Hydroxyapatite-Complexed Type I Collagen and Fibrinogen-Modified Porous Titanium Alloy Scaffold: Promoting Osteogenesis and Soft Tissue Integration
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of 3D Printed Ti-6Al-4V Scaffold and Composite Coatings
2.1.1. Preparation of 3D Printed Ti-6Al-4V Scaffold
2.1.2. Preparation of Multi-Element Doped Hydroxyapatite
2.1.3. Adhesion of Polydopamine and Multi-Element Doped Hydroxyapatite
2.1.4. Adhesion of Fibrinogen Coating
2.1.5. Adhesion of Type I Collagen Coating
2.2. Testing and Characterization
2.2.1. Surface Morphology Observation of Samples
2.2.2. Static Water Contact Angle Measurement on Sample Surfaces
2.2.3. Analysis of Surface Chemical Composition of Samples
2.3. Coating Stability Assessment
2.4. In Vitro Cell Experiments
2.4.1. Preparation of Experimental Samples
2.4.2. Cell Culturing
2.4.3. Tissue Compatibility Testing
2.4.4. Cell Adhesion and Spreading Experiment
2.4.5. Cell Proliferation Experiment
2.4.6. Alkaline Phosphatase Activity Assay
2.4.7. Measurement of Osteogenic Gene Expression Levels
2.4.8. Alizarin Red Staining (Late-Stage Detection of Cell Osteogenic Differentiation)
2.5. Statistical Analysis
3. Results
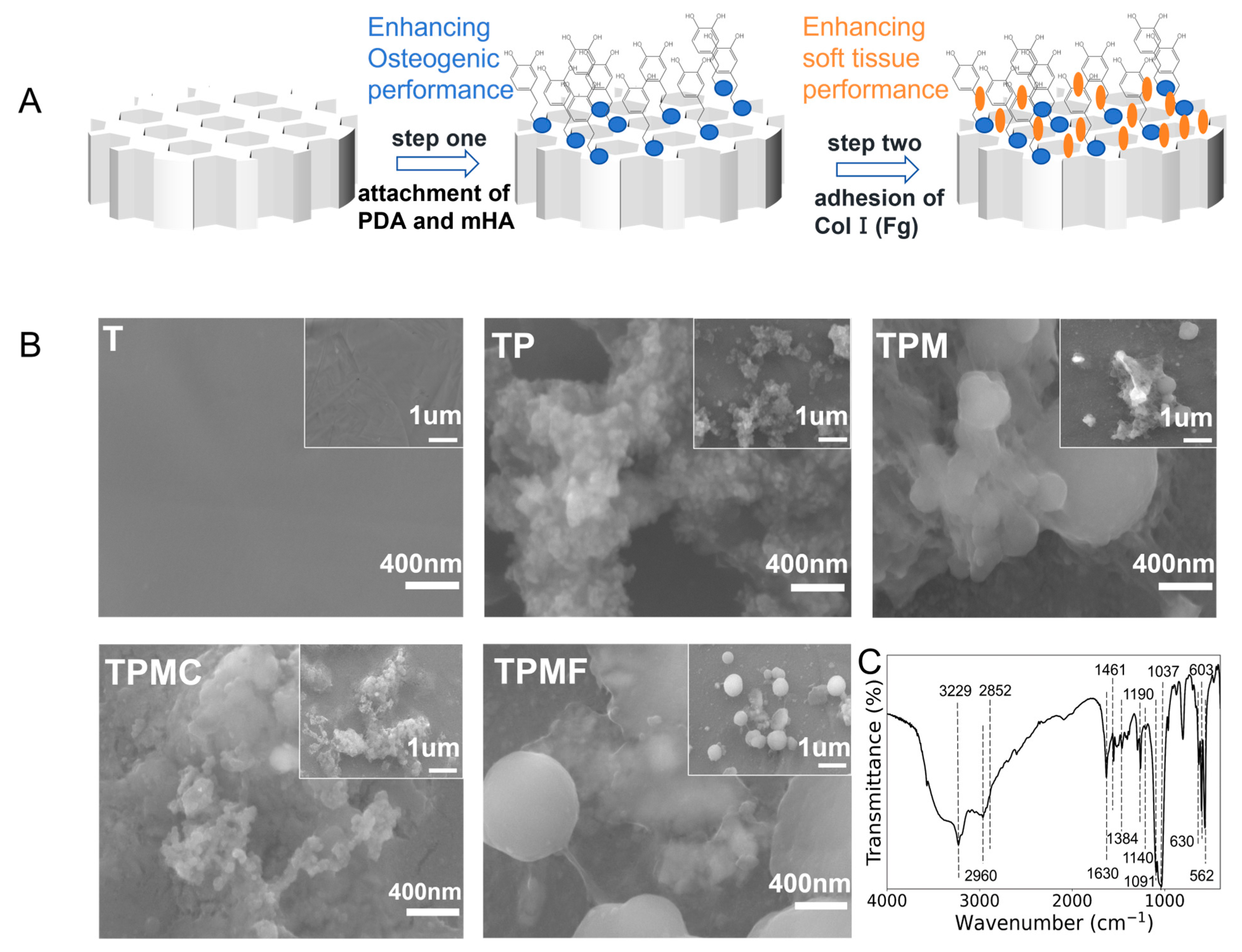
3.1. Preparation of 3D Printed Porous Titanium Alloy Scaffolds and Composite Coatings
3.2. Surface Characterization of the Samples
3.2.1. Surface Morphology Observation
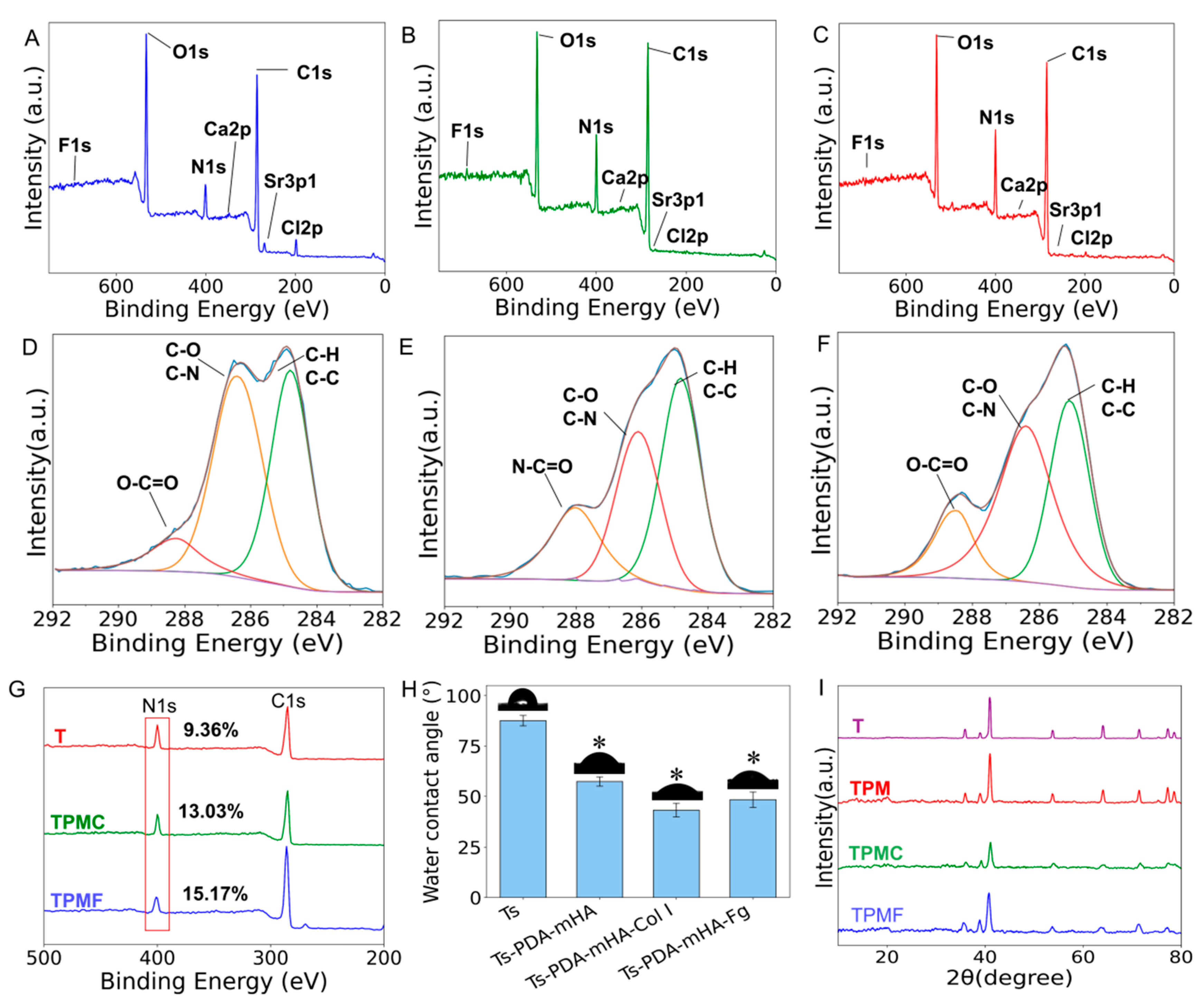
3.2.2. Surface Chemical Composition Analysis
3.2.3. Surface Hydrophilicity Testing
3.2.4. X-Ray Diffraction Analysis
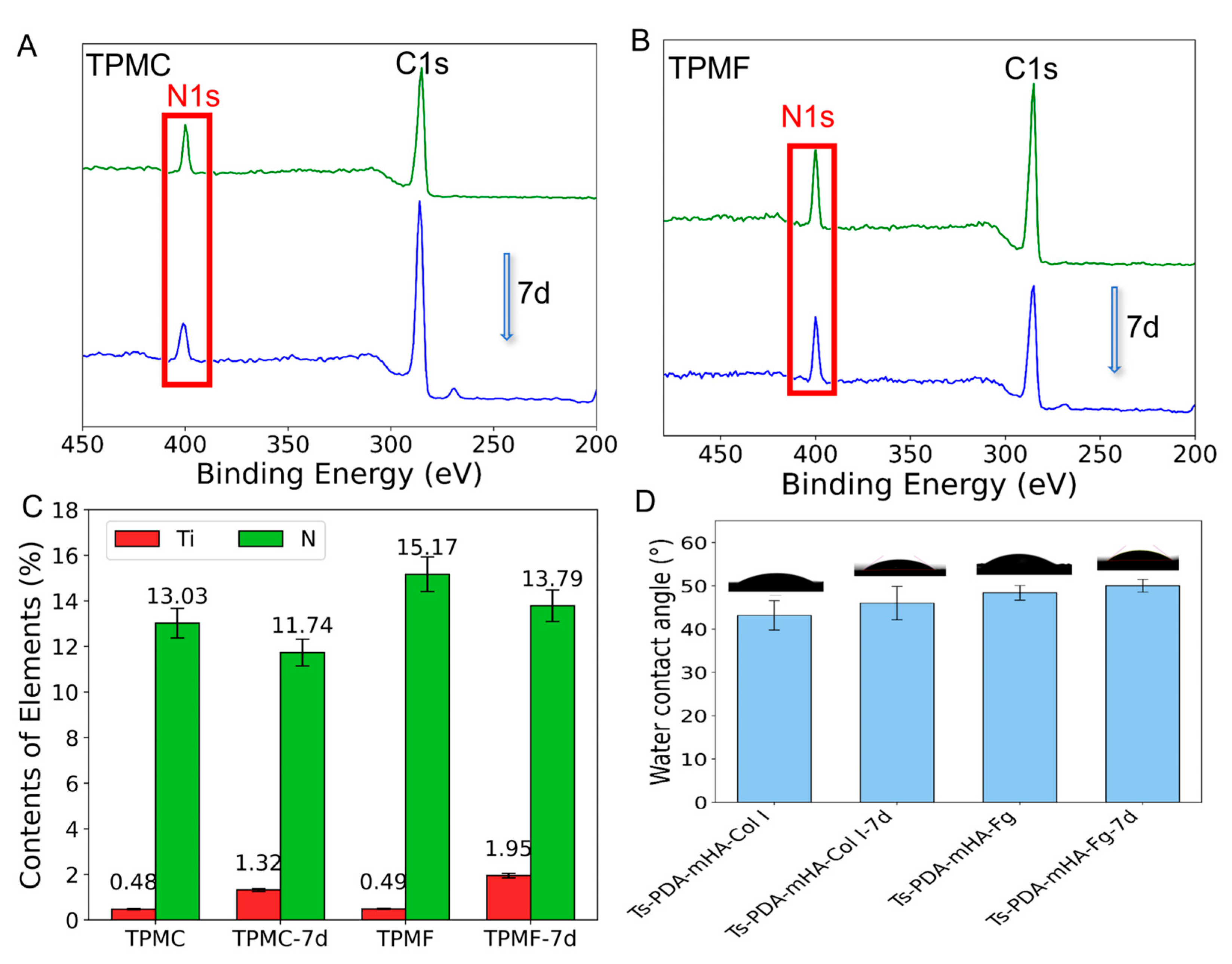
3.3. Coating Stability
3.4. In Vitro Cells Experiments
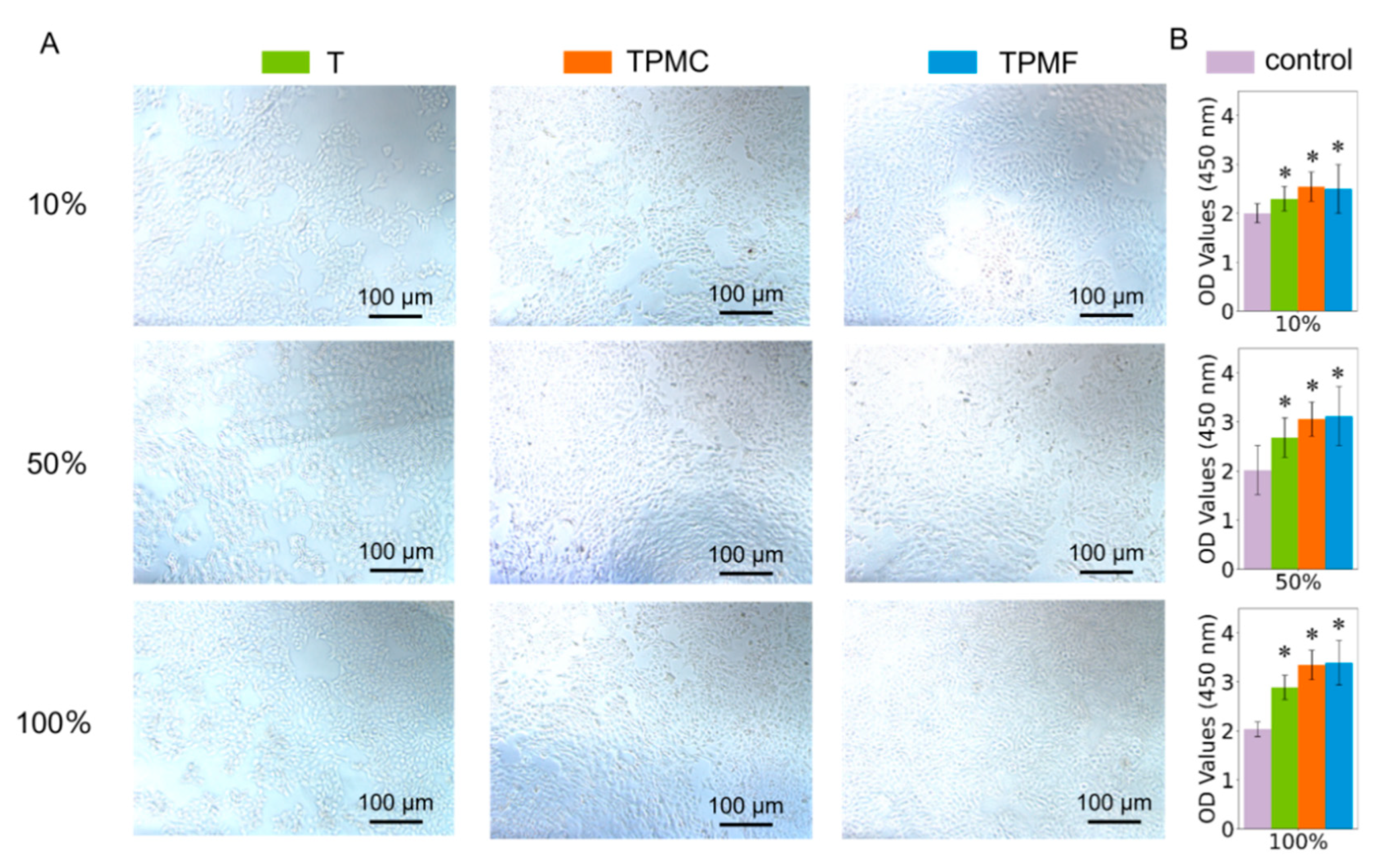
3.4.1. Cytotoxicity Assessment
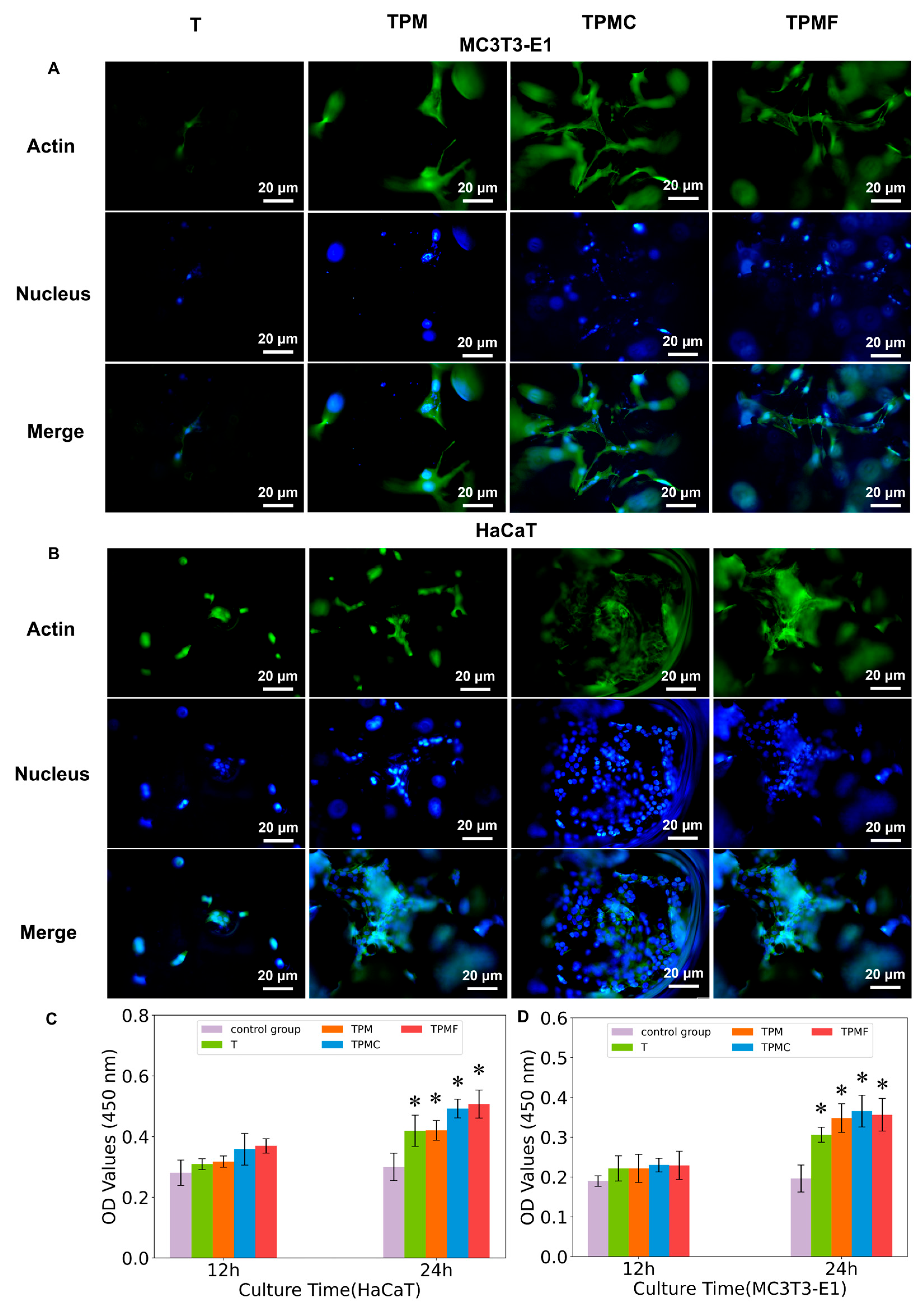
3.4.2. Cell Adhesion and Spreading
3.4.3. Cell Proliferation
3.4.4. Alkaline Phosphatase Activity
3.4.5. Osteogenesis-Related Gene Expressions
3.4.6. Alizarin Red S Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wu, W.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Zhu, J.; Ge, Z.; Cui, W. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater. Sci. Eng. C 2019, 103, 109858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kohn, J.; Yelick, P. Tyrofill–titanium implant constructs for the coordinated repair of rabbit mandible and tooth defects. Bioengineering 2023, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Smok, W.; Szewczenko, J.; Staszuk, M.; Chladek, G. Influence of hybrid surface modification on biocompatibility and physicochemical properties of ti-6al-4v eli titanium. J. Funct. Biomater. 2024, 15, 52. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhou, Y.; Ji, X.; Cheng, S.; Bian, D.; Fan, L.; Zhou, L.; Ning, C.; Zhang, Y. Biomimetic ti-6al-4v alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration. Bioact. Mater. 2021, 6, 3437–3448. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Lu, S.; Pan, D. Spatial topological structure design of porous ti–6al–4v alloy with low modulus and magnetic susceptibility. Nanomaterials 2023, 13, 3113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Zhou, Y.; Cai, J.; Sun, S.; Ma, J.; Wang, W.; Li, X.; Ma, L. The combination of a 3d-printed porous ti-6al-4v alloy scaffold and stem cell sheet technology for the construction of biomimetic engineered bone at an ectopic site. Mater. Today Bio 2022, 16, 100433. [Google Scholar] [CrossRef]
- Ng, S.L.; Das, S.; Ting, Y.; Wong, R.C.W.; Chanchareonsook, N. Benefits and biosafety of use of 3d-printing technology for titanium biomedical implants: A pilot study in the rabbit model. Int. J. Mol. Sci. 2021, 22, 8480. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Hou, W.; Nune, K.C.; Misra, R.D.K.; Correa-Rodriguez, V.L.; Guo, Z.; Hao, Y.; Yang, R.; Murr, L.E. Fabrication of open-cellular (porous) titanium alloy implants: Osseointegration, vascularization and preliminary human trials. Sci. China Mater. 2018, 61, 525–536. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Mas Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, A.; Domingues, R.M.A.; Tiainen, H.; Mendes, B.B.; Barrantes, A.; Reis, R.L.; Gomes, M.E.; Gomez Florit, M. Multifunctional surfaces for improving soft tissue integration. Adv. Healthc. Mater. 2021, 10, 2001985. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, D.; Bao, W.; Ding, R.; Shen, Z.; Huang, W.; Lu, Y.; Zhang, P.; Sun, Y.; Chen, H.; et al. Polydopamine-functionalized strontium alginate/hydroxyapatite composite microhydrogel loaded with vascular endothelial growth factor promotes bone formation and angiogenesis. ACS Appl. Mater. Interfaces 2024, 16, 4462–4477. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ding, R.; Jin, X.; Lu, Y.; Bao, W.; Zhao, Y.; Chen, S.; Shen, C.; Yang, Q.; Wang, Y. Strontium ion-functionalized nano-hydroxyapatite/chitosan composite microspheres promote osteogenesis and angiogenesis for bone regeneration. ACS Appl. Mater. Interfaces 2023, 15, 19951–19965. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.; Doan, V.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid. Interface. Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Gao, J.; Wang, M.; Shi, C.; Wang, L.; Zhu, Y.; Wang, D. A facile green synthesis of trace si, sr and f multi-doped hydroxyapatite with enhanced biocompatibility and osteoconduction. Mater. Lett. 2017, 196, 406–409. [Google Scholar] [CrossRef]
- Karanth, D.; Song, K.; Martin, M.L.; Meyer, D.R.; Dolce, C.; Huang, Y.; Holliday, L.S. Towards resorbable3d -printed scaffolds for craniofacial bone regeneration. Orthod. Craniofac. Res. 2023, 26, 188–195. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, X.; Wei, M. Biomimetic collagen/apatite coating formation on ti6al4v substrates. J. Biomed. Mater. Res. Part B 2012, 100, 871–881. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, D.; Wang, X.; He, Y.; Luan, W.; Qi, F.; Ding, J. Polydopamine-mediated covalent functionalization of collagen on a titanium alloy to promote biocompatibility with soft tissues. J. Mat. Chem. B 2019, 7, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium–tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Nuntapramote, T.; Brüggemann, D. Self-assembled fibrinogen scaffolds support cocultivation of human dermal fibroblasts and hacat keratinocytes. ACS Omega 2023, 8, 8650–8663. [Google Scholar] [CrossRef]
- Wang, X.; Lei, X.; Yu, Y.; Miao, S.; Tang, J.; Fu, Y.; Ye, K.; Shen, Y.; Shi, J.; Wu, H.; et al. Biological sealing and integration of a fibrinogen-modified titanium alloy with soft and hard tissues in a rat model. Biomater. Sci. 2021, 9, 5192–5208. [Google Scholar] [CrossRef]
- GB/T 16886.12-2017/ISO10993-12:012; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. Standards Press of China: Beijing, China, 2017.
- Zain, N.M.; Hussain, R.; Kadir, M.R.A. Surface modification of yttria stabilized zirconia via polydopamine inspired coating for hydroxyapatite biomineralization. Appl. Surf. Sci. 2014, 322, 169–176. [Google Scholar] [CrossRef]
- GB/T 16886.5-2017/ISO10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Standards Press of China: Beijing, China, 2017.
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X.; Shen, S.G. Mussel-inspired polydopamine coating: A general strategy to enhance osteogenic differentiation and osseointegration for diverse implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Liu, Z.; Wang, Y.; Dong, W.; Zhao, S.; Sun, D. Biomimetic design strategy of complex porous structure based on 3d printing ti-6al-4v scaffolds for enhanced osseointegration. Mater. Des. 2022, 218, 110721. [Google Scholar] [CrossRef]
- Yun, Y.J.; Kim, H.; Lee, D.; Um, S.; Chun, H.J. Polydopamine-mediated surface modifications of poly l-lactic acid with hydroxyapatite, heparin and bone morphogenetic protein-2 and their effects on osseointegration. J. Ind. Eng. Chem. 2018, 67, 244–254. [Google Scholar] [CrossRef]
- Middleton, C.A.; Pendegrass, C.J.; Gordon, D.; Jacob, J.; Blunn, G.W. Fibronectin silanized titanium alloy: A bioinductive and durable coating to enhance fibroblast attachment in vitro. J. Biomed. Mater. Res. Part A 2007, 83, 1032–1038. [Google Scholar] [CrossRef]
- Abdallah, M.N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for optimizing the soft tissue seal around osseointegrated implants. Adv. Healthc. Mater. 2017, 6, 1700549. [Google Scholar] [CrossRef]
- Chien, C.; Tsai, W. Poly(dopamine)-assisted immobilization of arg-gly-asp peptides, hydroxyapatite, and bone morphogenic protein-2 on titanium to improve the osteogenesis of bone marrow stem cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. Bmp2 regulates osterix through msx2 and runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formationin vivo andin vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Shen, T.; Yang, W.; Shen, X.; Chen, W.; Tao, B.; Yang, X.; Yuan, J.; Liu, P.; Cai, K. Polydopamine-assisted hydroxyapatite and lactoferrin multilayer on titanium for regulating bone balance and enhancing antibacterial property. ACS Biomater. Sci. Eng. 2018, 4, 3211–3223. [Google Scholar] [CrossRef]

| Groups | DA-Tris (mg/mL) | mHA-Tris(mg/mL) |
|---|---|---|
| T-PDA (TP) | 2 | - |
| T-PDA-mHA (TPM) | 2 | 4 |
| Gene | Sequence |
|---|---|
| GADPH | Upstream: GAGTCGGTGTGAACGGATTTG |
| Downstream: TGTAGACCAGTTAGTTGAGGTCA | |
| ALP | Upstream: CACGGCGTCCATGAGCAGAAC |
| Downstream: CAGGCACAGTGGTCAAGGTTGG | |
| RunX2 | Upstream: ATTGCAGGCTTCGTGGTTGAGG |
| Downstream: TGGCTGTATGGTGAGGCTGGTAG | |
| OCN | Upstream: GCTCGGCTTTGGCTGCTCTC |
| Downstream: AGCTGCTGTGACATCCATACTTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, W.; Tian, G.; Han, X.; Gao, J.; Zhu, Y.; Xu, X. Hydroxyapatite-Complexed Type I Collagen and Fibrinogen-Modified Porous Titanium Alloy Scaffold: Promoting Osteogenesis and Soft Tissue Integration. Micromachines 2025, 16, 692. https://doi.org/10.3390/mi16060692
Tao W, Tian G, Han X, Gao J, Zhu Y, Xu X. Hydroxyapatite-Complexed Type I Collagen and Fibrinogen-Modified Porous Titanium Alloy Scaffold: Promoting Osteogenesis and Soft Tissue Integration. Micromachines. 2025; 16(6):692. https://doi.org/10.3390/mi16060692
Chicago/Turabian StyleTao, Wenhao, Gang Tian, Xu Han, Jianyong Gao, Yingchun Zhu, and Xiaogang Xu. 2025. "Hydroxyapatite-Complexed Type I Collagen and Fibrinogen-Modified Porous Titanium Alloy Scaffold: Promoting Osteogenesis and Soft Tissue Integration" Micromachines 16, no. 6: 692. https://doi.org/10.3390/mi16060692
APA StyleTao, W., Tian, G., Han, X., Gao, J., Zhu, Y., & Xu, X. (2025). Hydroxyapatite-Complexed Type I Collagen and Fibrinogen-Modified Porous Titanium Alloy Scaffold: Promoting Osteogenesis and Soft Tissue Integration. Micromachines, 16(6), 692. https://doi.org/10.3390/mi16060692






