Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface
Abstract
1. Introduction
2. Materials and Methods
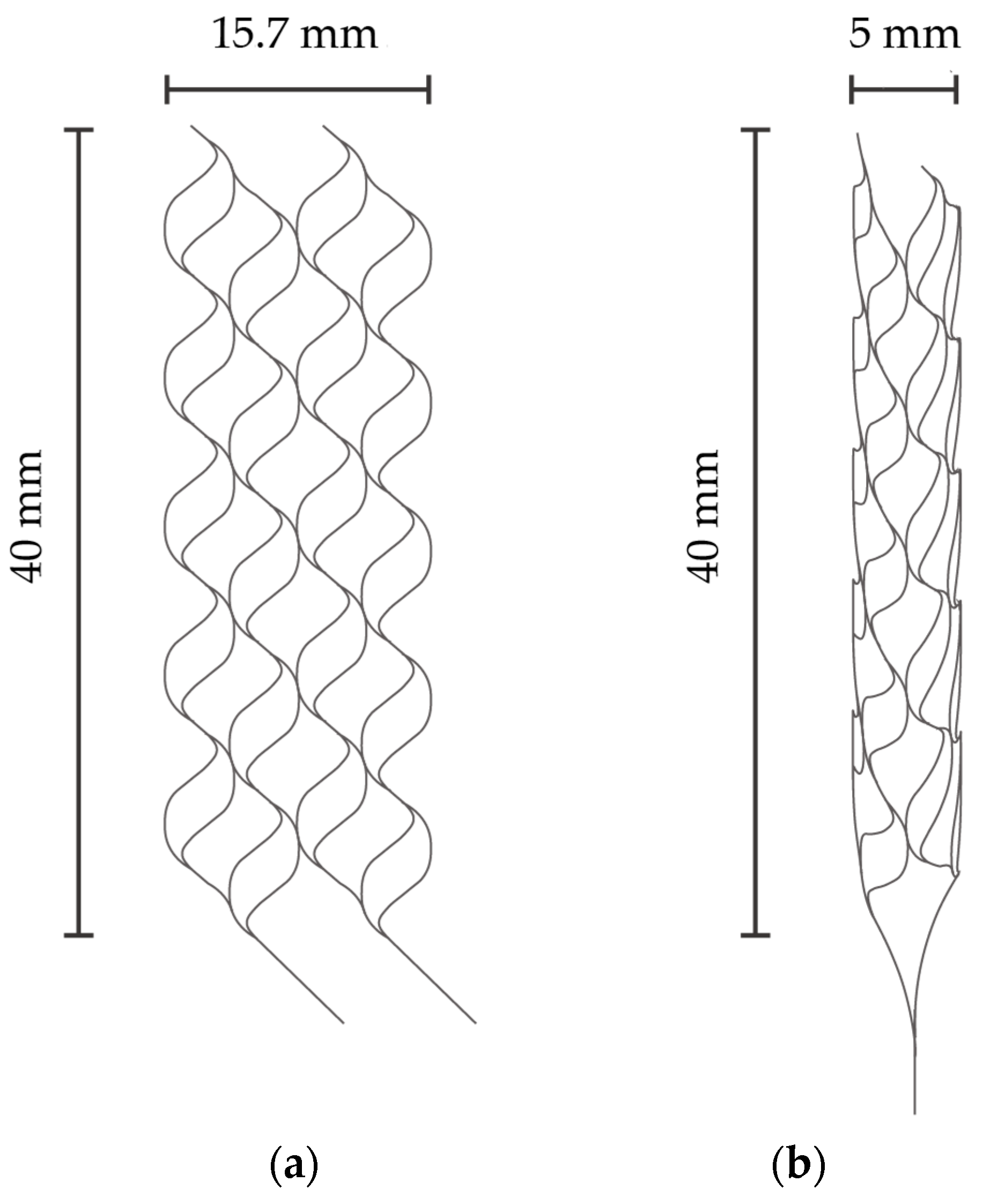
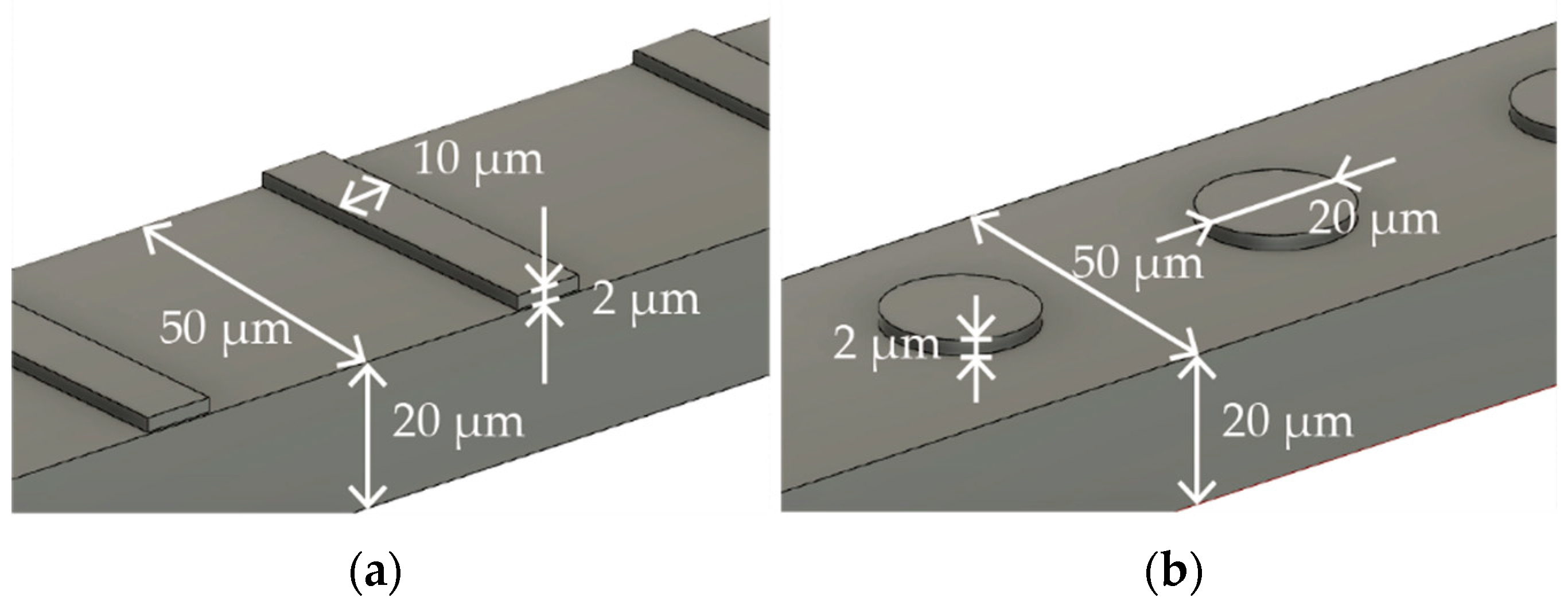
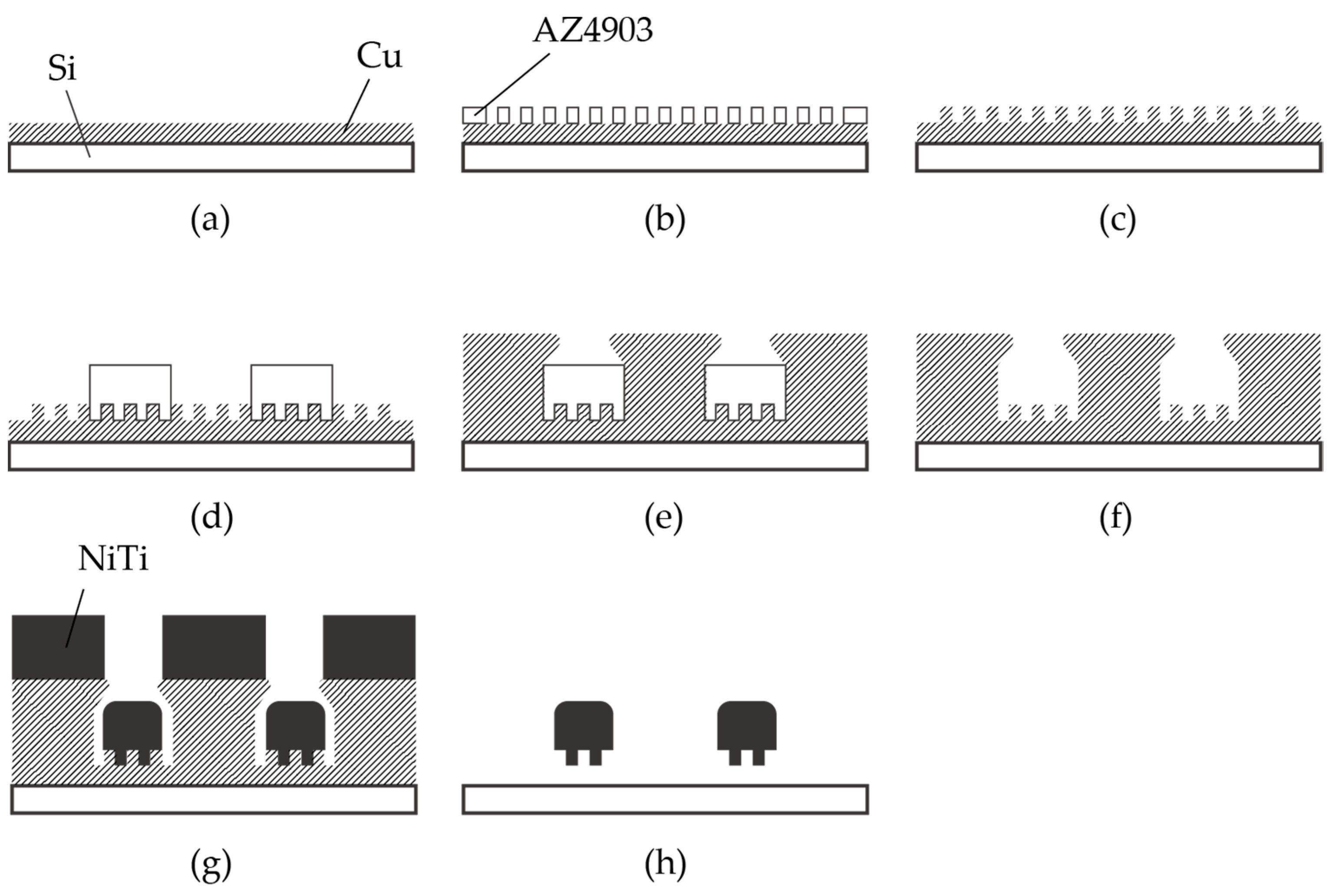
2.1. Fabrication Process of Nitinol Stent Retrievers with Surface Micro-Patterns
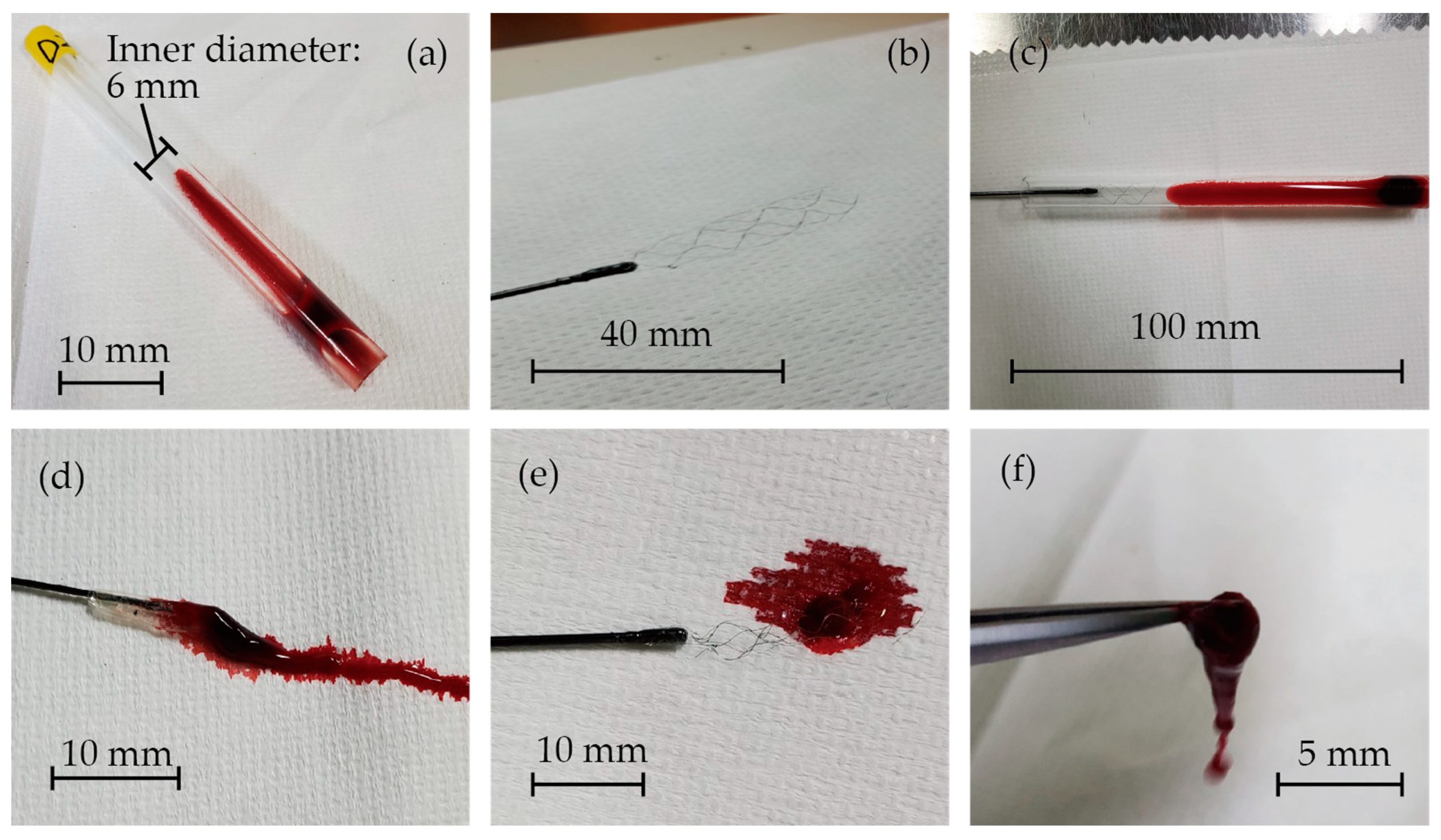
2.2. In Vitro Experiments with Pig Blood Clots
3. Results and Discussion
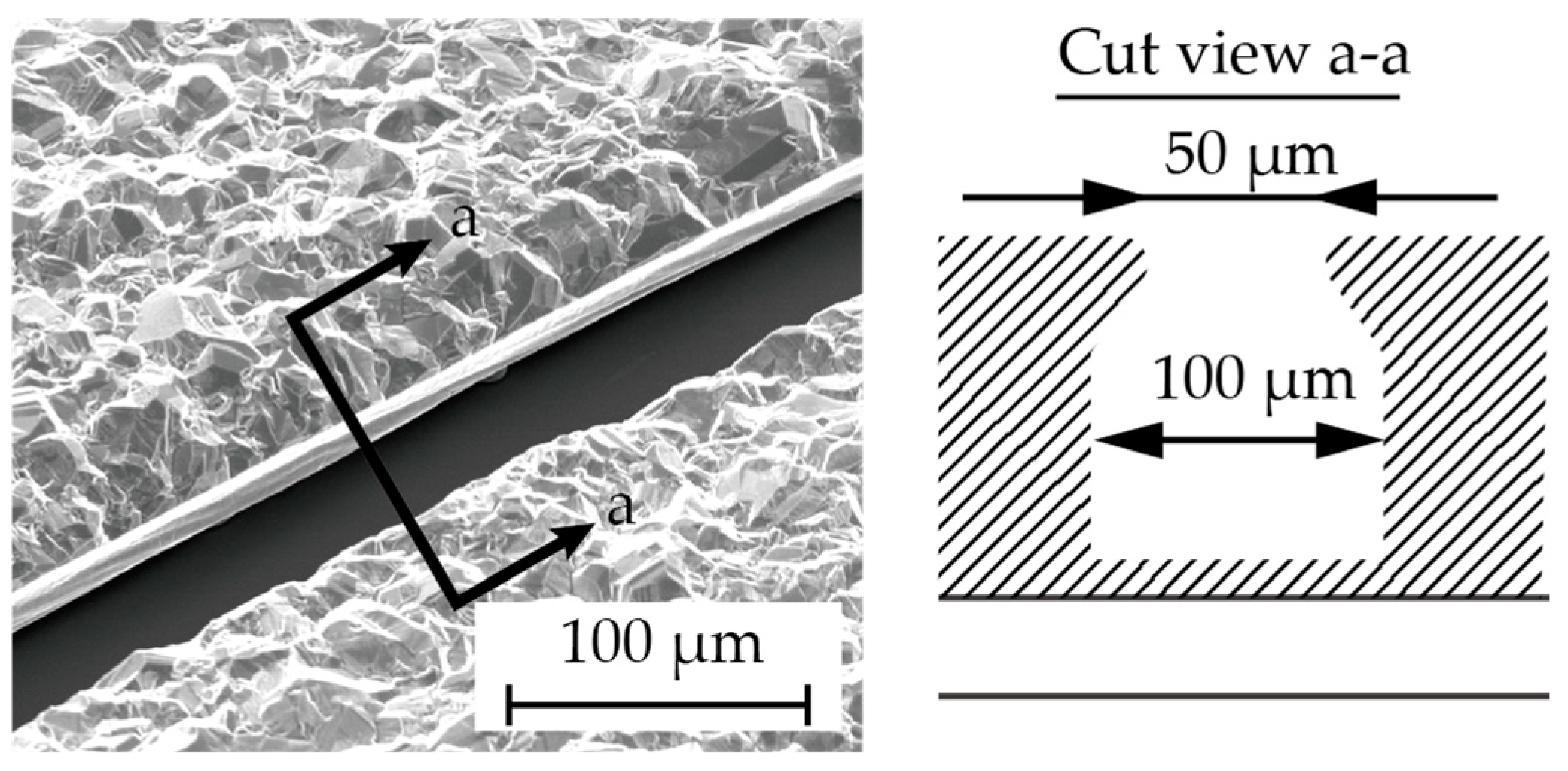
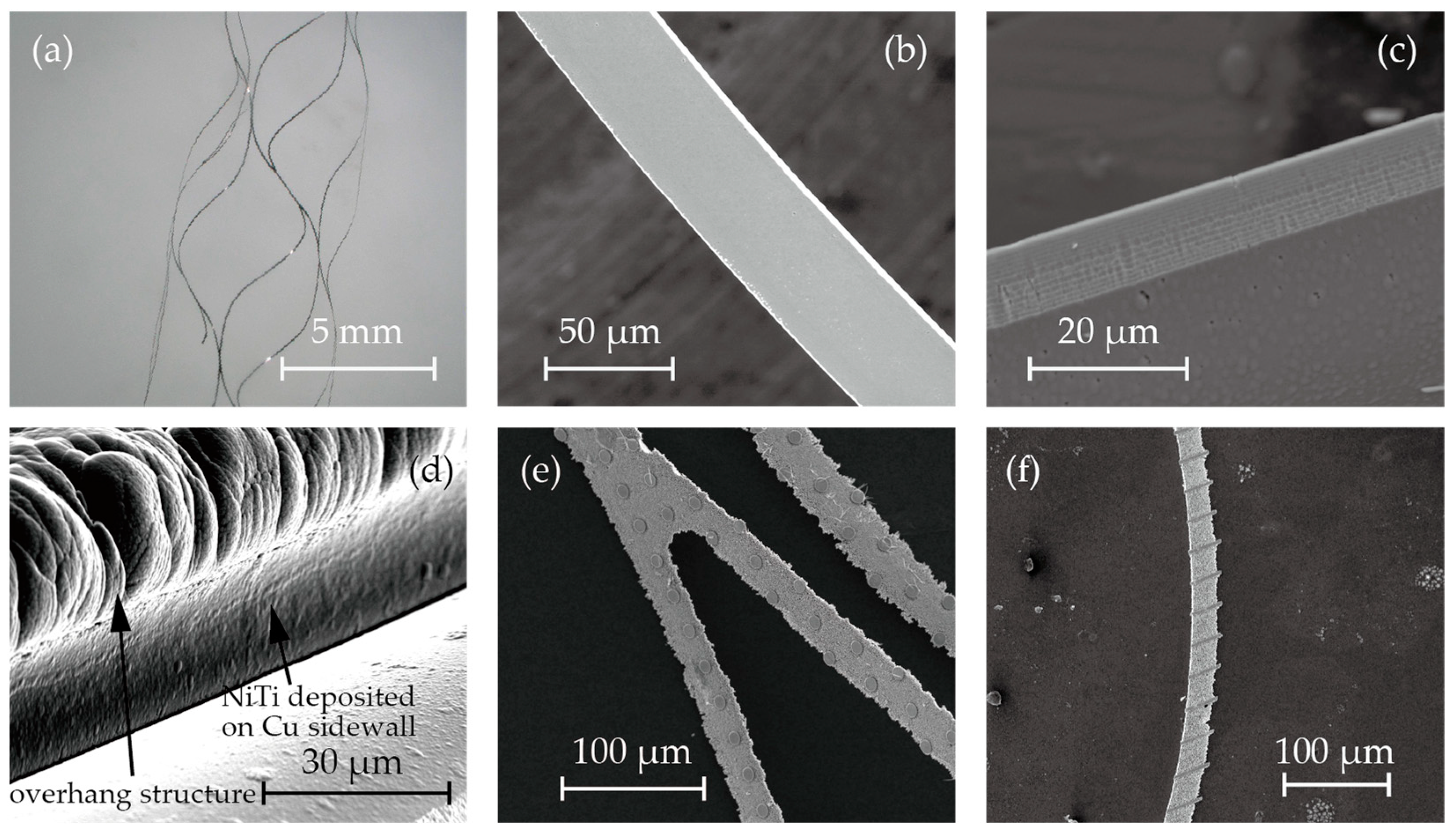
3.1. Fabrication of Nitinol Stent Retrievers with Surface Micro-Patterns
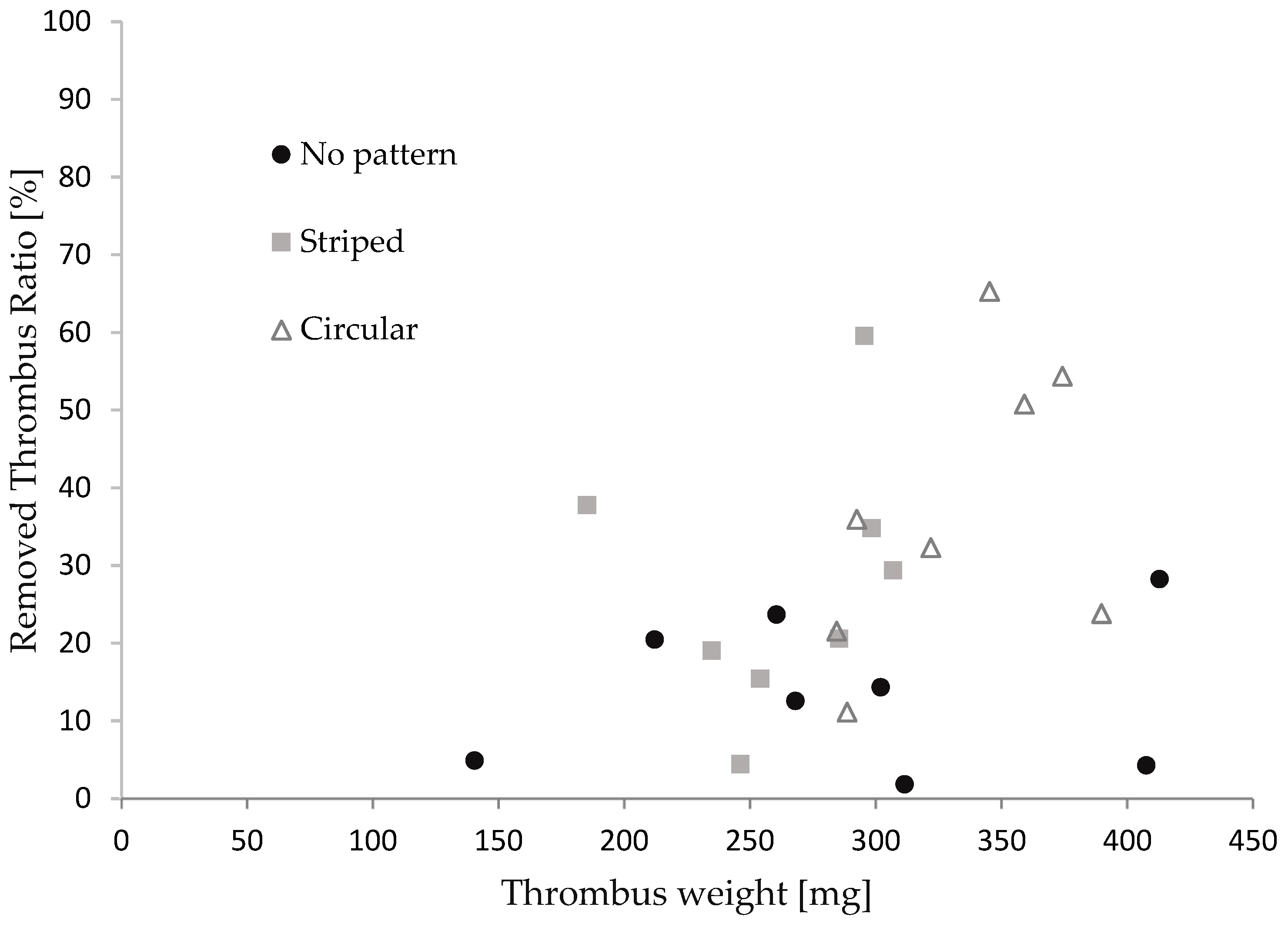
3.2. Assessment of the Thrombectomy Performance through In Vitro Experiments
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morotti, A.; Poli, L.; Costa, P. Acute Stroke. Semin. Neurol. 2019, 39, 061–072. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Murray, V.; Berge, E.; Del Zoppo, G.J. Thrombolysis for Acute Ischaemic Stroke. Cochrane Database Syst. Rev. 2014, CD000213. [Google Scholar] [CrossRef]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; De Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Mays, W.; Elijovich, L. Complications of Mechanical Thrombectomy in Acute Ischemic Stroke. Neurology 2021, 97, S115–S125. [Google Scholar] [CrossRef] [PubMed]
- Weafer, F.M.; Duffy, S.; Machado, I.; Gunning, G.; Mordasini, P.; Roche, E.; McHugh, P.E.; Gilvarry, M. Characterization of Strut Indentation during Mechanical Thrombectomy in Acute Ischemic Stroke Clot Analogs. J. Neurointerventional Surg. 2019, 11, 891–897. [Google Scholar] [CrossRef]
- Pereira, V.M.; Gralla, J.; Davalos, A.; Bonafé, A.; Castaño, C.; Chapot, R.; Liebeskind, D.S.; Nogueira, R.G.; Arnold, M.; Sztajzel, R.; et al. Prospective, Multicenter, Single-Arm Study of Mechanical Thrombectomy Using Solitaire Flow Restoration in Acute Ischemic Stroke. Stroke 2013, 44, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- The IMS II Trial Investigators The Interventional Management of Stroke (IMS) II Study. Stroke 2007, 38, 2127–2135. [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary Stents: A Materials Perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef]
- Cooper, A.I. Conjugated Microporous Polymers. Adv. Mater. 2009, 21, 1291–1295. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-Induced Activation of Covalent Bonds in Mechanoresponsive Polymeric Materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An Overview of Nitinol Medical Applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Stoeckel, D. Nitinol Medical Devices and Implants. Minim. Invasive Ther. Allied Technol. 2000, 9, 81–88. [Google Scholar] [CrossRef]
- Castleman, L.S.; Motzkin, S.M.; Alicandri, F.P.; Bonawit, V.L.; Johnson, A.A. Biocompatibility of Nitinol Alloy as an Implant Material. J. Biomed. Mater. Res. 1976, 10, 695–731. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; de Vries, J.; Busscher, H.J.; Uges, D.R.; van Horn, J.R. Electrochemical and Surface Characterization of a Nickel-Titanium Alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, C.; Tabrizian, M.; Yahia, L.; Bilodeau, L.; Piron, D.L. Improvement of the Corrosion Resistance of NiTi Stents by Surface Treatments. MRS Online Proc. Libr. (OPL) 1997, 459, 363–368. [Google Scholar] [CrossRef]
- Russell, S.M. SMST-2000: Proceedings of the International Conference on Shape Memory and Superelastic Technologies; ASM International: Almere, the Netherlands, 2001; ISBN 978-1-61503-106-1. [Google Scholar]
- Wadood, A. Brief Overview on Nitinol as Biomaterial. Adv. Mater. Sci. Eng. 2016, 2016, e4173138. [Google Scholar] [CrossRef]
- Robertson, S.W.; Pelton, A.R.; Ritchie, R.O. Mechanical Fatigue and Fracture of Nitinol. Int. Mater. Rev. 2012, 57, 1–37. [Google Scholar] [CrossRef]
- Dotter, C.T.; Buschmann, R.W.; McKinney, M.K.; Rösch, J. Transluminal Expandable Nitinol Coil Stent Grafting: Preliminary Report. Radiology 1983, 147, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.C.; Gu, L. The Impact of Wire Stent Fabrication Technique on the Performance of Stent Placement. J. Med. Devices 2012, 6, 011007. [Google Scholar] [CrossRef]
- Kripalani, K.; Jain, P. Comprehensive Study of Laser Cladding by Nitinol Wire. Mater. Today Proc. 2021, 37, 656–664. [Google Scholar] [CrossRef]
- Raval, A.; Choubey, A.; Engineer, C.; Kothwala, D. Development and Assessment of 316LVM Cardiovascular Stents. Mater. Sci. Eng. A 2004, 386, 331–343. [Google Scholar] [CrossRef]
- Liu, W.; Du, W.; Liao, J. Application of Fiber Laser Used in the Field of Stent Cutting and Micromachining. Proc. SPIE 2005, 5629, 263–270. [Google Scholar]
- Fu, C.H.; Liu, J.F.; Guo, A. Statistical Characteristics of Surface Integrity by Fiber Laser Cutting of Nitinol Vascular Stents. Appl. Surf. Sci. 2015, 353, 291–299. [Google Scholar] [CrossRef]
- Muhammad, N.; Whitehead, D.; Boor, A.; Oppenlander, W.; Liu, Z.; Li, L. Picosecond Laser Micromachining of Nitinol and Platinum–Iridium Alloy for Coronary Stent Applications. Appl. Phys. A 2012, 106, 607–617. [Google Scholar] [CrossRef]
- Guo, Y.; Klink, A.; Fu, C.; Snyder, J. Machinability and Surface Integrity of Nitinol Shape Memory Alloy. CIRP Ann. 2013, 62, 83–86. [Google Scholar] [CrossRef]
- Katona, B.; Bognár, E.; Balázs, B.; Nagy, P.; Hirschberg, K. Chemical Etching of Nitinol Stents. Acta Bioeng. Biomech. 2013, 15, 3–8. [Google Scholar] [CrossRef]
- Demir, A.G.; Previtali, B.; Biffi, C.A. Fibre Laser Cutting and Chemical Etching of AZ31 for Manufacturing Biodegradable Stents. Adv. Mater. Sci. Eng. 2013, 2013, e692635. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, Y.; Wu, H.; Wei, L.; Zhou, G.; Zhang, Y.; Deng, L.; Cheng, Y.; Li, M.; et al. Endovascular Metal Devices for the Treatment of Cerebrovascular Diseases. Adv. Mater. 2019, 31, 1805452. [Google Scholar] [CrossRef]
- Weinert, K.; Petzoldt, V.; Kötter, D. Turning and Drilling of NiTi Shape Memory Alloys. CIRP Ann. 2004, 53, 65–68. [Google Scholar] [CrossRef]
- Lima de Miranda, R.; Zamponi, C.; Quandt, E. Rotational UV Lithography Device for Cylindrical Substrate Exposure. Rev. Sci. Instrum. 2009, 80, 015103. [Google Scholar] [CrossRef]
- Lima De Miranda, R.; Zamponi, C.; Quandt, E. Fabrication of TiNi Thin Film Stents. Smart Mater. Struct. 2009, 18, 104010. [Google Scholar] [CrossRef]
- Lima De Miranda, R.; Zamponi, C.; Quandt, E. Micropatterned Freestanding Superelastic TiNi Films. Adv. Eng. Mater. 2013, 15, 66–69. [Google Scholar] [CrossRef]
- Bechtold, C.; Lima De Miranda, R.; Quandt, E. Capability of Sputtered Micro-Patterned NiTi Thick Films. Shap. Mem. Superelasticity 2015, 1, 286–293. [Google Scholar] [CrossRef]
- Velvaluri, P.; Soor, A.; Plucinsky, P.; de Miranda, R.L.; James, R.D.; Quandt, E. Origami-Inspired Thin-Film Shape Memory Alloy Devices. Sci. Rep. 2021, 11, 10988. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-Memory Materials and Hybrid Composites for Smart Systems: Part I Shape-Memory Materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Quandt, E.; Halene, C.; Holleck, H.; Feit, K.; Kohl, M.; Schloβmacher, P.; Skokan, A.; Skrobanck, K.D. Sputter Deposition of TiNi, TiNiPd and TiPd Films Displaying the Two-Way Shape-Memory Effect. Sens. Actuators A Phys. 1996, 53, 434–439. [Google Scholar] [CrossRef]
- Ho, K.K.; Carman, G.P. Sputter deposition of NiTi thin film shape memory alloy using a heated target. Thin Solid Film 2000, 370, 18–29. [Google Scholar] [CrossRef]
- ASTM-F2063; Standard Specification for Wrought Nickel-Titanium Shape Memory Alloys for Medical Devices and Surgical Implants. ASTM International: West Conshohocken, PA, USA, 2018. Available online: https://www.document-center.com/standards/show/ASTM-F2063 (accessed on 28 January 2024).
- Oberg, E.; Jones, F.D.; Rogers, F.E. Machinery’s Handbook for Machine Shop and Drafting-Room: A Reference Book on Machine Design and Shop Practice for the Mechanical Engineer, Draftsman, Toolmaker and Machinist; Industrial Press: New York, NY, USA, 1915. [Google Scholar]
- Srivatsa, S.; Duan, Y.; Sheppard, J.P.; Pahwa, S.; Pace, J.; Zhou, X.; Bambakidis, N.C. Cerebral Vessel Anatomy as a Predictor of First-Pass Effect in Mechanical Thrombectomy for Emergent Large-Vessel Occlusion. J. Neurosurg. 2020, 134, 576–584. [Google Scholar] [CrossRef]
- Nikoubashman, O.; Nikoubashman, A.; Büsen, M.; Wiesmann, M. Necessary Catheter Diameters for Mechanical Thrombectomy with ADAPT. Am. J. Neuroradiol. 2017, 38, 2277–2281. [Google Scholar] [CrossRef]
- Davison, M.A.; Ouyang, B.; Keppetipola, K.M.; Chen, M. Arterial Diameter and the Gender Disparity in Stroke Thrombectomy Outcomes. J. NeuroInterventional Surg. 2018, 10, 949–952. [Google Scholar] [CrossRef]
- Pigoń, K.; Tomecka, N.; Korner, D.; Pękała, M.; Grzegorczyn, S.; Konka, A.; Nowalany-Kozielska, E.; Tomasik, A. In Vitro Comparison of Several Thrombus Removal Tools. JCDD 2023, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Morr, S.; Natarajan, S.K.; Lin, N.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Thrombus Density Predicts Successful Recanalization with Solitaire Stent Retriever Thrombectomy in Acute Ischemic Stroke. J. NeuroInterventional Surg. 2014, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Machi, P.; Jourdan, F.; Ambard, D.; Reynaud, C.; Lobotesis, K.; Sanchez, M.; Bonafé, A.; Costalat, V. Experimental Evaluation of Stent Retrievers’ Mechanical Properties and Effectiveness. J. NeuroIntervent. Surg. 2017, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kaesmacher, J.; Boeckh-Behrens, T.; Simon, S.; Maegerlein, C.; Kleine, J.F.; Zimmer, C.; Schirmer, L.; Poppert, H.; Huber, T. Risk of Thrombus Fragmentation during Endovascular Stroke Treatment. AJNR Am. J. Neuroradiol. 2017, 38, 991–998. [Google Scholar] [CrossRef]
- Fereidoonnezhad, B.; Dwivedi, A.; Johnson, S.; McCarthy, R.; McGarry, P. Blood Clot Fracture Properties Are Dependent on Red Blood Cell and Fibrin Content. Acta Biomater. 2021, 127, 213–228. [Google Scholar] [CrossRef]
- Jolugbo, P.; Ariëns, R.A.S. Thrombus Composition and Efficacy of Thrombolysis and Thrombectomy in Acute Ischemic Stroke. Stroke 2021, 52, 1131–1142. [Google Scholar] [CrossRef]
- Mereuta, O.M.; Rossi, R.; Douglas, A.; Gil, S.M.; Fitzgerald, S.; Pandit, A.; McCarthy, R.; Gilvarry, M.; Ceder, E.; Dunker, D.; et al. Characterization of the ‘White’ Appearing Clots That Cause Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106127. [Google Scholar] [CrossRef]
- Gralla, J.; Schroth, G.; Remonda, L.; Fleischmann, A.; Fandino, J.; Slotboom, J.; Brekenfeld, C. A Dedicated Animal Model for Mechanical Thrombectomy in Acute Stroke. Am. J. Neuroradiol. 2006, 27, 1357–1361. [Google Scholar]
- Chueh, J.Y.; Wakhloo, A.K.; Hendricks, G.H.; Silva, C.F.; Weaver, J.P.; Gounis, M.J. Mechanical Characterization of Thromboemboli in Acute Ischemic Stroke and Laboratory Embolus Analogs. Am. J. Neuroradiol. 2011, 32, 1237–1244. [Google Scholar] [CrossRef]
- Luo, Z.H.; Chung, A.; Choi, G.; Lin, Y.H.; Uchida, B.T.; Pavcnik, D.; Loriaux, M.M.; Nesbit, G.M.; Keller, F.S.; Rösch, J. Creation of Fibrinogen-Enhanced Experimental Blood Clots to Evaluate Mechanical Thrombectomy Devices for Treatment of Acute Stroke: An In Vitro Study. J. Vasc. Interv. Radiol. 2012, 23, 1077–1083. [Google Scholar] [CrossRef]
- Fitzgerald, S.T.; Liu, Y.; Dai, D.; Mereuta, O.M.; Abbasi, M.; Larco, J.L.A.; Douglas, A.S.; Kallmes, D.F.; Savastano, L.; Doyle, K.M.; et al. Novel Human Acute Ischemic Stroke Blood Clot Analogs for In Vitro Thrombectomy Testing. Am. J. Neuroradiol. 2021, 42, 1250–1257. [Google Scholar] [CrossRef]
- Karpiouk, A.B.; Aglyamov, S.R.; Mallidi, S.; Shah, J.; Scott, W.G.; Rubin, J.M.; Emelianov, S.Y. Combined Ultrasound and Photoacoustic Imaging to Detect and Stage Deep Vein Thrombosis: Phantom and Ex Vivo Studies. J. Biomed. Opt. 2008, 13, 054061. [Google Scholar] [CrossRef]
- Tajikawa, T.; Hirono, M.; Tanaka, M.; Yano, R.; Nagira, K. Model Blood for Simulating Red Thrombus Formation Owing to Stagnant Blood Flow Using Hypercoagulable Skim Milk Solution. J. Biomech. Sci. Eng. 2024, 19, 23–00350. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Reddy, A.S.; Gebrezgiabhier, D.; Davis, E.; Cockrum, J.; Gemmete, J.J.; Chaudhary, N.; Griauzde, J.M.; Pandey, A.S.; et al. Analysis of Human Emboli and Thrombectomy Forces in Large-Vessel Occlusion Stroke. J. Neurosurg. 2020, 134, 893–901. [Google Scholar] [CrossRef]
- Kaneko, N.; Komuro, Y.; Yokota, H.; Tateshima, S. Stent Retrievers with Segmented Design Improve the Efficacy of Thrombectomy in Tortuous Vessels. J NeuroIntervent Surg 2019, 11, 119–122. [Google Scholar] [CrossRef]
- Dick, A.; Neuerburg, J.; Schmitz-Rode, T.; Alliger, H.; Günther, R.W. Declotting of Embolized Temporary Vena Cava Filter by Ultrasound and the Angiojet: Comparative Experimental In Vitro Studies. Investig. Radiol. 1998, 33, 91. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.F.; Andersson, T.; Baxter, B.; Bendszus, M.; Brouwer, P.; Brinjikji, W.; Campbell, B.C.; Costalat, V.; Dávalos, A.; Demchuk, A.; et al. Analyses of Thrombi in Acute Ischemic Stroke: A Consensus Statement on Current Knowledge and Future Directions. Int. J. Stroke 2017, 12, 606–614. [Google Scholar] [CrossRef]
| Electroplating Conditions | |
|---|---|
| Plating bath | 1 M, CuSO4 |
| Current density | 4.5 mA/cm2 |
| Plating rate | 70 nm/min |
| Sputtering Conditions | |
|---|---|
| Pressure | 0.27 Pa |
| Power | 2.5 kW |
| Ar flow rate | 40 sccm |
| Deposition time | 300 s |
| Interval time | 30 min |
| Sputtering rate | 4 nm/s |
| Stent Retriever Type | No Pattern | Striped | Circular | |||
|---|---|---|---|---|---|---|
| Thrombus size | small | large | small | large | small | large |
| (n = 4) | (n = 4) | (n = 5) | (n = 3) | (n = 3) | (n = 5) | |
| The average of removed thrombus mass [%] X ± SD | 15.43 ± 8.43 | 12.19 ± 12.00 | 19.46 ± 12.02 | 41.26 ± 16.09 | 22.88 ± 12.46 | 45.32 ± 16.89 |
| Significance (Mann-Whiteney) |  (p < 0.05) | |||||
 (p < 0.05) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, S.; Ban, Y.; Ota, T.; Miki, N. Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface. Micromachines 2024, 15, 213. https://doi.org/10.3390/mi15020213
Kato S, Ban Y, Ota T, Miki N. Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface. Micromachines. 2024; 15(2):213. https://doi.org/10.3390/mi15020213
Chicago/Turabian StyleKato, Shogo, Yuzuki Ban, Takashi Ota, and Norihisa Miki. 2024. "Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface" Micromachines 15, no. 2: 213. https://doi.org/10.3390/mi15020213
APA StyleKato, S., Ban, Y., Ota, T., & Miki, N. (2024). Microfabricated Nitinol Stent Retrievers with a Micro-Patterned Surface. Micromachines, 15(2), 213. https://doi.org/10.3390/mi15020213







