Quantum Simulation Study of Ultrascaled Label-Free DNA Sensors Based on Sub-10 nm Dielectric-Modulated TMD FETs: Sensitivity Enhancement Through Downscaling
Abstract
1. Introduction
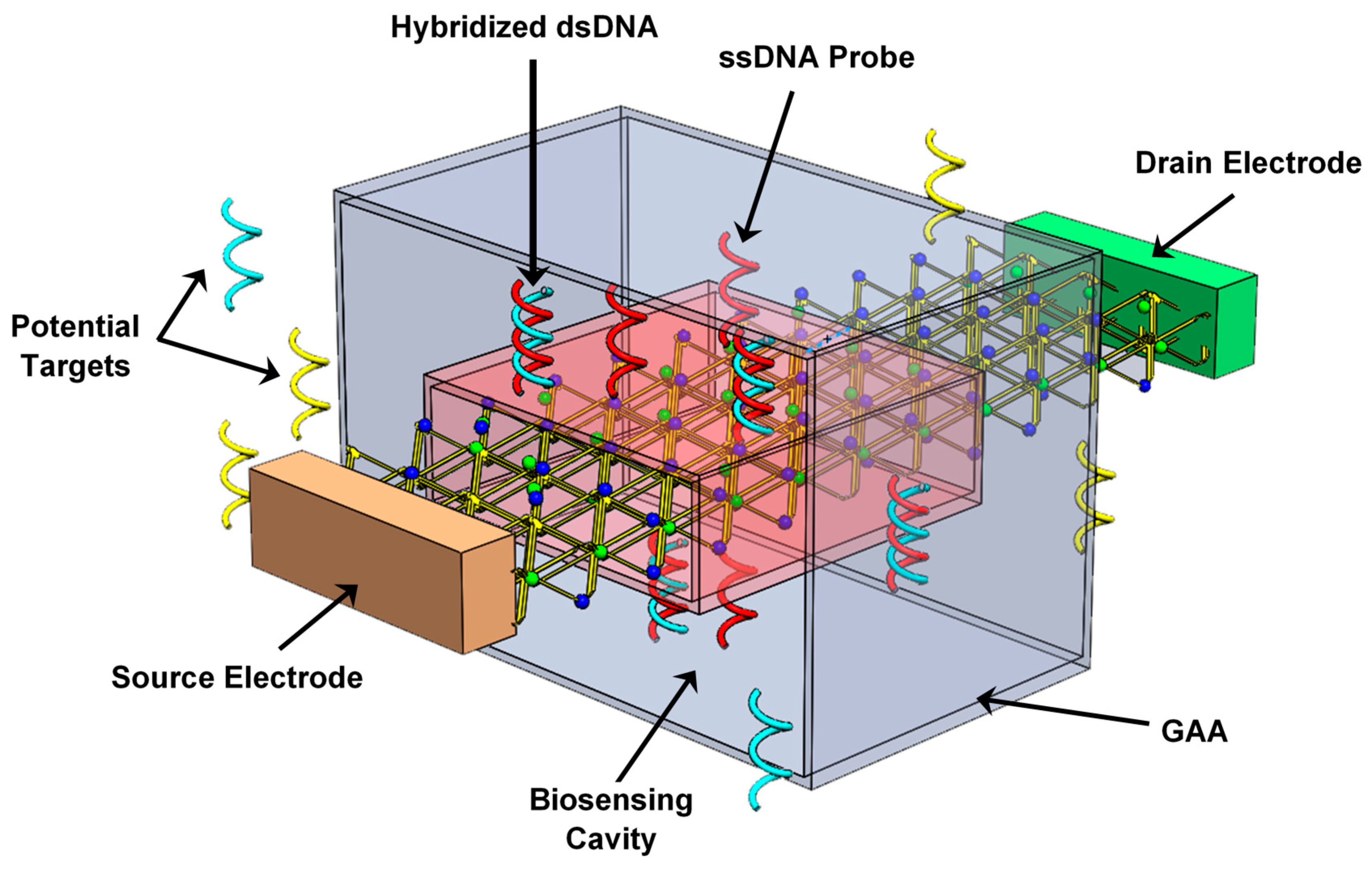
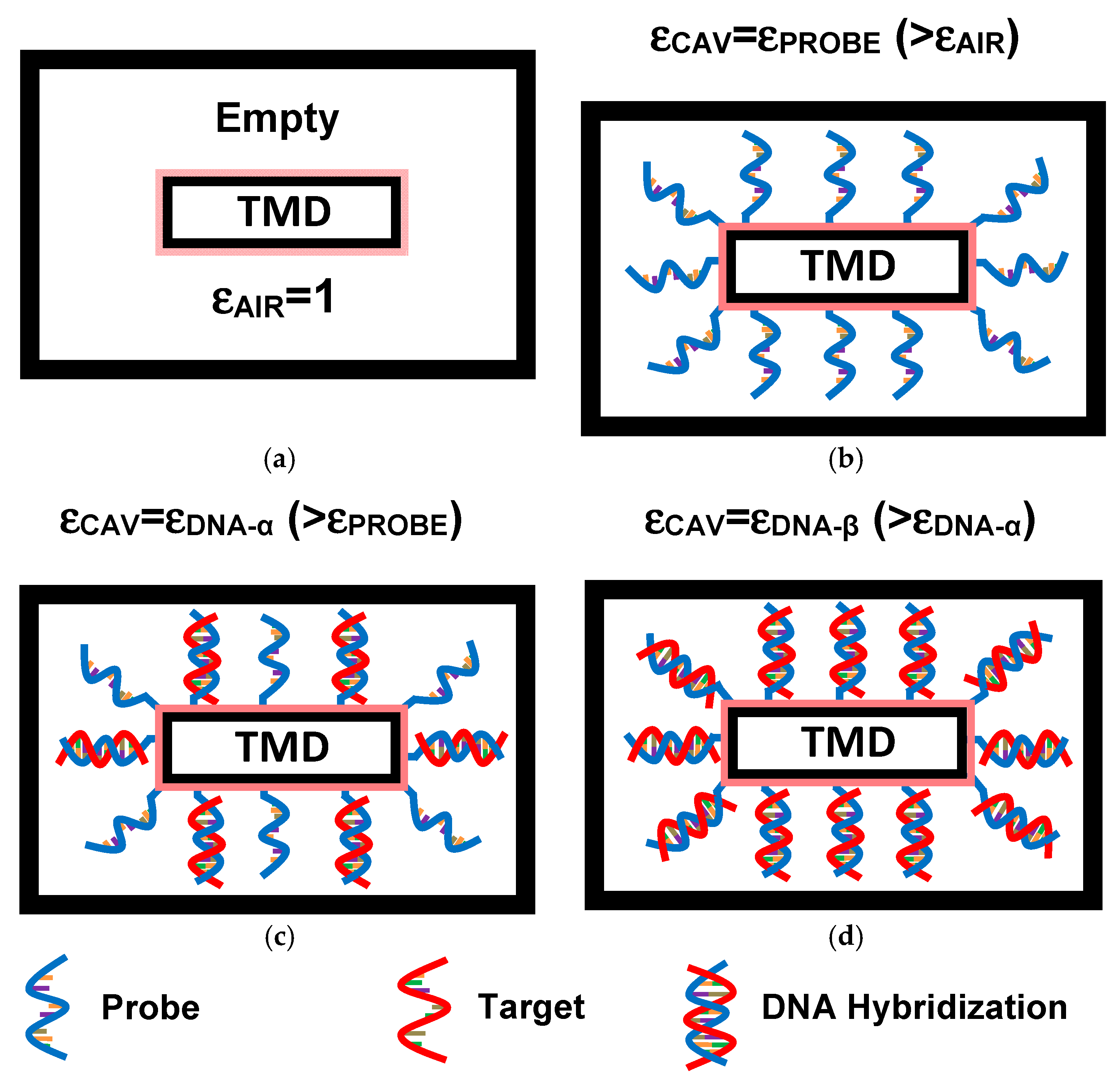
2. Nanobiosensor Structure and Principle of DM-Based Label-Free DNA Detection
3. Quantum Simulation Approach
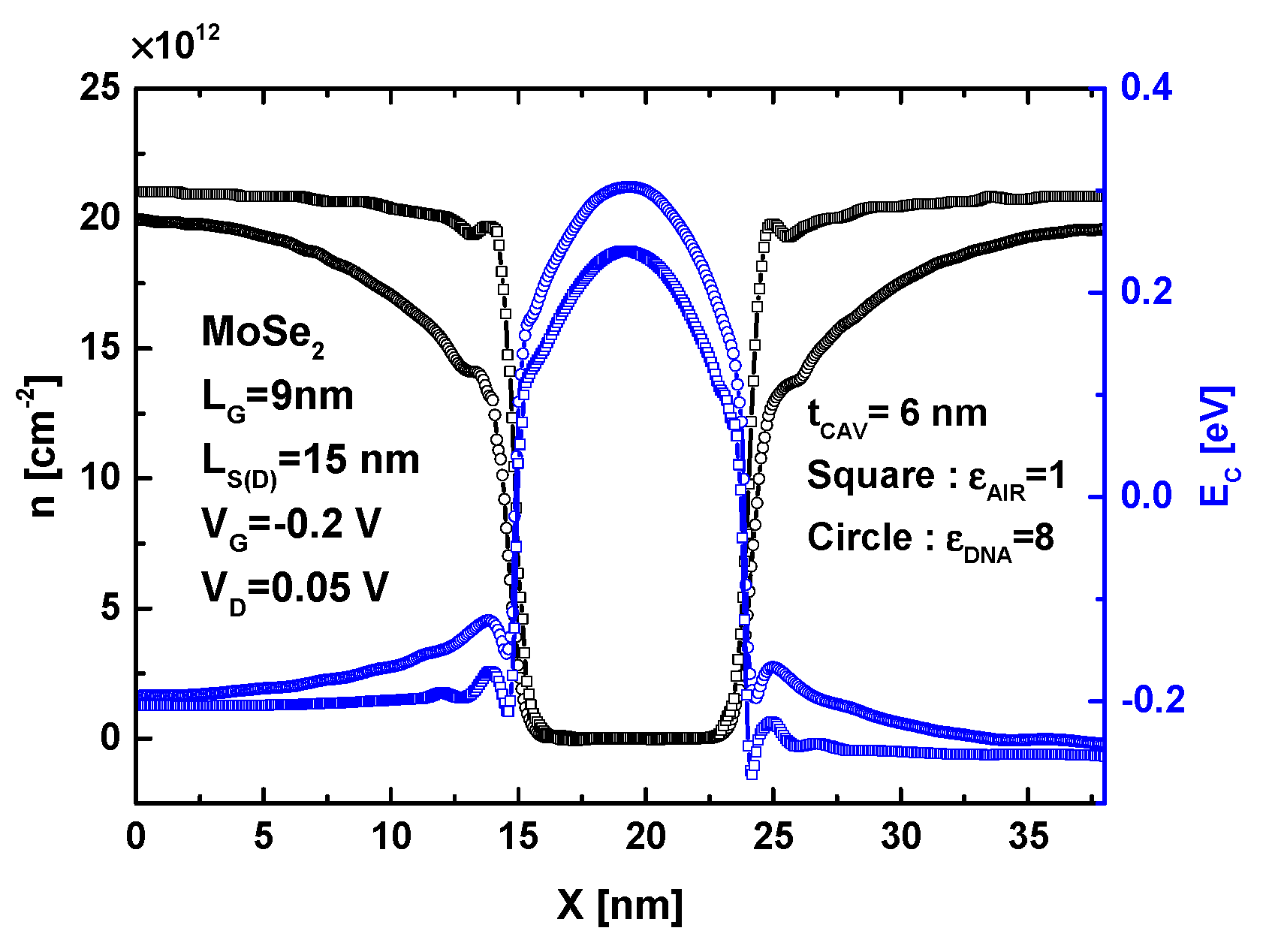
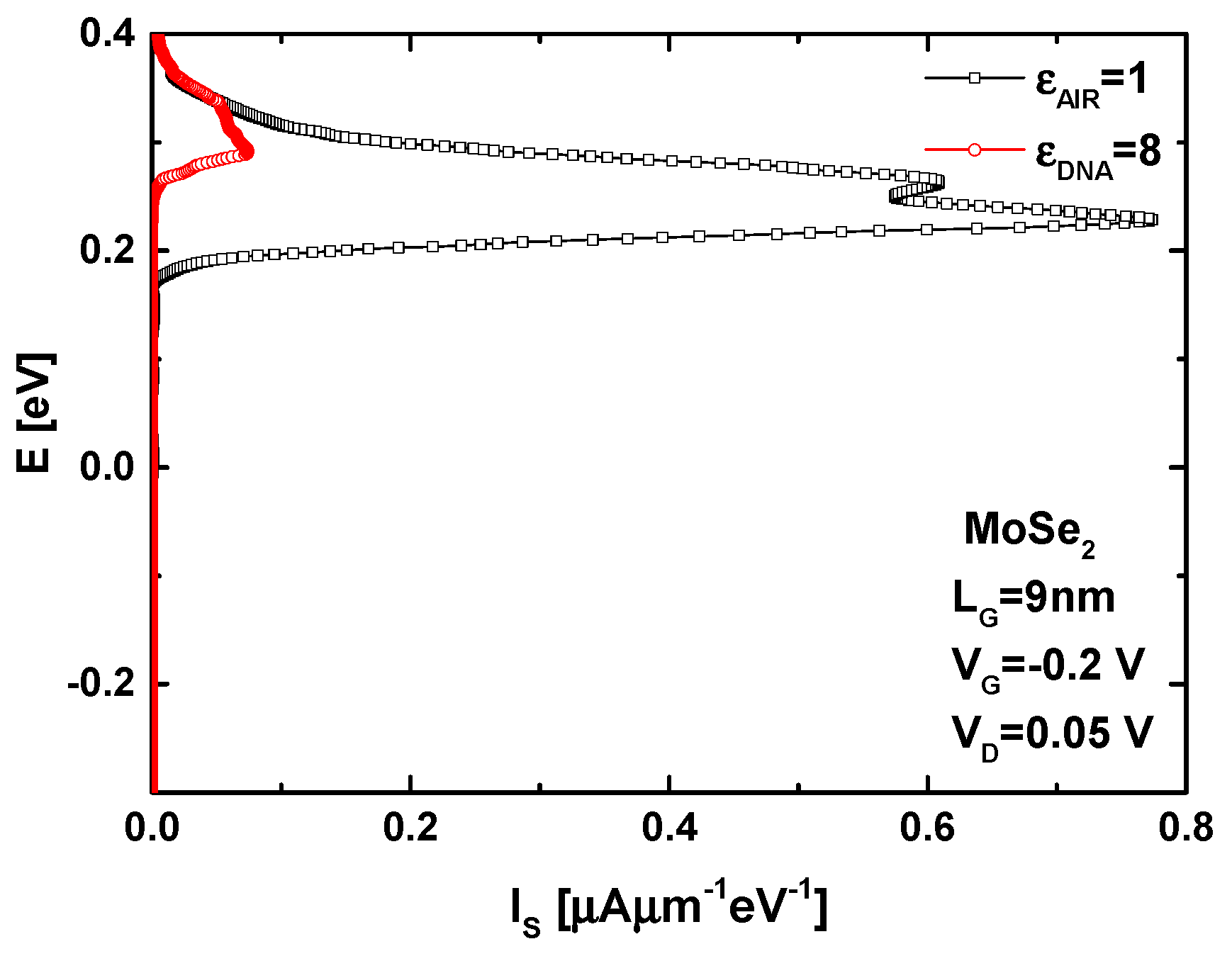
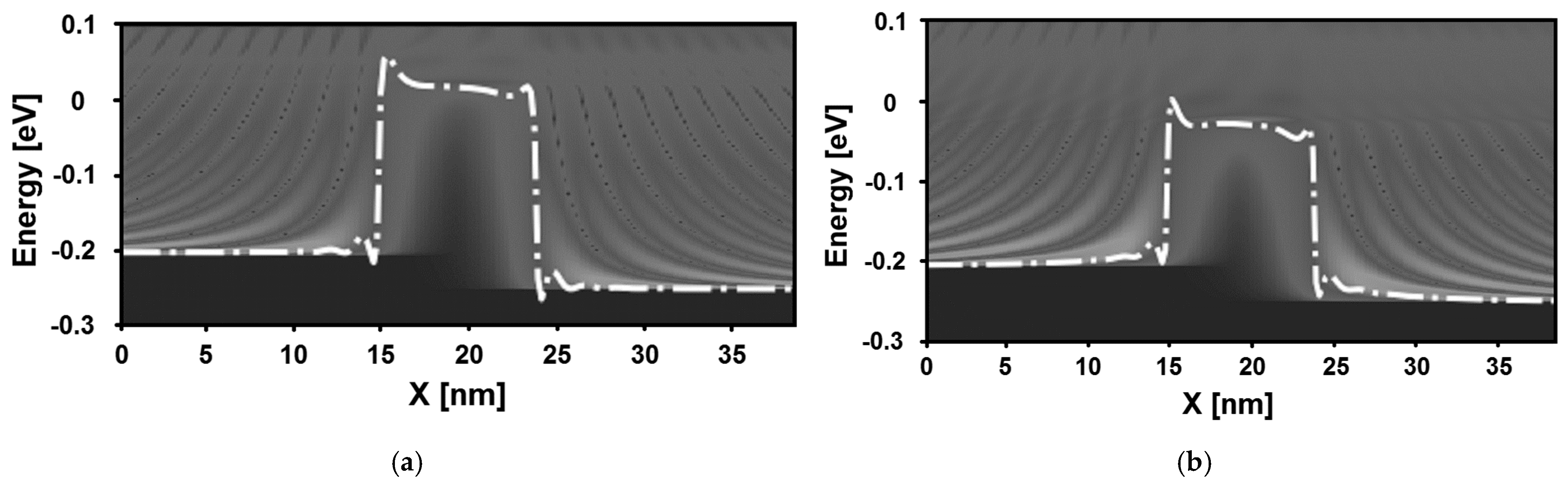
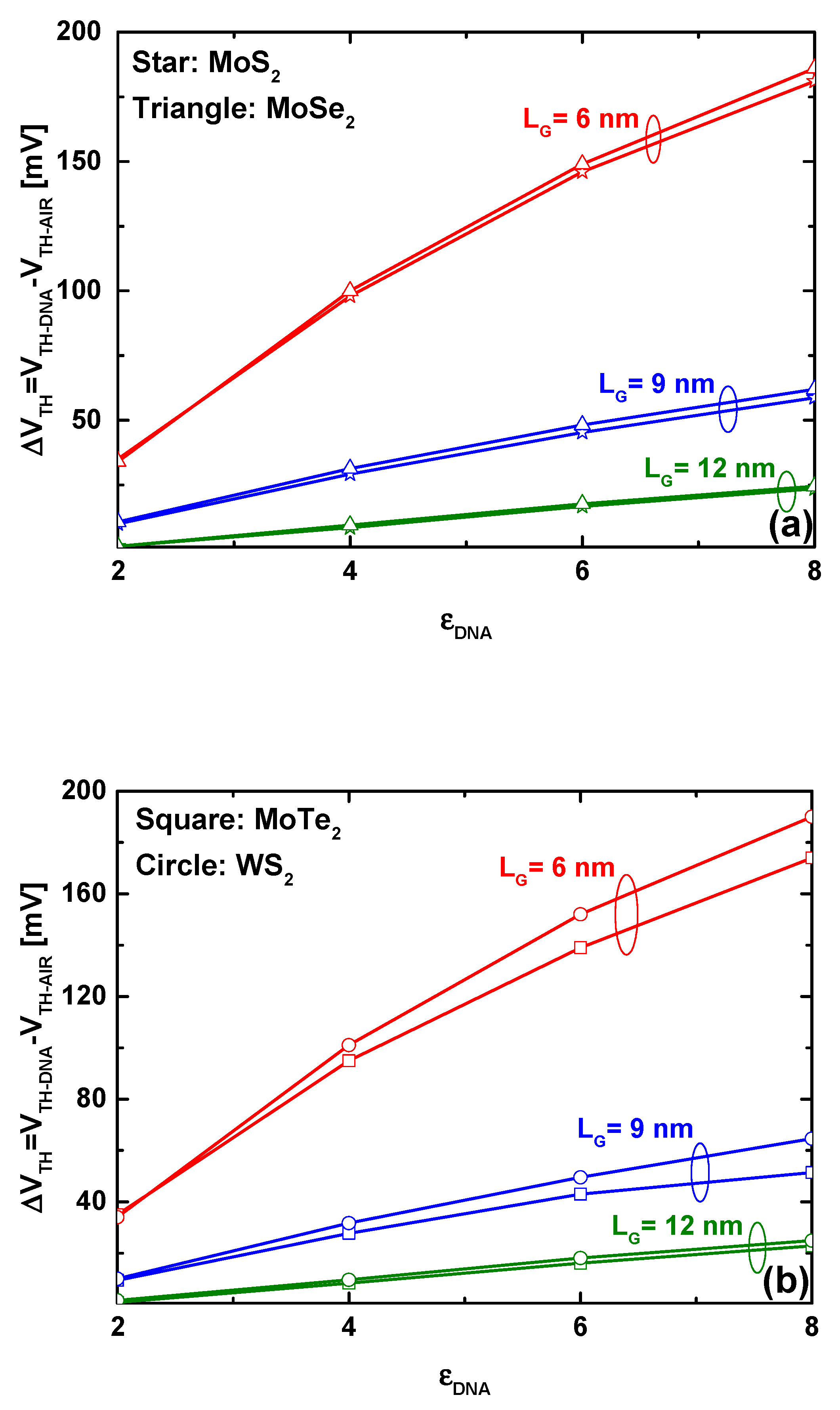
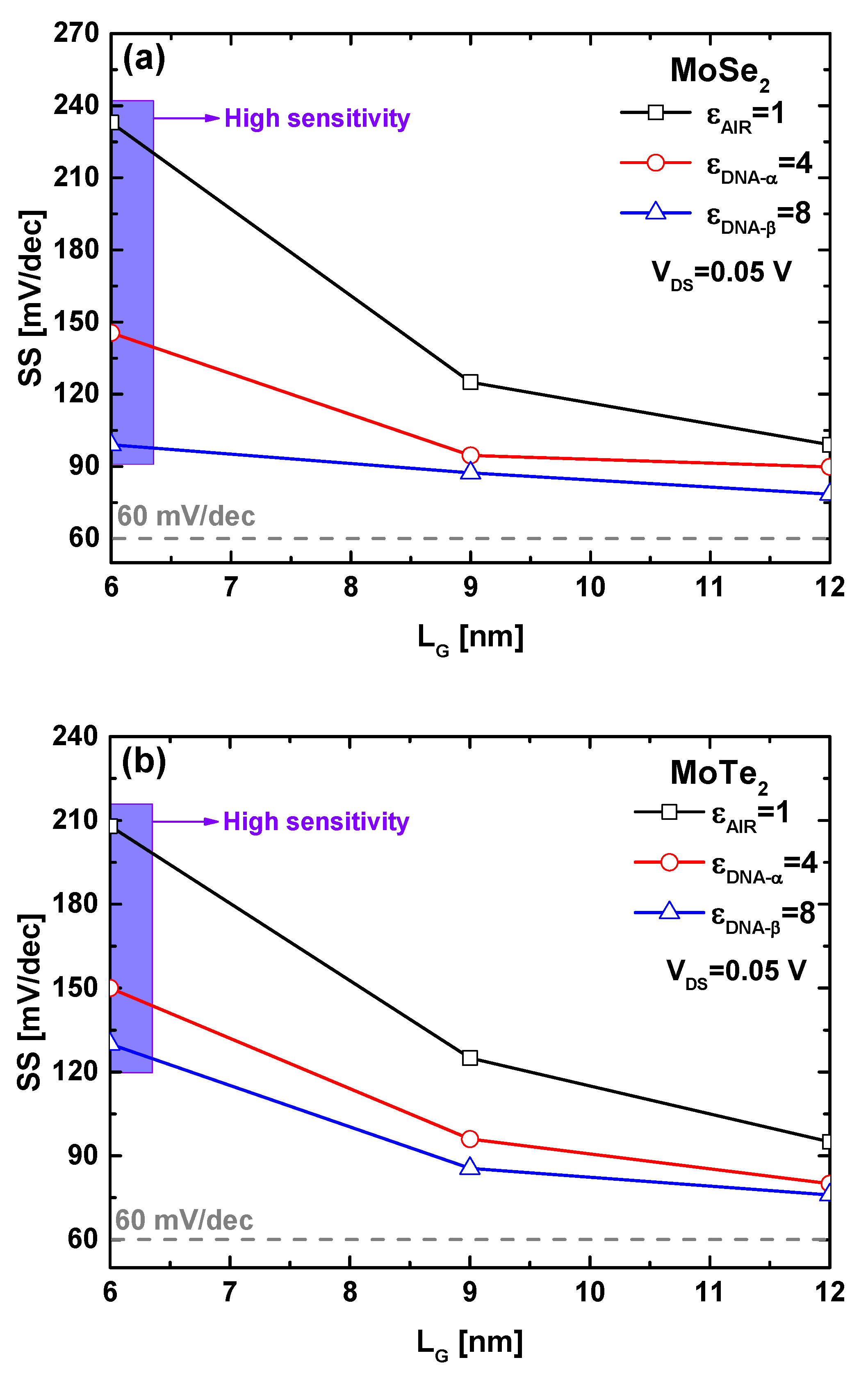
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergveld, P. Thirty Years of ISFETOLOGY. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Sun, C.; Ren, D.; Chen, J.; Li, Y. Signal Amplification Strategies for DNA-Based Surface Plasmon Resonance Biosensors. Biosens. Bioelectron. 2018, 117, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Fortunato, E.; Baptista, P. Field Effect Sensors for Nucleic Acid Detection: Recent Advances and Future Perspectives. Sensors 2015, 15, 10380–10398. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Park, S.-M. DNA Hybridization Sensors Based on Electrochemical Impedance Spectroscopy as a Detection Tool. Sensors 2009, 9, 9513–9532. [Google Scholar] [CrossRef] [PubMed]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing Based on Field-Effect Transistors (FET): Recent Progress and Challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon Nanowire Field-Effect Transistor-Based Biosensors for Biomedical Diagnosis and Cellular Recording Investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Poghossian, A.; Schöning, M.J. Recent Progress in Silicon-Based Biologically Sensitive Field-Effect Devices. Curr. Opin. Electrochem. 2021, 29, 100811. [Google Scholar] [CrossRef]
- Lu, N.; Gao, A.; Zhou, H.; Wang, Y.; Yang, X.; Wang, Y.; Li, T. Progress in Silicon Nanowire-Based Field-Effect Transistor Biosensors for Label-Free Detection of DNA. Chin. J. Chem. 2016, 34, 308–316. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z. Biosensors Based on Two-Dimensional MoS2. ACS Sens. 2015, 1, 5–16. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-Dimensional Materials in Biomedical, Biosensing and Sensing Applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Gu, X.; Xu, Y.; Shen, J.; Zhu, D.; Chao, J.; Fan, C.; Wang, L. Two-Dimensional Nanomaterials for Biosensing Applications. TrAC Trends Anal. Chem. 2019, 119, 115610. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Huang, K.-J.; Wu, X. Recent Advances in Transition-Metal Dichalcogenides Based Electrochemical Biosensors: A Review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef]
- Mia, A.K.; Meyyappan, M.; Giri, P.K. Two-Dimensional Transition Metal Dichalcogenide Based Biosensors: From Fundamentals to Healthcare Applications. Biosensors 2023, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-Dimensional Transition Metal Dichalcogenide Nanomaterials for Biosensing Applications. Mater. Chem. Front. 2017, 1, 24–36. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Molybdenum Disulfide-Based Field Effect Transistor Biosensors for Medical Diagnostics: Exploring a Decade of Advancements (2014–2024). TrAC Trends Anal. Chem. 2024, 176, 117742. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Huang, H. Evolution Application of Two-Dimensional MoS2-Based Field-Effect Transistors. Nanomaterials 2022, 12, 3233. [Google Scholar] [CrossRef]
- Im, H.; Huang, X.-J.; Gu, B.; Choi, Y.-K. A Dielectric-Modulated Field-Effect Transistor for Biosensing. Nat. Nanotech 2007, 2, 430–434. [Google Scholar] [CrossRef]
- Therriault, D. Filling the Gap. Nat. Nanotech 2007, 2, 393–394. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Z.; Yang, G.-M.; Li, J.; Li, M.-Q.; Liu, J.-H.; Huang, X.-J. Electrical Nanogap Devices for Biosensing. Mater. Today 2010, 13, 28–41. [Google Scholar] [CrossRef]
- Kim, C.-H.; Jung, C.; Park, H.G.; Choi, Y.-K. Novel dielectric modulated field-effect transistor for label-free DNA detection. Biochip J. 2008, 2, 127–134. [Google Scholar]
- Kim, C.-H.; Jung, C.; Lee, K.-B.; Park, H.G.; Choi, Y.-K. Label-Free DNA Detection with a Nanogap Embedded Complementary Metal Oxide Semiconductor. Nanotechnology 2011, 22, 135502. [Google Scholar] [CrossRef]
- Im, M.; Ahn, J.-H.; Han, J.-W.; Park, T.J.; Lee, S.Y.; Choi, Y.-K. Development of a Point-of-Care Testing Platform With a Nanogap-Embedded Separated Double-Gate Field Effect Transistor Array and Its Readout System for Detection of Avian Influenza. IEEE Sens. J. 2011, 11, 351–360. [Google Scholar] [CrossRef]
- Kim, S.; Baek, D.; Kim, J.-Y.; Choi, S.-J.; Seol, M.-L.; Choi, Y.-K. A Transistor-Based Biosensor for the Extraction of Physical Properties from Biomolecules. Appl. Phys. Lett. 2012, 101, 073703. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ahn, J.-H.; Lee, K.-B.; Jung, C.; Park, H.G.; Choi, Y.-K. A New Sensing Metric to Reduce Data Fluctuations in a Nanogap-Embedded Field-Effect Transistor Biosensor. IEEE Trans. Electron. Devices 2012, 59, 2825–2831. [Google Scholar] [CrossRef]
- Kannan, N.; Kumar, M.J. Dielectric-Modulated Impact-Ionization MOS Transistor as a Label-Free Biosensor. IEEE Electron. Device Lett. 2013, 34, 1575–1577. [Google Scholar] [CrossRef]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-Dimensional Layered MoS2 Biosensors Enable Highly Sensitive Detection of Biomolecules. Sci. Rep. 2014, 4, 7352. [Google Scholar] [CrossRef]
- Mei, J.; Li, Y.-T.; Zhang, H.; Xiao, M.-M.; Ning, Y.; Zhang, Z.-Y.; Zhang, G.-J. Molybdenum Disulfide Field-Effect Transistor Biosensor for Ultrasensitive Detection of DNA by Employing Morpholino as Probe. Biosens. Bioelectron. 2018, 110, 71–77. [Google Scholar] [CrossRef]
- Ono, T.; Kanai, Y.; Inoue, K.; Watanabe, Y.; Nakakita, S.; Kawahara, T.; Suzuki, Y.; Matsumoto, K. Electrical Biosensing at Physiological Ionic Strength Using Graphene Field-Effect Transistor in Femtoliter Microdroplet. Nano Lett. 2019, 19, 4004–4009. [Google Scholar] [CrossRef]
- Tamersit, K.; Djeffal, F. Double-Gate Graphene Nanoribbon Field-Effect Transistor for DNA and Gas Sensing Applications: Simulation Study and Sensitivity Analysis. IEEE Sens. J. 2016, 16, 4180–4191. [Google Scholar] [CrossRef]
- Tamersit, K.; Djeffal, F. Carbon Nanotube Field-Effect Transistor With Vacuum Gate Dielectric for Label-Free Detection of DNA Molecules: A Computational Investigation. IEEE Sens. J. 2019, 19, 9263–9270. [Google Scholar] [CrossRef]
- Tamersit, K. Dielectric-Modulated Junctionless Carbon Nanotube Field-Effect Transistor as a Label-Free DNA Nanosensor: Achieving Ultrahigh Sensitivity in the Band-to-Band Tunneling Regime. IEEE Sens. J. 2024, 24, 2915–2922. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-Time Reliable Determination of Binding Kinetics of DNA Hybridization Using a Multi-Channel Graphene Biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef]
- Afzalian, A.; Flandre, D. Ultra-Scaled Si Nanowire Biosensors for Single DNA Molecule Detection. Sensors 2023, 23, 5405. [Google Scholar] [CrossRef]
- Datta, S. Nanoscale Device Modeling: The Green’s Function Method. Superlattices Microstruct. 2000, 28, 253–278. [Google Scholar] [CrossRef]
- Datta, S. Quantum Transport: Atom to Transistor; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Datta, S. Electronic Transport in Mesoscopic Systems; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Anantram, M.P.; Lundstrom, M.S.; Nikonov, D.E. Modeling of Nanoscale Devices. Proc. IEEE 2008, 96, 1511–1550. [Google Scholar] [CrossRef]
- Rahman, A.; Guo, J.; Datta, S.; Lundstrom, M.S. Theory of Ballistic Nanotransistors. IEEE Trans. Electron. Devices 2003, 50, 1853–1864. [Google Scholar] [CrossRef]
- Tamersit, K.; Jooq, M.K.Q.; Moaiyeri, M.H. Analog/RF Performance Assessment of Ferroelectric Junctionless Carbon Nanotube FETs: A Quantum Simulation Study. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114915. [Google Scholar] [CrossRef]
- Tamersit, K. New Nanoscale Band-to-Band Tunneling Junctionless GNRFETs: Potential High-Performance Devices for the Ultrascaled Regime. J. Comput. Electron. 2021, 20, 1147–1156. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Guo, J. On Monolayer MoS2 Field-Effect Transistors at the Scaling Limit. IEEE Trans. Electron. Devices 2013, 60, 4133–4139. [Google Scholar] [CrossRef]
- Wu, T.; Guo, J. Multiscale Modeling of Semimetal Contact to Two-Dimensional Transition Metal Dichalcogenide Semiconductor. Appl. Phys. Lett. 2022, 121, 023507. [Google Scholar] [CrossRef]
- Dong, Z.; Guo, J. Assessment of 2-D Transition Metal Dichalcogenide FETs at Sub-5-nm Gate Length Scale. IEEE Trans. Electron. Devices 2017, 64, 622–628. [Google Scholar] [CrossRef]
- Lam, K.-T.; Cao, X.; Guo, J. Device Performance of Heterojunction Tunneling Field-Effect Transistors Based on Transition Metal Dichalcogenide Monolayer. IEEE Electron. Device Lett. 2013, 34, 1331–1333. [Google Scholar] [CrossRef]
- Svizhenko, A.; Anantram, M.P.; Govindan, T.R.; Biegel, B.; Venugopal, R. Two-Dimensional Quantum Mechanical Modeling of Nanotransistors. J. Appl. Phys. 2002, 91, 2343–2354. [Google Scholar] [CrossRef]
- Tamersit, K.; Kouzou, A.; Bourouba, H.; Kennel, R.; Abdelrahem, M. Synergy of Electrostatic and Chemical Doping to Improve the Performance of Junctionless Carbon Nanotube Tunneling Field-Effect Transistors: Ultrascaling, Energy-Efficiency, and High Switching Performance. Nanomaterials 2022, 12, 462. [Google Scholar] [CrossRef]
- Tamersit, K. Improved Switching Performance of Nanoscale P-i-n Carbon Nanotube Tunneling Field-Effect Transistors Using Metal-Ferroelectric-Metal Gating Approach. ECS J. Solid State Sci. Technol. 2021, 10, 031004. [Google Scholar] [CrossRef]
- Liu, L.; Kumar, S.B.; Ouyang, Y.; Guo, J. Performance Limits of Monolayer Transition Metal Dichalcogenide Transistors. IEEE Trans. Electron. Devices 2011, 58, 3042–3047. [Google Scholar] [CrossRef]
- Majumdar, K.; Hobbs, C.; Kirsch, P.D. Benchmarking Transition Metal Dichalcogenide MOSFET in the Ultimate Physical Scaling Limit. IEEE Electron. Device Lett. 2014, 35, 402–404. [Google Scholar] [CrossRef]
- Tamersit, K.; Ramezani, Z.; Amiri, I.S. Improved Performance of Sub-10-Nm Band-to-Band Tunneling n-i-n Graphene Nanoribbon Field-Effect Transistors Using Underlap Engineering: A Quantum Simulation Study. J. Phys. Chem. Solids 2022, 160, 110312. [Google Scholar] [CrossRef]
- Tamersit, K.; Madan, J.; Kouzou, A.; Pandey, R.; Kennel, R.; Abdelrahem, M. Role of Junctionless Mode in Improving the Photosensitivity of Sub-10 Nm Carbon Nanotube/Nanoribbon Field-Effect Phototransistors: Quantum Simulation, Performance Assessment, and Comparison. Nanomaterials 2022, 12, 1639. [Google Scholar] [CrossRef]
- O’Brien, K.P.; Naylor, C.H.; Dorow, C.; Maxey, K.; Penumatcha, A.V.; Vyatskikh, A.; Zhong, T.; Kitamura, A.; Lee, S.; Rogan, C.; et al. Process Integration and Future Outlook of 2D Transistors. Nat. Commun. 2023, 14, 6400. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.K. Bio-Inspired Computing—A Review of Algorithms and Scope of Applications. Expert Syst. Appl. 2016, 59, 20–32. [Google Scholar] [CrossRef]
- Tamersit, K.; Kotti, M.; Fakhfakh, M. A New Pressure Microsensor Based on Dual-Gate Graphene Field-Effect Transistor with a Vertically Movable Top-Gate: Proposal, Analysis, and Optimization. AEU—Int. J. Electron. Commun. 2020, 124, 153346. [Google Scholar] [CrossRef]
- Bendib, T.; Djeffal, F. Electrical Performance Optimization of Nanoscale Double-Gate MOSFETs Using Multiobjective Genetic Algorithms. IEEE Trans. Electron. Devices 2011, 58, 3743–3750. [Google Scholar] [CrossRef]
- Kalra, S.; Kumar, M.J.; Dhawan, A. Dielectric-Modulated Field Effect Transistors for DNA Detection: Impact of DNA Orientation. IEEE Electron. Device Lett. 2016, 37, 1485–1488. [Google Scholar] [CrossRef]
- Parihar, M.S.; Kranti, A. Enhanced Sensitivity of Double Gate Junctionless Transistor Architecture for Biosensing Applications. Nanotechnology 2015, 26, 145201. [Google Scholar] [CrossRef]
- Anvarifard, M.K.; Ramezani, Z.; Amiri, I.S.; Tamersit, K.; Nejad, A.M. Profound Analysis on Sensing Performance of Nanogap SiGe Source DM-TFET Biosensor. J. Mater. Sci. Mater. Electron. 2020, 31, 22699–22712. [Google Scholar] [CrossRef]
- Tamersit, K.; Moaiyeri, M.H.; Jooq, M.K.Q. Leveraging Negative Capacitance Ferroelectric Materials for Performance Boosting of Sub-10 Nm Graphene Nanoribbon Field-Effect Transistors: A Quantum Simulation Study. Nanotechnology 2022, 33, 465204. [Google Scholar] [CrossRef]
- Jooq, M.K.Q.; Moaiyeri, M.H.; Tamersit, K. A New Design Paradigm for Auto-Nonvolatile Ternary SRAMs Using Ferroelectric CNTFETs: From Device to Array Architecture. IEEE Trans. Electron. Devices 2022, 69, 6113–6120. [Google Scholar] [CrossRef]
- Tamersit, K.; Jooq, M.K.Q.; Moaiyeri, M.H. Computational Investigation of Negative Capacitance Coaxially Gated Carbon Nanotube Field-Effect Transistors. IEEE Trans. Electron. Devices 2021, 68, 376–384. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamersit, K.; Kouzou, A.; Rodriguez, J.; Abdelrahem, M. Quantum Simulation Study of Ultrascaled Label-Free DNA Sensors Based on Sub-10 nm Dielectric-Modulated TMD FETs: Sensitivity Enhancement Through Downscaling. Micromachines 2025, 16, 690. https://doi.org/10.3390/mi16060690
Tamersit K, Kouzou A, Rodriguez J, Abdelrahem M. Quantum Simulation Study of Ultrascaled Label-Free DNA Sensors Based on Sub-10 nm Dielectric-Modulated TMD FETs: Sensitivity Enhancement Through Downscaling. Micromachines. 2025; 16(6):690. https://doi.org/10.3390/mi16060690
Chicago/Turabian StyleTamersit, Khalil, Abdellah Kouzou, José Rodriguez, and Mohamed Abdelrahem. 2025. "Quantum Simulation Study of Ultrascaled Label-Free DNA Sensors Based on Sub-10 nm Dielectric-Modulated TMD FETs: Sensitivity Enhancement Through Downscaling" Micromachines 16, no. 6: 690. https://doi.org/10.3390/mi16060690
APA StyleTamersit, K., Kouzou, A., Rodriguez, J., & Abdelrahem, M. (2025). Quantum Simulation Study of Ultrascaled Label-Free DNA Sensors Based on Sub-10 nm Dielectric-Modulated TMD FETs: Sensitivity Enhancement Through Downscaling. Micromachines, 16(6), 690. https://doi.org/10.3390/mi16060690








