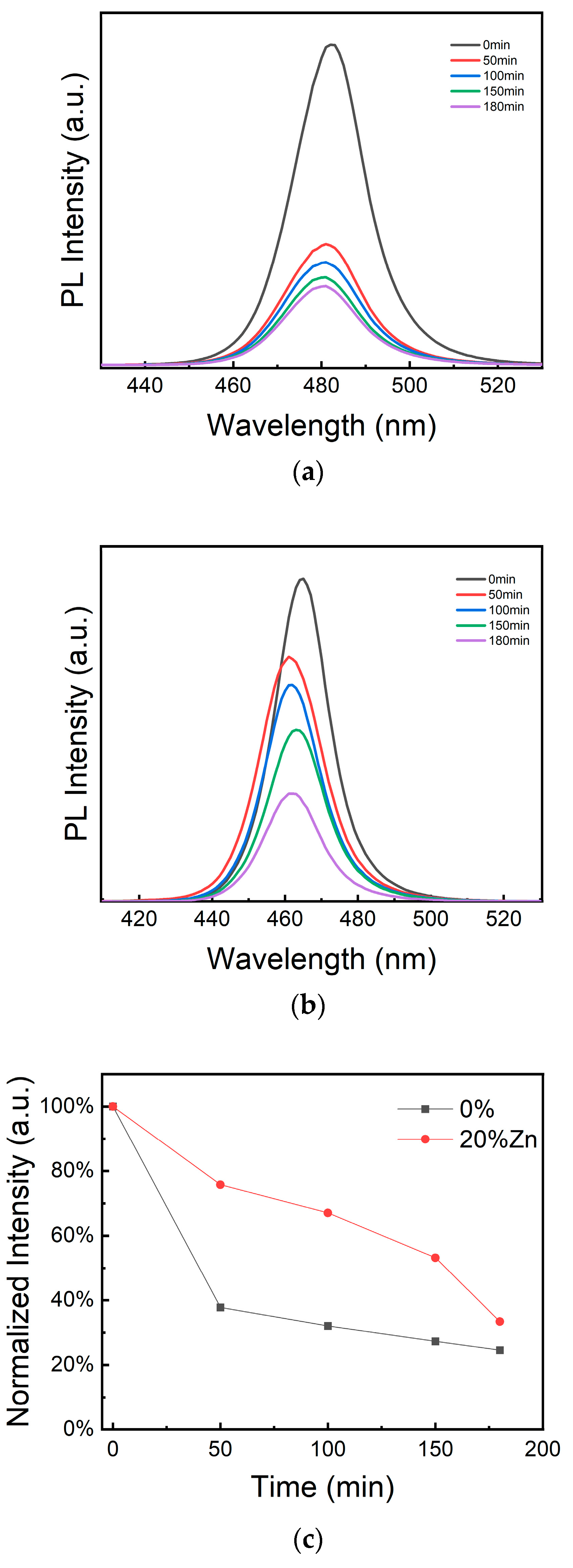
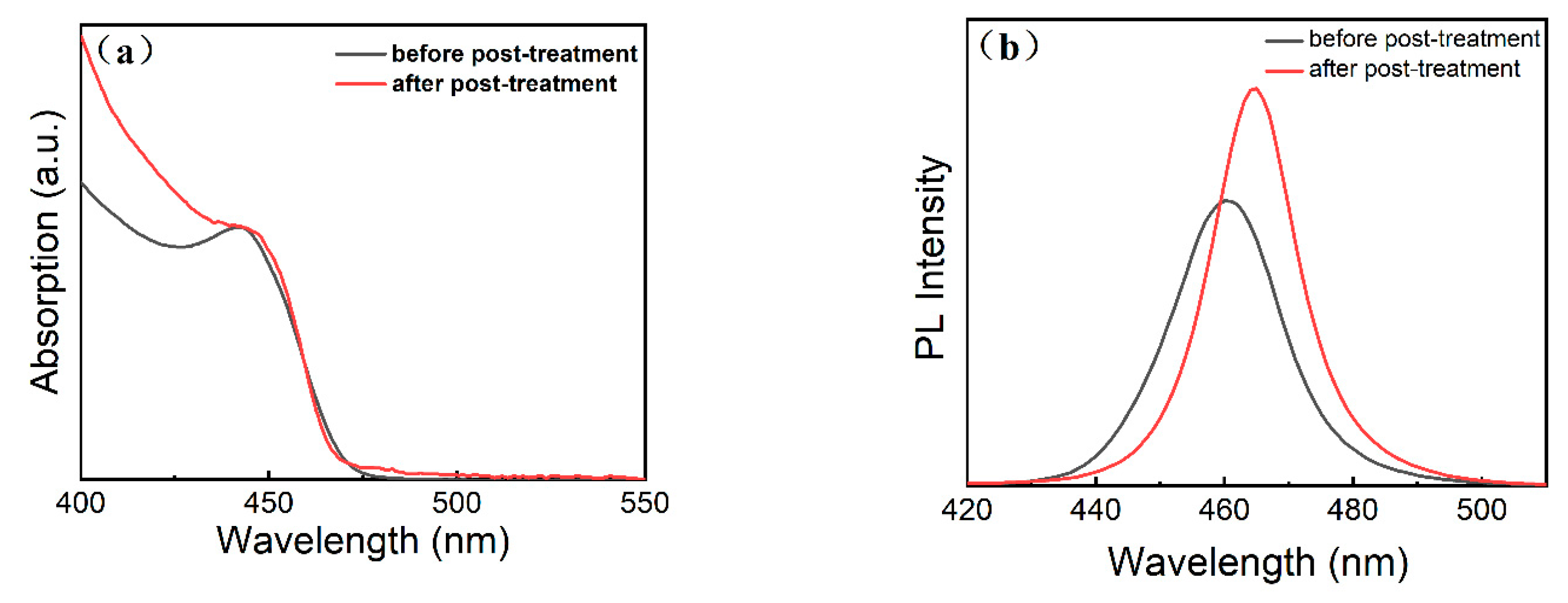
| Sample | A1 [%] | τ1 [ns] | A2 [%] | τ2 [ns] | A3 [%] | τ3 [ns] | τave [ns] | PLQY |
|---|---|---|---|---|---|---|---|---|
| X = 0% | 1.21 | 0.87 | 0.34 | 4.84 | 0.06 | 26.71 | 12.02 | 85% |
| X = 5% | 1.09 | 0.8 | 0.41 | 4.03 | 0.09 | 21.67 | 11.09 | 81% |
| X = 10% | 1.21 | 0.79 | 0.32 | 4.35 | 0.08 | 21.56 | 10.80 | 78% |
| X = 20% | 2.1 | 0.8 | 0.26 | 4.96 | 0.06 | 24.76 | 9.99 | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, B.; Fang, Y.; Wang, J.; Yuan, X.; Lang, J.; Cao, J.; Hua, J.; Yang, X. Bright Blue Light Emission of ZnCl2-Doped CsPbCl1Br2 Perovskite Nanocrystals with High Photoluminescence Quantum Yield. Micromachines 2025, 16, 920. https://doi.org/10.3390/mi16080920
Feng B, Fang Y, Wang J, Yuan X, Lang J, Cao J, Hua J, Yang X. Bright Blue Light Emission of ZnCl2-Doped CsPbCl1Br2 Perovskite Nanocrystals with High Photoluminescence Quantum Yield. Micromachines. 2025; 16(8):920. https://doi.org/10.3390/mi16080920
Chicago/Turabian StyleFeng, Bo, Youbin Fang, Jin Wang, Xi Yuan, Jihui Lang, Jian Cao, Jie Hua, and Xiaotian Yang. 2025. "Bright Blue Light Emission of ZnCl2-Doped CsPbCl1Br2 Perovskite Nanocrystals with High Photoluminescence Quantum Yield" Micromachines 16, no. 8: 920. https://doi.org/10.3390/mi16080920
APA StyleFeng, B., Fang, Y., Wang, J., Yuan, X., Lang, J., Cao, J., Hua, J., & Yang, X. (2025). Bright Blue Light Emission of ZnCl2-Doped CsPbCl1Br2 Perovskite Nanocrystals with High Photoluminescence Quantum Yield. Micromachines, 16(8), 920. https://doi.org/10.3390/mi16080920






