Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly
Abstract
:1. Introduction
- (a)
- Creating several simple microfluidic chambers/chips, within each, a cell monoculture is hosted, which is connected either through microchannels or tubing [5,6,7,8,9,10] (Figure 1a). This design enables an easy and simple connection of many organ modules using external channels/tubing with the possibility of selectively switching the flow into the individual chip (ON/OFF) or directing the flow using external fluidic valves. In addition, modules can be connected/disconnected at the desired time (e.g., after cell differentiation). However, the organization of cell types in this design does not mimic that in vivo, where the cell–cell interfacing distance (i.e., the channel/tubing) between the various types of cells is relatively large. This design is suitable to link cells/tissues, which are separated a distance between each other such as heart–liver, intestine–liver, etc., but do not provide a physiologically relevant cell–cell interfacing when used to model the heterogeneous cellular structure of an individual organ, such as the liver which contains several cell types (hepatocytes, hepatic stellate cells, sinusoidal endothelial cells, and Kupffer cells), or interfacing the parenchyma and nonparenchymal cells or immune cells where cells are very close to each other. Zhang et al., 2017 [11] reported an integrated modular sensing platform through a fluidics-routing breadboard, which operates multiorgan-on-a-chip units. The system comprises built-in pneumatic valves, micro bioreactors for housing organoids, and biochemical, physical, and optical sensors. The individual modules are selectively connected/disconnected with Teflon tubes. The system was tested for long-term monitoring of drug-induced organ toxicity in hepatic and cardiac organoids. Few commercial platforms became available. For example, TissUse GmbH (Berlin, Germany) introduced a variety of HUMIMIC Chips, such as HUMIMIC Chip2, HUMIMIC Chip3, HUMIMIC Chip4, and HUMIMIC Chip XX/XY, for different in vitro modeling purposes, which enable the integration of 2, 3, and 4 organs [12].
- (b)
- Integration of multiorgans on a single chip. In this design, the chip comprises multiple fluidic chambers, with each compartment hosting a specific organ, tissue, or homogeneous single cell type culture (Figure 1b). Each chamber may host one or two cell types within single or multiple compartments (e.g., two stacked compartments separated by a porous membrane) that emulate a specific organ. The fluidic chambers are linked either by physical or biological barriers that ensure continuous organ-organ crosstalk [13,14,15,16,17,18,19,20]. This design facilitates efficient cell–cell and tissue-tissue interaction between heterogeneous cell cultures, and allows spatial organization of different types of cells/tissue in 2D and 3D architectures with physiologically relevant orientation. In addition, the fluidic architecture allows the recruitment of circulating immune cells with a high spatiotemporal resolution of cell–cell interaction [3,13,15]. However, the limited diffusion through the physical/biological barriers limits the number of fluidic chambers, and hence, the number of cell types within the system. Therefore, the biological complexity of the system would require a more sophisticated design of the fluidic chip to enable efficient fluidic exchange or diffusion through the multichamber system.
- (c)
- Simple OOC chips within each 2–3 fluidic compartments can be integrated by externally linking them through tubing and valves (Figure 1c). This design allows for integrating relatively complex individual organ models (e.g., liver) within individual chips and integrating several organ modules in one perfusion system (e.g., intestine, liver, skin, heart, and kidney).
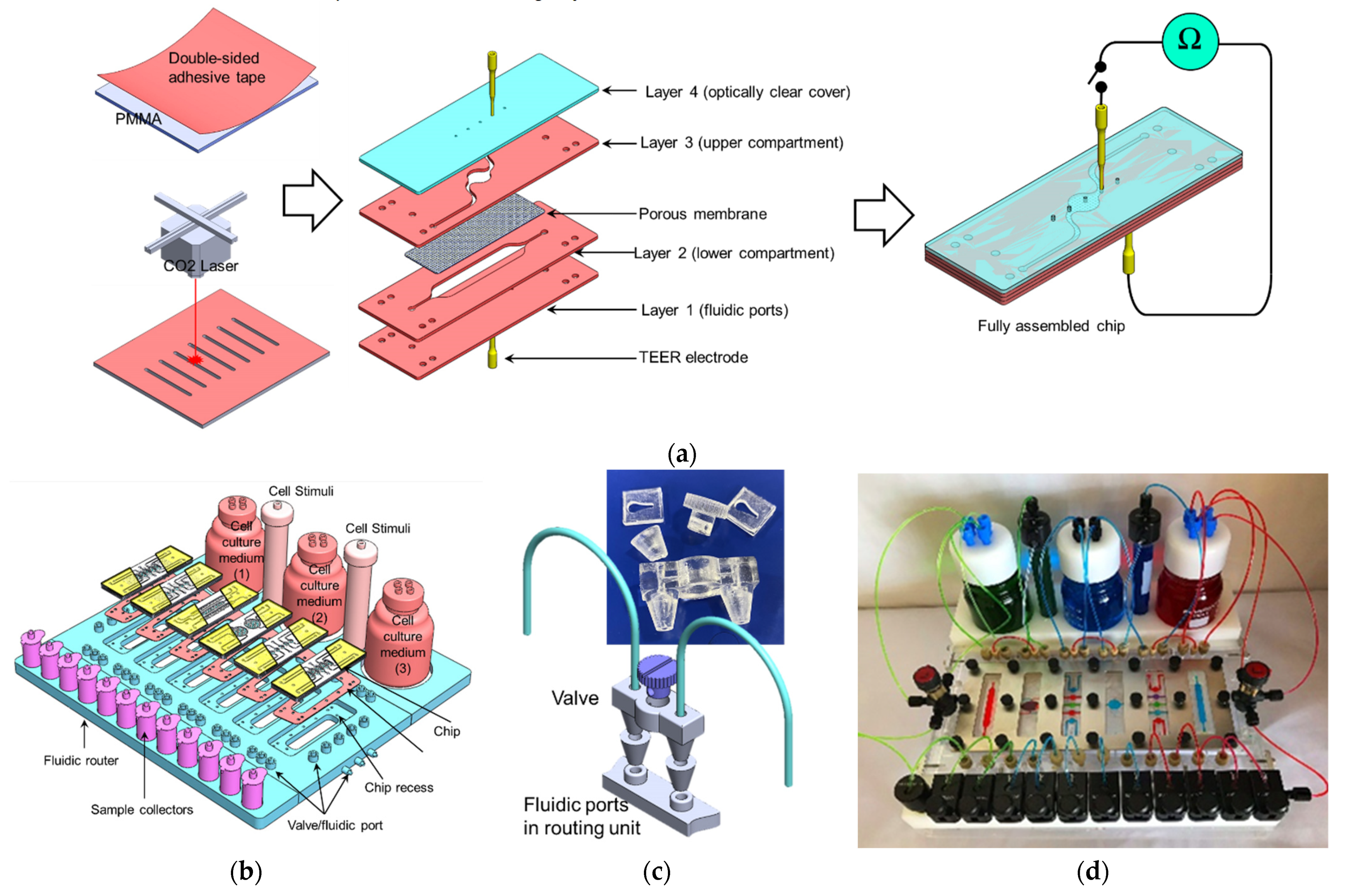
2. Fabrication
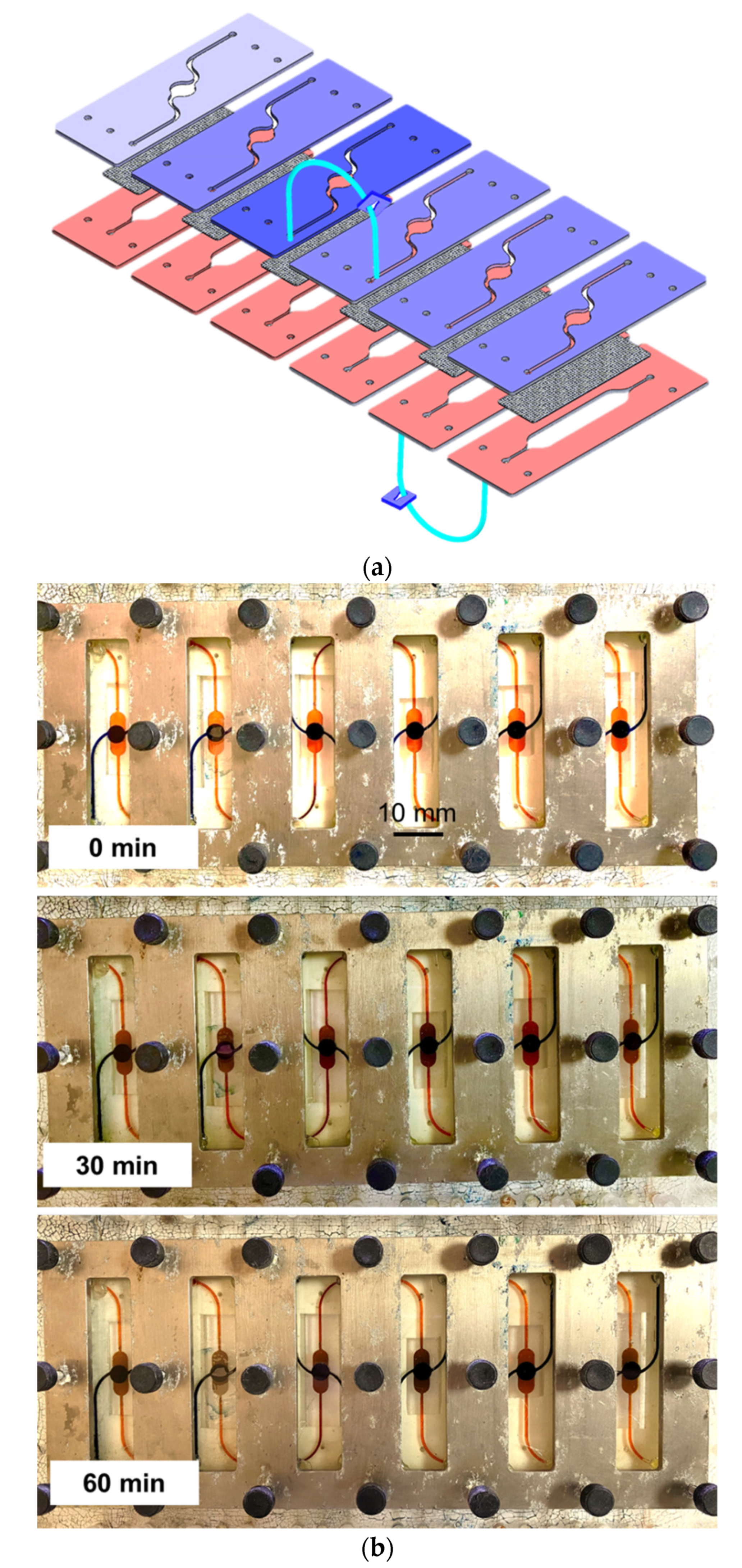
2.1. Fabrication of the 3D Microfluidic Chip
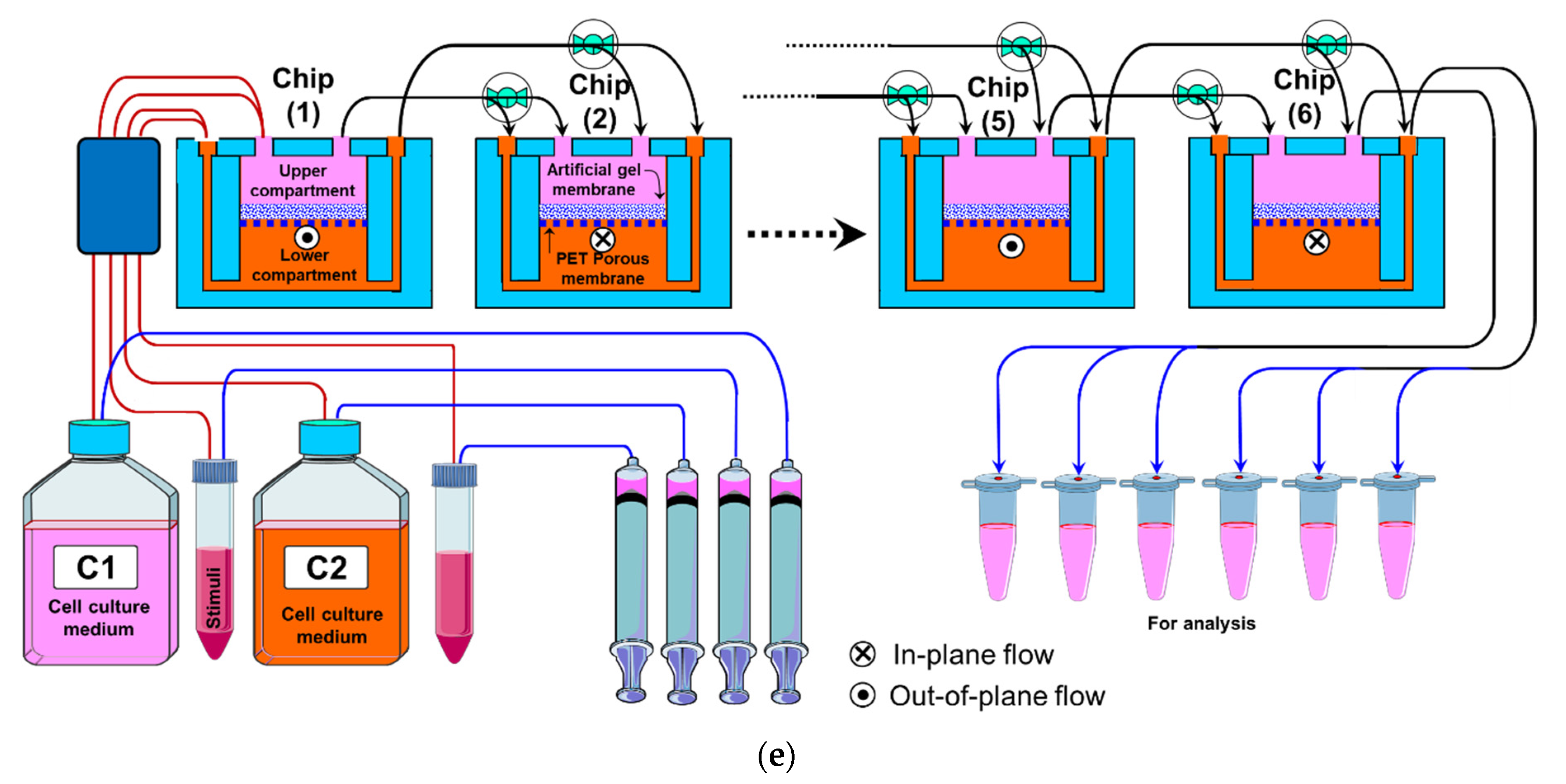
2.2. Fabrication of the Fluidic Routing Unit
- (1)
- Six recesses with dimensions that enable accommodating the chip and channels network (with an individual channel diameter of 500 µm). Within each recess, a set of L-shaped channels were made to connect the chip ports to the external tubing and the injection syringes.
- (2)
- A set of 3D-printed fluidic valves: The valve was designed using SolidWorks software (Dassault Systèmes, Vélizy-Villacoublay, France) and printed using an ASIGA MAX UV 3D printer (NSW, Australia). The valve comprises a rotating cylindrical rod with a 500 µm perpendicular through hole. When the hole is aligned with the fluidic channel, it allows the fluid to pass through (OPEN). Rotating the rod 90°, the fluidic channel and the valve’s hole will be perpendicular to each other, which would block the flow through the channel (CLOSE) (Figure 2c).
- (3)
- The fluidic router also allows for connecting the chips to cell culture media and stimuli reservoirs as shown in Figure 2b,d,e. Pinch valves were used to control the flow from the reservoirs.
3. Materials and Methods
3.1. The Perfusion Setup
3.2. TEER Measurements
3.3. Trans-Membrane Permeability
3.4. Cell Culture
3.4.1. Immune Cells
3.4.2. Epithelial Cells
3.4.3. Cell Viability
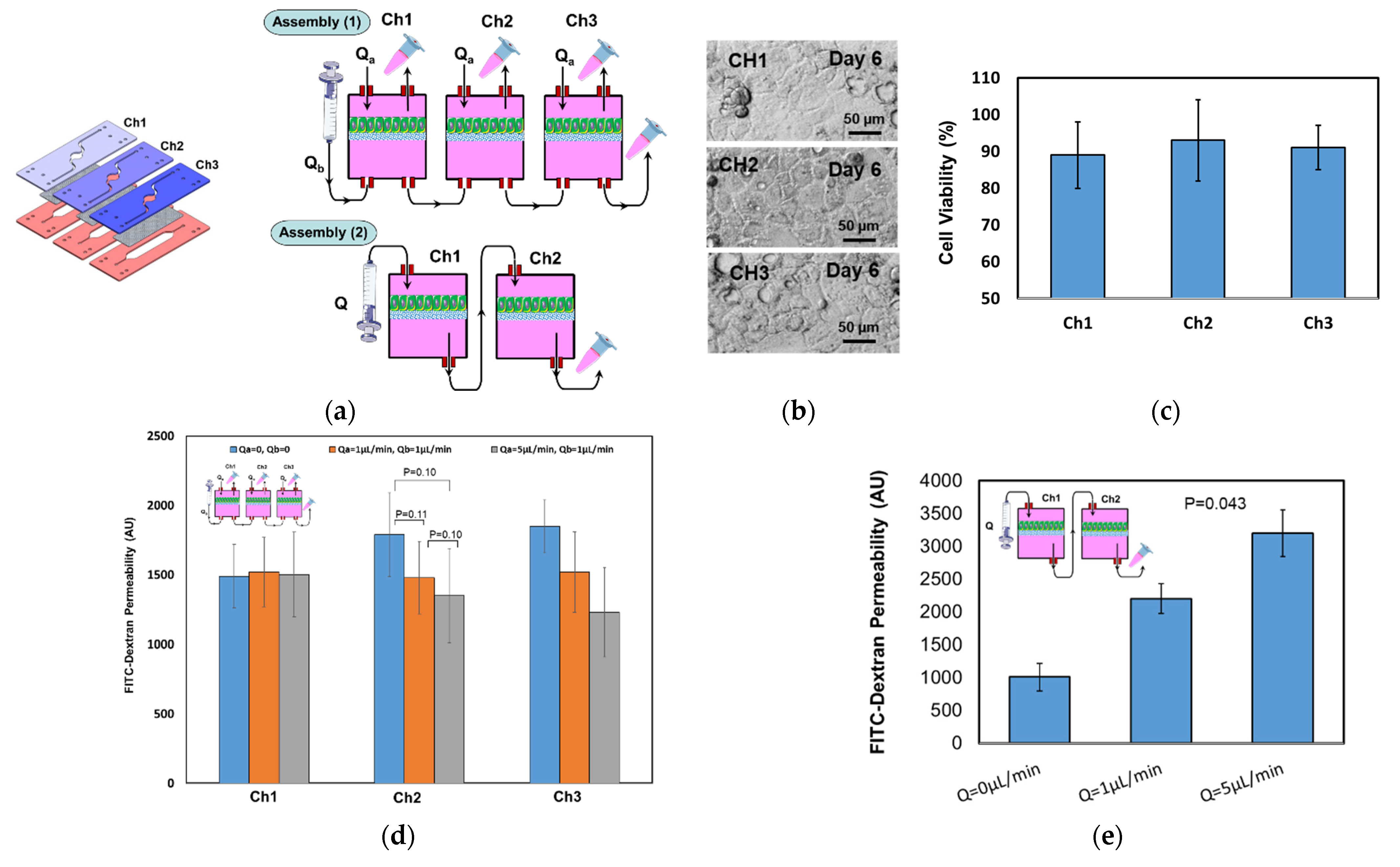
4. Results and Discussion
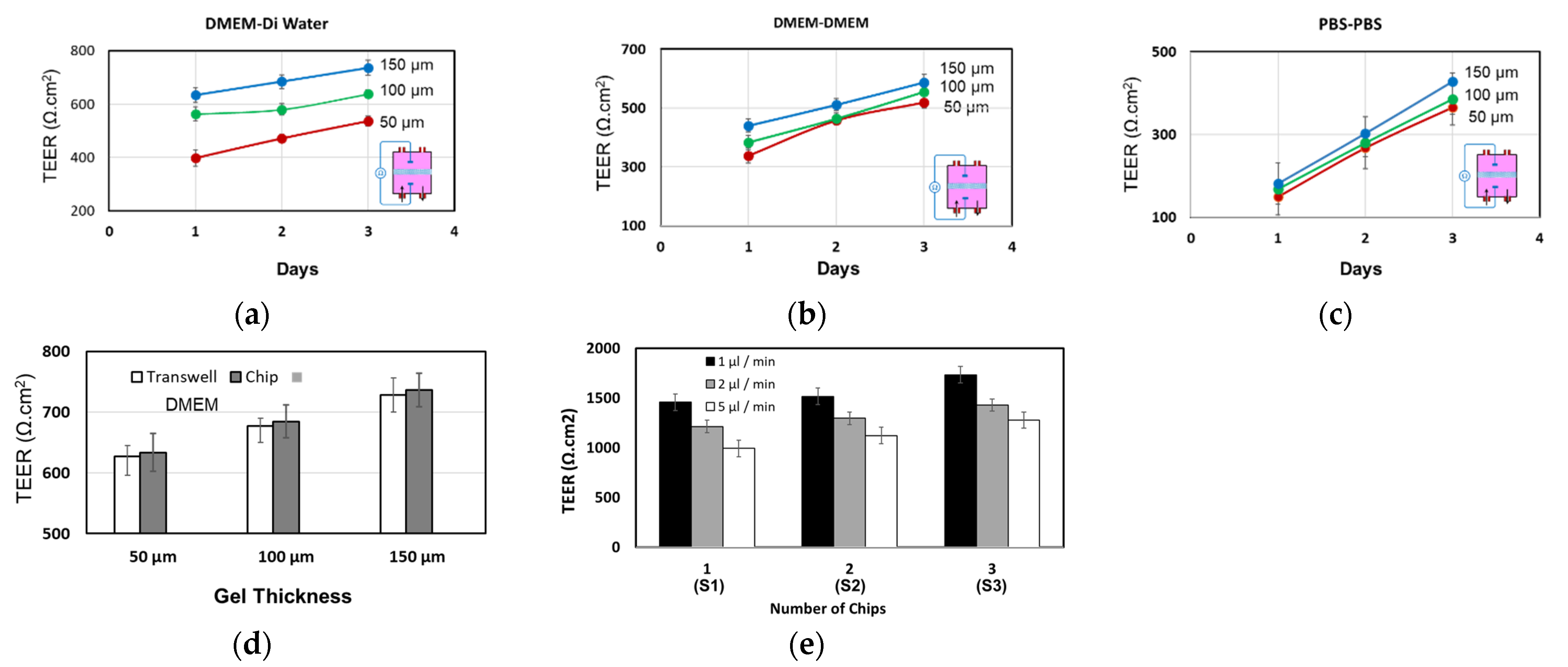
4.1. Fluidic Characterization
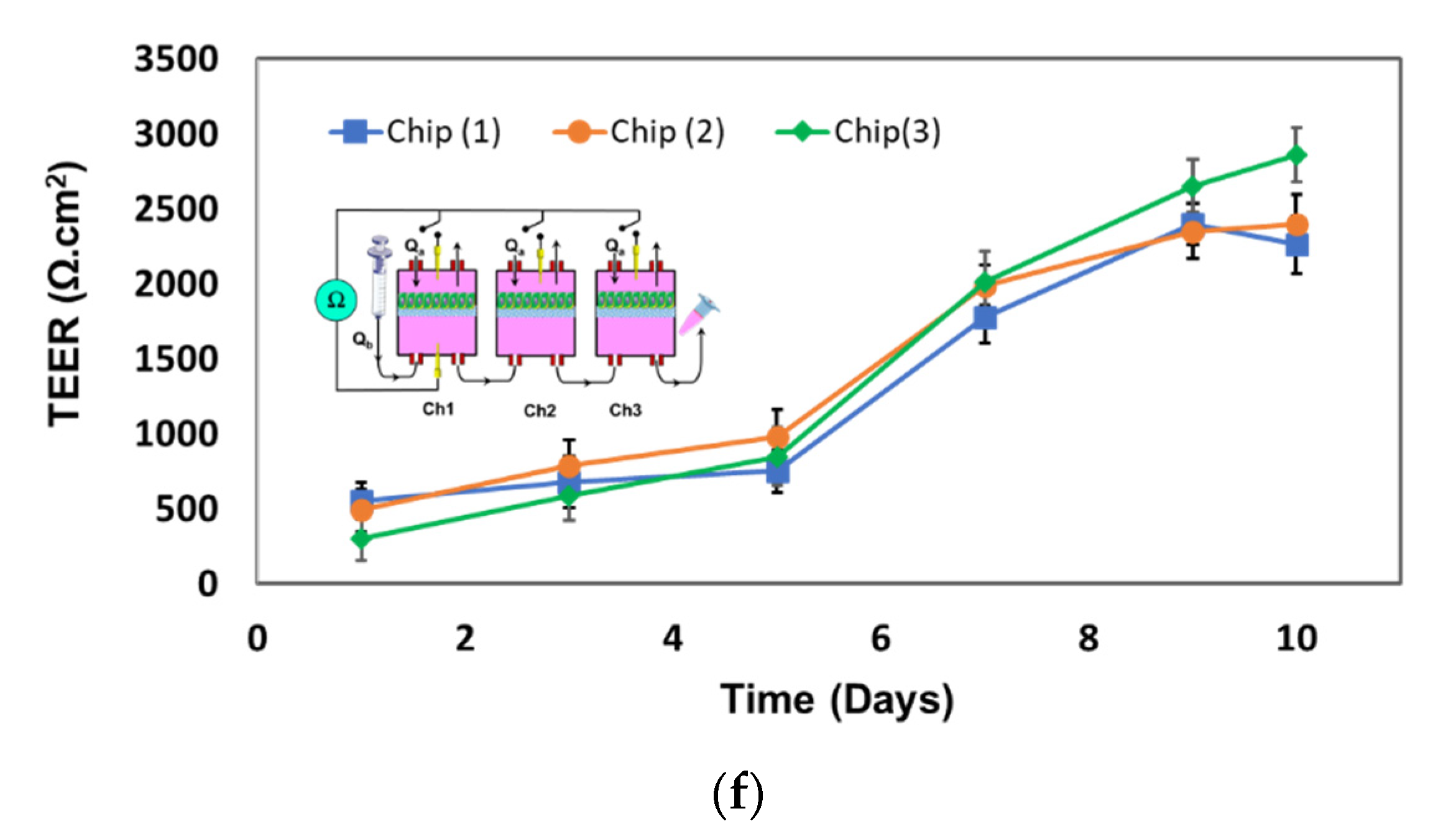
4.2. Tranepithelial Electrical Resistance (TEER) Characterization
4.3. Permeability Characterization
4.4. Epithelial Cell Culture Testing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, M.; Evans, D. Tissue engineering: The future of stem cells. Top. Tissue Eng. 2005, 2, 1–22. [Google Scholar]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef] [PubMed]
- Lelièvrea, S.A.; Kwok, T.; Chittiboyina, S. Architecture in 3D cell culture: An essential feature for in vitro toxicology. Toxicol. Vitr. 2017, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Kama, C.; Shuler, C.M.L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef]
- Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2018, 18, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688. [Google Scholar] [CrossRef] [Green Version]
- McAleer, C.W.; Long, C.J.; Elbrecht, D.; Sasserath, T.; Bridges, L.R.; Rumsey, J.W.; Martin, C.; Schnepper, M.; Wang, Y.; Schuler, F.; et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci. Transl. Med. 2019, 11, eaav1386. [Google Scholar] [CrossRef] [PubMed]
- Edington, C.D.; Chen, W.L.K.; Geishecker, E.; Kassis, T.; Soenksen, L.R.; Bhushan, B.M.; Freake, D.; Kirschner, J.; Maass, C.; Tsamandouras, N.; et al. Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci. Rep. 2018, 8, 4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A.A.; Andrade-Navarro, M.A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; et al. Liver-Kidney-on-Chip To Study Toxicity of Drug Metabolites. ACS Biomater. Sci. Eng. 2017, 4, 78–89. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chaea, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 14, E2293–E2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.tissuse.com/en/humimic/chips (accessed on 1 September 2021).
- Ramadan, Q.; Jafarpoorchekab, H.; Huang, C.; Silacci, P.; Carrara, S.; Koklü, G.; Ghaye, J.; Ramsden, J.; Ruffert, C.; Vergeres, G.; et al. NutriChip: Nutrition analysis meets microfluidics. Lab Chip 2012, 13, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Ramadan, Q.; Wacker, J.B.; Tekin, H.C.; Ruffert, C.; Vergères, G.; Silacci, P.; Gijs, M.A.M. Microfluidic chip for monitoring Ca2+transport through a confluent layer of intestinal cells. RSC Adv. 2014, 4, 52887–52891. [Google Scholar] [CrossRef]
- Ramadan, Q.; Jing, L. Characterization of tight junction disruption and immune response modulation in a miniaturized Caco-2/U937 coculture-based in vitro model of the human intestinal barrier. Biomed. Microdevices 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Sajay, B.N.G.; Yin, C.S.; Ramadan, Q. Optimization of micro-fabricated porous membranes for intestinal epithelial cell culture andin vitromodeling of the human intestinal barrier. J. Micromech. Microeng. 2017, 27, 124004. [Google Scholar] [CrossRef]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospichd, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Gröger, M.; et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef]
- Shin, W.J.; Hinojosa, C.D.; Ingber, D.E.; Kim, H.J. Human Intestinal Morphogenesis Controlled by Transepithelial Morphogen Gradient and Flow-Dependent Physical Cues in a Microengineered Gut-on-a-Chip. IScience 2019, 15, 391–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.C.; Shin, W.; Koh, D.; Wu, A.; Ambrosini, Y.M.; Min, S.; Eckhardt, S.G.; Fleming, R.Y.D.; Kim, S.; Park, S.; et al. Three-Dimensional Regeneration of Patient-Derived Intestinal Organoid Epithelium in a Physiodynamic Mucosal Interface-on-a-Chip. Micromachines 2020, 11, 663. [Google Scholar] [CrossRef]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2017, 21, 326–340. [Google Scholar] [CrossRef]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Nguyen, K.; Kerns, E.; Yan, Z.; Yu, K.R.; Shah, P.; Jadhav, A.; Xu, X. Highly predictive and interpretable models for PAMPA permeability. Bioorganic Med. Chem. 2017, 25, 1266–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Dawas, S.; Alawami, H.; Zourob, M.; Ramadan, Q. Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly. Micromachines 2021, 12, 1542. https://doi.org/10.3390/mi12121542
Abu-Dawas S, Alawami H, Zourob M, Ramadan Q. Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly. Micromachines. 2021; 12(12):1542. https://doi.org/10.3390/mi12121542
Chicago/Turabian StyleAbu-Dawas, Sadeq, Hawra Alawami, Mohammed Zourob, and Qasem Ramadan. 2021. "Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly" Micromachines 12, no. 12: 1542. https://doi.org/10.3390/mi12121542
APA StyleAbu-Dawas, S., Alawami, H., Zourob, M., & Ramadan, Q. (2021). Design and Fabrication of Low-Cost Microfluidic Chips and Microfluidic Routing System for Reconfigurable Multi-(Organ-on-a-Chip) Assembly. Micromachines, 12(12), 1542. https://doi.org/10.3390/mi12121542









