Molecular “Yin-Yang” Machinery of Synthesis of the Second and Third Fullerene C60 Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.2.1. UV–Vis–NIR and FTIR
2.2.2. TEM and HAADF-STEM
2.2.3. AFM/MFM
2.3. Methods
2.3.1. Symmetry
2.3.2. Harmony
2.3.3. Perfection
3. Results
3.1. FD-C60 (The First Derivative of the C60)
3.2. TEM Images of SD-C60 and TD-C60 Derivatives of C60
3.3. FTIR Spectra of SD-C60 and TD-C60 Derivatives of C60
3.4. MFM Spectra of SD-C60 and TD-C60 Derivative of C60
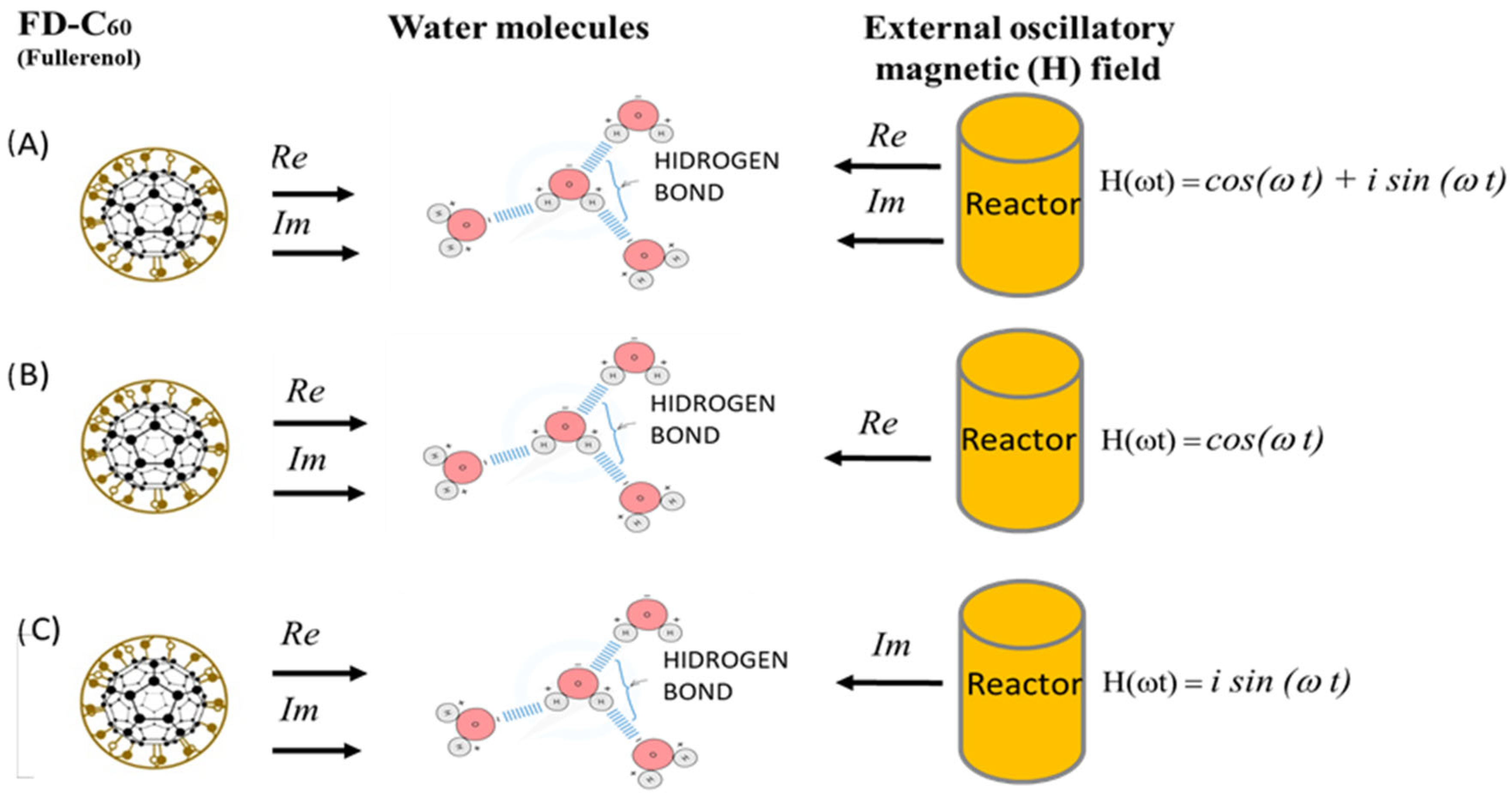
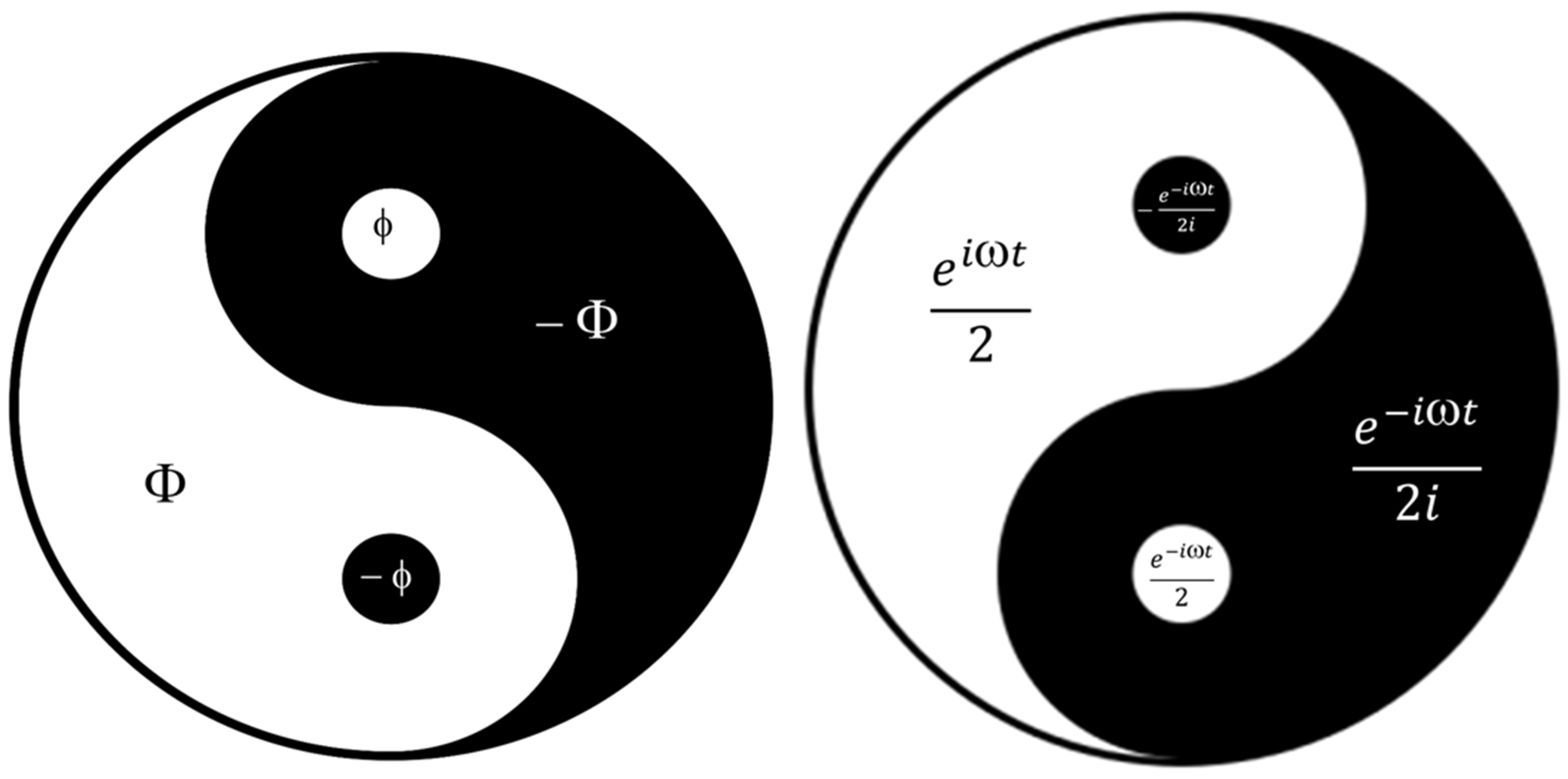
3.5. “Yin-Yang” Machinery of C60 Molecule Derivatives Synthesis
4. Discussion
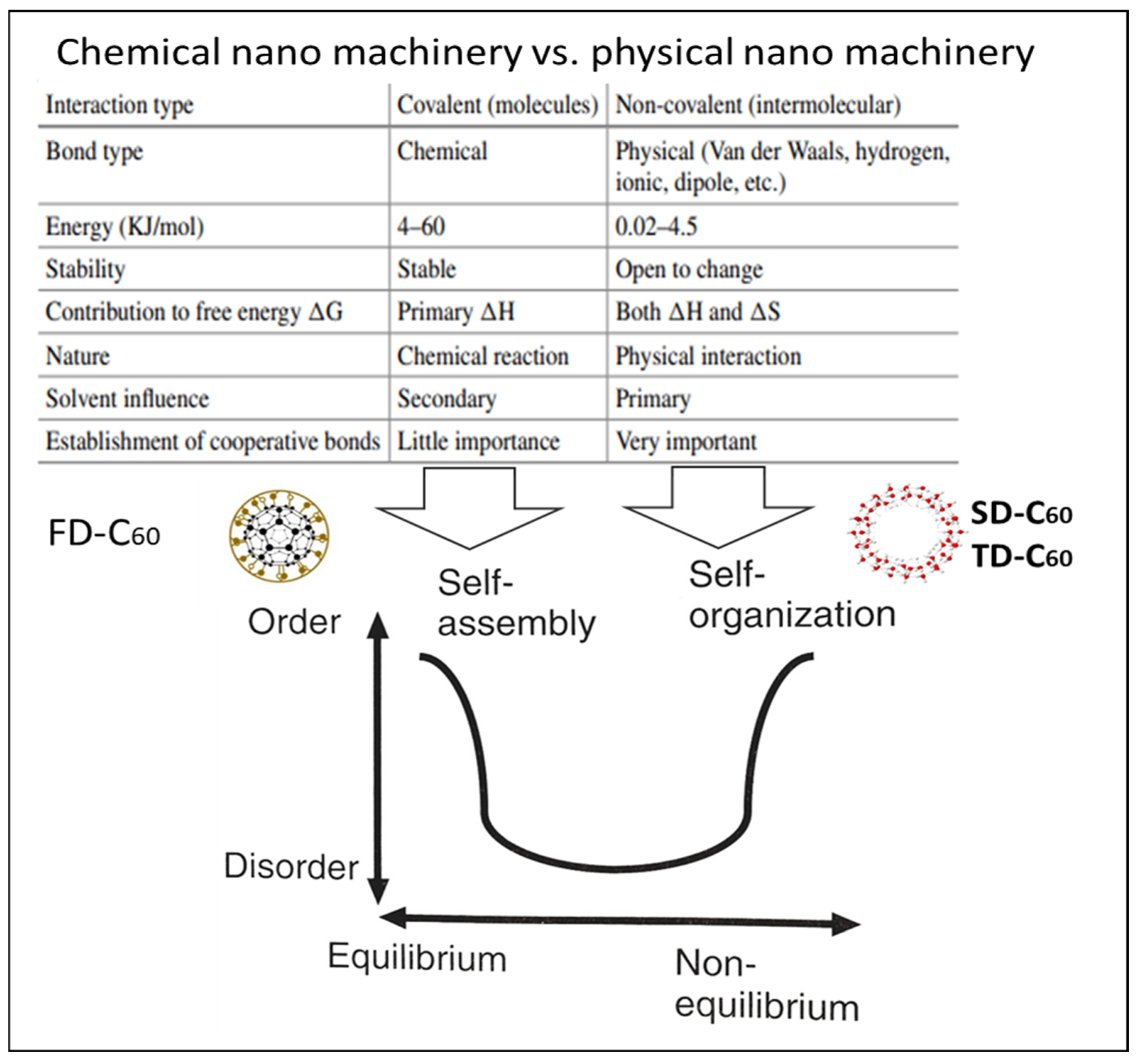
4.1. Physical Nano-Machinery vs. Chemical Nano-Machinery
4.2. Effects of C60 Molecule Derivatives in Biomedical Applications
4.3. The Further Direction of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C60 | Spherical molecular crystal (icosahedral symmetry) with 60 carbon atoms arranged on the surface of the sphere. Ground size (carbon positions) is 0.71 nm with π-electron size approximately 1 nm. Inside has a cage of approximately 0.3 nm. Possess both classical and quantum properties (wave-particle). In crystal state rotate 3 × 1010 s−1, while in solution 1.8 × 1010 s−1. |
| FD-C60 | The first derivative molecule C60 (or fullerenol). Molecule C60 is covalently added with OH groups (C60(OH)x, x = 12 to 48). |
| SD-C60 | The second derivative of C60. There are two substructures, first with water layers around FD-C60 and second, with Fibonacci open water linear chains. |
| TD–C60 | The third derivative of C60. There are two substructures. First, close cyclic water structure (“bubbles”, “micelle”) and second, close water chains (pentagonal and hexagonal). |
| 3HFWC Hyper harmonized hydrolyzed fullerene water complex | |
| DDS | Drug Delivery System |
| ROS | Reactive oxygen species |
| SHP | Symmetry-Harmony-Perfection |
| TME | Tumor microenvironment |
| TEM | Transmission electron microscopy |
| AFM | Atomic Force Microscopy |
| MFM | Magnetic Force Microscopy |
| FTIR | Fourier Transforms Infra-Red Spectroscopy |
| QCS Quantum-classical substance | |
| 13NMR | Nuclear magnetic resonance based on carbon isotope 13C |
| HPL Hyperpolarized light | |
| ΔH | Enthalpy |
| ΔS | Entropy |
| H | Magnetic field strength (Henry) |
| I | Electrical current |
| C | Capacitance |
| L | Inductance |
| R | Resistance |
| ω | The angular velocity of the oscillation |
| f | Frequency, f = ω/2π |
References
- Jain, K.; Mehra, N.K.; Jain, N.K. Potentials and emerging trends in nanopharmacology. Curr. Opin. Pharmacol. 2014, 15, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.E.M. Nanotechnology in pharmacology: Advances and Applications in Drag Delivery. J. Pharmacol. Clin. Res. 2023, 9, 555773. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.Y.; Upasani, R.B.; Swirczewski, J.W. Process of Forming Polysubstituted. Fullerenes.U.S. Patent 5,177,248, 5 January 1993. [Google Scholar]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Isakovic, A.; Markovic, Z.; Todorovic-Markovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z.; et al. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef]
- Bogdanović, G.; Kojić, V.; Đorđević, A.; Čanadanović-Brunet, J.; Vojinović-Miloradov, M.; Baltić, V.V. Modulating activity of fullerol C60(OH)22 on doxorubicin-induced cytotoxicity. Toxicol. Vitr. 2004, 18, 629–637. [Google Scholar] [CrossRef]
- Jiao, F.; Liu, Y.; Qu, Y.; Li, W.; Zhou, G.; Ge, C.; Li, Y.; Sun, B.; Chen, C. Studies on anti-tumor and antimetastatic activities of fullerenol in a mouse breast cancer model. Carbon 2010, 48, 2231–2243. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, F.; Qiu, Y.; Li, W.; Qu, Y.; Tian, C.; Li, Y.; Bai, R.; Lao, F.; Zhao, Y.; et al. Immunostimulatory properties and enhanced TNF- α mediated cellular immunity for tumor therapy by C60(OH)20 nanoparticles. Nanotechnology 2009, 20, 415102. [Google Scholar] [CrossRef]
- Yamawaki, H.; Iwai, N. Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am. J. Physiol.-Cell Physiol. 2006, 290, C1495–C1502. [Google Scholar] [CrossRef]
- Johnson-Lyles, D.N.; Peifley, K.; Lockett, S.; Neun, B.W.; Hansen, M.; Clogston, J.; Stern, S.T.; McNeil, S.E. Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol. Appl. Pharmacol. 2010, 248, 249–258. [Google Scholar] [CrossRef]
- Isaacs, E.D.; Shukla, A.; Platzman, P.M.; Hamann, D.R.; Barbiellini, B.; Tulk, C.A. Covalency of the Hydrogen Bond in Ice: A Direct X-Ray Measurement. Phys. Rev. Lett. 1999, 82, 600–603. [Google Scholar] [CrossRef]
- Dresselhous, M.S.; Dresselhous, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Elsevier BV: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Koruga, D. Composition of Matter Containing Harmonized Hydroxyl Modified Fullerene Substance. U.S. Patent 8,058,483 B2, 15 November 2011. [Google Scholar]
- Koruga, D. Compositions Comprising Hyper Harmonised Hydroxyl Modified Fullerene Substances. International Patent WO 2021/110234 A1, 10 June 2021. [Google Scholar]
- Matija, L.R.; Stankovic, I.M.; Puric, M.; Miličić, M.; Maksimović-Ivanić, D.; Mijatovic, S.; Krajnović, T.; Gordic, V.; Koruga, D.L. The Second Derivative of Fullerene C60 (SD-C60) and Biomolecular Machinery of Hydrogen Bonds: Water-Based Nanomedicine. Micromachines 2023, 14, 2152. [Google Scholar] [CrossRef] [PubMed]
- Koruga, D.; Stanković, I.; Matija, L.; Kuhn, D.; Christ, B.; Dembski, S.; Jevtić, N.; Janać, J.; Pavlović, V.; De Wever, B. Comparative Studies of the Structural and Physicochemical Properties of the First Fullerene Derivative FD-C60 (Fullerenol) and Second Fullerene Derivate SD-C60 (3HFWC). Nanomaterials 2024, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Minch, M.J. An Introduction to Hydrogen Bonding (Jeffrey, George A.). J. Chem. Educ. 1999, 76, 759. [Google Scholar] [CrossRef]
- Koruga, D. Qi Engineering: Classical-Quantum Biophysics, Acupuncture and Chaakras; Grafopen: Belgrade, Serbia, 2024; ISBN 978-86-83615-44-5. [Google Scholar]
- Arndt, M.; Nairz, O.; Vos-Andreae, J.; Keller, C.; van der Zouw, G.; Zeilinger, A. Wave–particle duality of C60 molecules. Nature 1999, 401, 680–682. [Google Scholar] [CrossRef]
- Kostić, L. The Basic Principle; The City Library “Karlo Bjelicki”: Sombor, Serbia, 2015. [Google Scholar]
- Zia, D.; Dehghan, N.; D’Errico, A.; Sciarrino, F.; Karimi, E. Interferometric imaging of amplitude and phase of spatial biphoton states. Nat. Photon 2023, 17, 1009–1016. [Google Scholar] [CrossRef]
- Mansoori, G.A. Principles of Nanotechnology: Molecular-Based Study of Condensed Matter in Small Systems; World Scientific: Singapore, 2005. [Google Scholar]
- Malsch, N.H. Biomedical Nanotechnology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Krishna, V.; Singh, A.; Sharma, P.; Iwakuma, N.; Wang, Q.; Zhang, Q.; Knapik, J.; Jiang, H.; Grobmyer, S.R.; Koopman, B.; et al. Polyhydroxy Fullerenes for Non-Invasive Cancer Imaging and Therapy. Small 2010, 6, 2236–2241. [Google Scholar] [CrossRef]
- Chen, A.; Grobmyer, S.R.; Krishna, V.B. Photothermal Response of Polyhydroxy Fullerenes. ACS Omega 2020, 5, 14444–14450. [Google Scholar] [CrossRef]
- Pickering, K.D.; Wiesner, M.R. Fullerol-Sensitized Production of Reactive Oxygen Species in Aqueous Solution. Environ. Sci. Technol. 2005, 39, 1359–1365. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of α-tocopherol. Free Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.B.; Shenoy, R.U.K.; Kajampady, M.K.; DCruz, C.E.; Shirodkar, R.K.; Kumar, L.; Verma, R. Fullerenes for the treatment of cancer: An emerging tool. Environ. Sci. Pollut. Res. 2022, 29, 58607–58627. [Google Scholar] [CrossRef] [PubMed]
- Markelić, M.; Drača, D.; Krajnović, T.; Jović, Z.; Vuksanović, M.; Koruga, D.; Mijatović, S.; Maksimović-Ivanić, D. Combined Action of Hyper-Harmonized Hydroxylated Fullerene Water Complex and Hyperpolarized Light Leads to Melanoma Cell Reprogramming In Vitro. Nanomaterials 2022, 12, 1331. [Google Scholar] [CrossRef]
- Markelić, M.; Mojić, M.; Bovan, D.; Jelača, S.; Jović, Z.; Purić, M.; Koruga, D.; Mijatović, S.; Maksimović-Ivanić, D. Melanoma Cell Reprogramming and Awakening of Antitumor Immunity as a Fingerprint of Hyper-Harmonized Hydroxylated Fullerene Water Complex (3HFWC) and Hyperpolarized Light Application In Vivo. Nanomaterials 2023, 13, 372. [Google Scholar] [CrossRef]
- Koruga, D. Hyperpolarized Light: Fundamentals of Nanobiomedical Phptonics; Zepter Book World: Belgrade, Serbia, 2018; ISBN 78-86-7494-136-2. [Google Scholar]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Arima, Y.; Nobusue, H.; Saya, H. Targeting of cancer stem cells by differentiation therapy. Cancer Sci. 2020, 111, 2689–2695. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes. Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S.; Taylor, B.; Neal, J.W. Tumor Evolution, Heterogeneity, and Therapy for Our Patients with Advanced Cancer: How Far Have We Come? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Ruscetti, M.; Leibold, J.; Bott, M.J.; Fennell, M.; Kulick, A.; Salgado, N.R.; Chen, C.C.; Ho, Y.J.; Sanchez-Rivera, F.J.; Feucht, J.; et al. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 2018, 362, 1416–1422. [Google Scholar] [CrossRef]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Kotsafti, A.; Scarpa, M.; Castagliuolo, I.; Scarpa, M. Reactive Oxygen Species and Antitumor Immunity—From Surveillance to Evasion. Cancers 2020, 12, 1748. [Google Scholar] [CrossRef]
- Wilson, D.L.; Ahlawat, J.; Narayan, M. The Role of Fullerenes in Neurodegenerative Disorders. J. Nanotheranostics 2024, 5, 1–12. [Google Scholar] [CrossRef]
- Perovic, M.; Ciric, J.; Matovic, V.; Srbovan, M.; Koruga, D.; Kanazir, S.; Ivkovic, S. The presymptomatic treatment with 3HFWC nanosubstance decreased plaque load in 5XFAD mouse model of Alzheimer’s disease. CNS Neurosci. Ther. 2023, 30, e14188. [Google Scholar] [CrossRef]
- Ivkovic, S.; Koruga, D. Role of fullerenols derivative 3HFWC in the treatment of Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1641–1642. [Google Scholar] [CrossRef]
- Xu, K. Stepwise oscillatory circuits of DNA molecules. J. Biol. Phys. 2009, 35, 223–230. [Google Scholar] [CrossRef]
- Johnson, R.D.; Yannoni, C.S.; Dorn, H.C.; Salem, J.R.; Bethune, D.S. C60 Rotation in the Solid State: Dynamics of a Faceted Spherical Top. Science 1992, 255, 1235–1238. [Google Scholar] [CrossRef]
- Kettle, S.F.A. Symmetry and Structure; John Willey and Sons: Chichester, UK, 1995. [Google Scholar]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koruga, D.L.; Matija, L.R.; Stanković, I.M.; Pavlović, V.B.; Dinić, A.P. Molecular “Yin-Yang” Machinery of Synthesis of the Second and Third Fullerene C60 Derivatives. Micromachines 2025, 16, 770. https://doi.org/10.3390/mi16070770
Koruga DL, Matija LR, Stanković IM, Pavlović VB, Dinić AP. Molecular “Yin-Yang” Machinery of Synthesis of the Second and Third Fullerene C60 Derivatives. Micromachines. 2025; 16(7):770. https://doi.org/10.3390/mi16070770
Chicago/Turabian StyleKoruga, Djuro Lj., Lidija R. Matija, Ivana M. Stanković, Vladimir B. Pavlović, and Aleksandra P. Dinić. 2025. "Molecular “Yin-Yang” Machinery of Synthesis of the Second and Third Fullerene C60 Derivatives" Micromachines 16, no. 7: 770. https://doi.org/10.3390/mi16070770
APA StyleKoruga, D. L., Matija, L. R., Stanković, I. M., Pavlović, V. B., & Dinić, A. P. (2025). Molecular “Yin-Yang” Machinery of Synthesis of the Second and Third Fullerene C60 Derivatives. Micromachines, 16(7), 770. https://doi.org/10.3390/mi16070770





