Design and Fabrication of Micro Saw Enabling Root-Side Cutting of Bone
Abstract
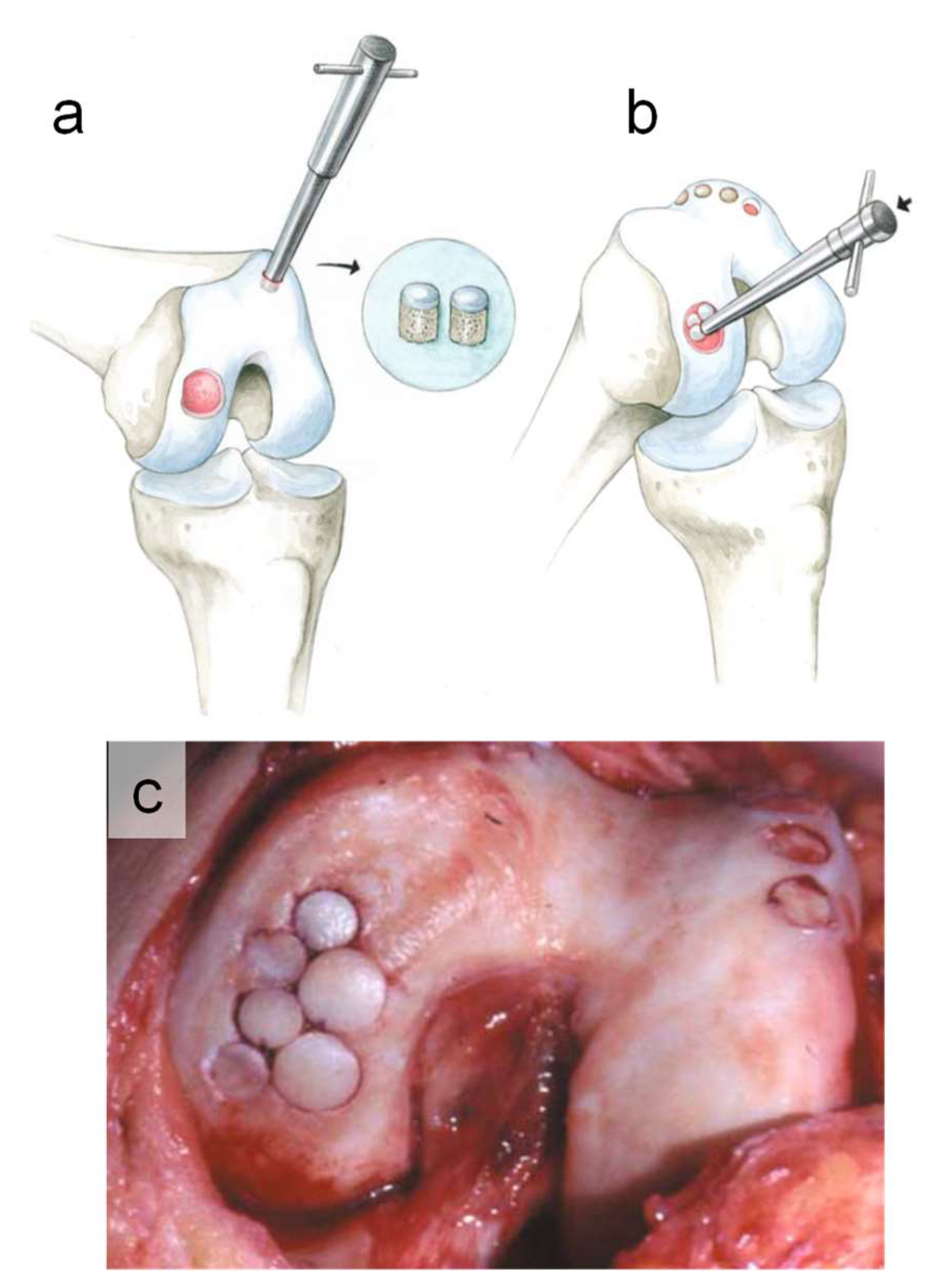
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Design of Micro Saw
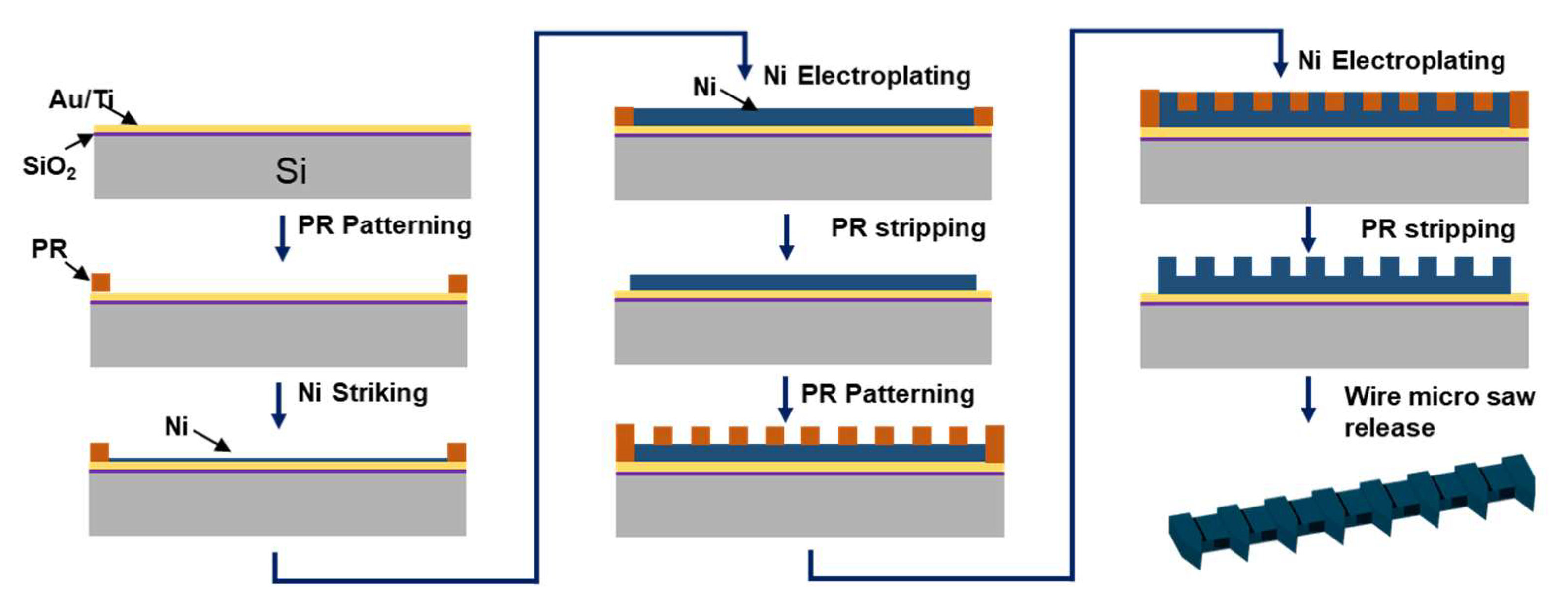
2.3. Fabrication of Micro Saw
2.4. Materials Characterization
3. Results and Discussion
3.1. Morphology and Chemical Analysis
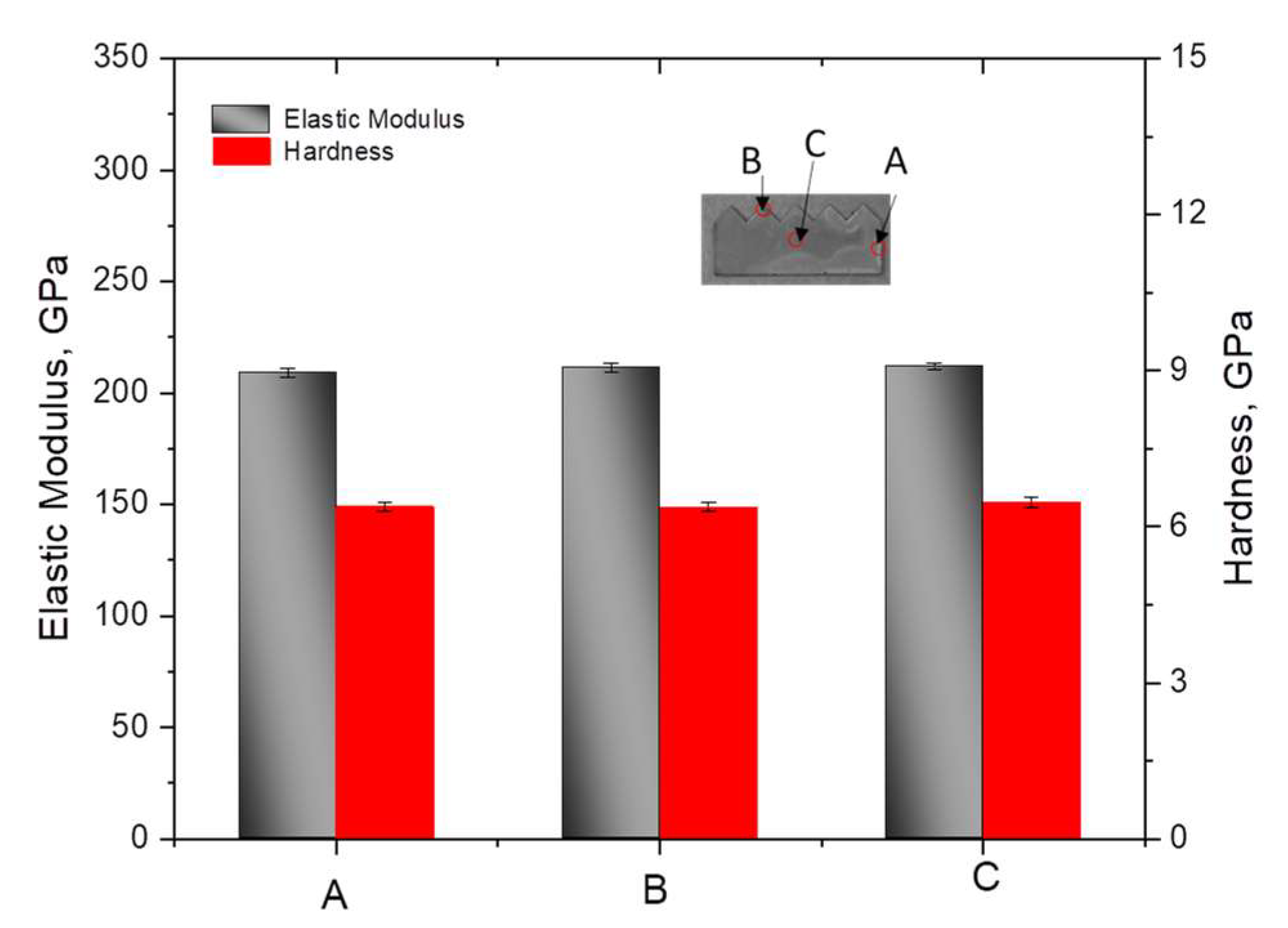
3.2. Mechanical Analysis
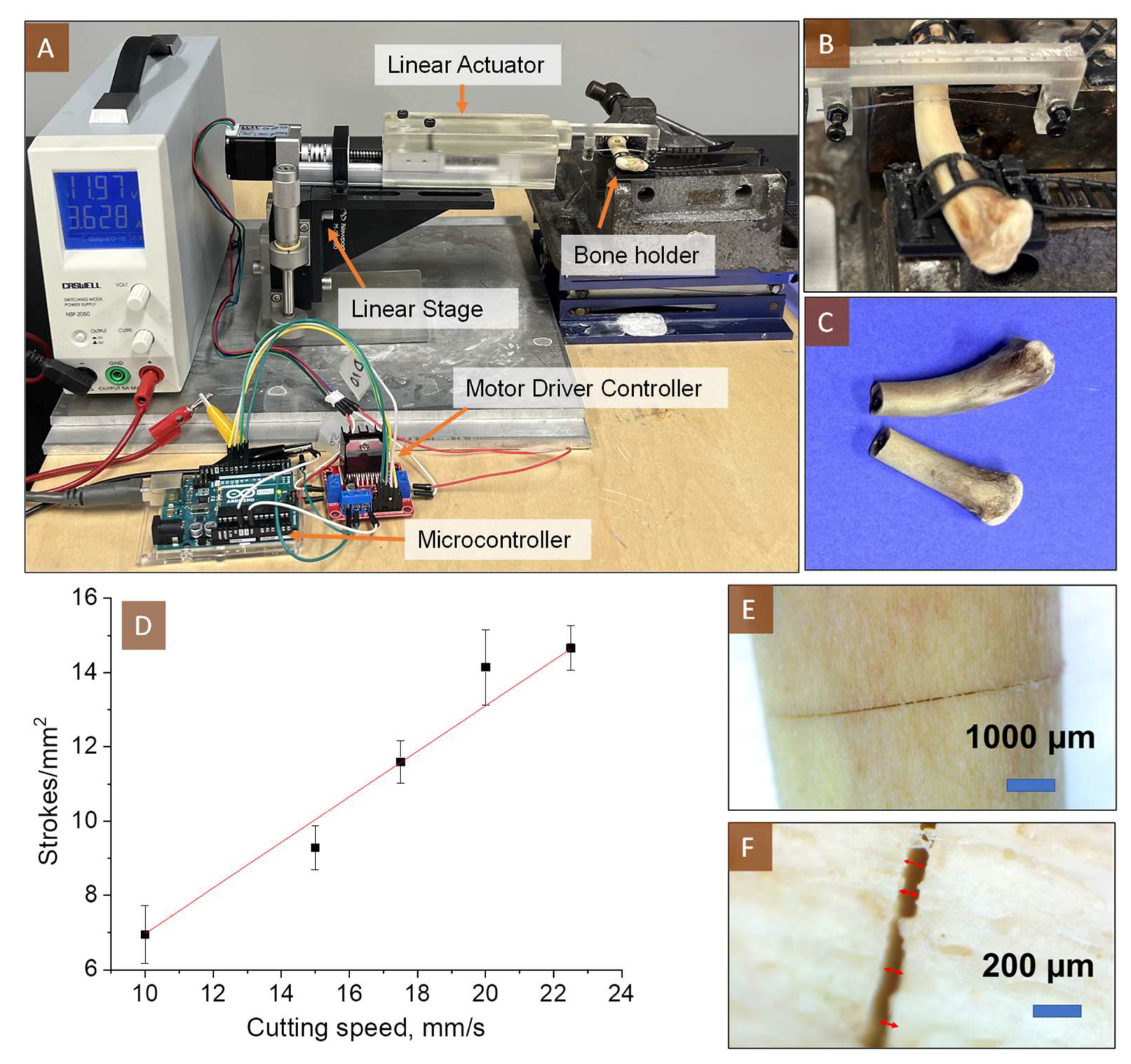
3.3. Bone Cutting Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205. [Google Scholar]
- Hulth, A. Experimental osteoarthritis: A survey. Acta Orthop. Scand. 1982, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.M.; Lee, S.; Levy, D.; Poland, S.; Smith, M.; Scalise, N.; Cvetanovich, G.L.; Cole, B.J. Osteochondral allograft transplantation of the knee: Analysis of failures at 5 years. Am. J. Sport. Med. 2017, 45, 864–874. [Google Scholar] [CrossRef]
- Camp, C.L.; Stuart, M.J.; Krych, A.J. Current concepts of articular cartilage restoration techniques in the knee. Sport. Health 2014, 6, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Tanksley, J.A.; Miller, M.D. Osteochondral autograft transplantation: A review of the surgical technique and outcomes. Sport. Med. Arthrosc. Rev. 2016, 24, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Gross, A.E. Immunogenicity of allograft articular cartilage. JBJS 1974, 56, 297–304. [Google Scholar] [CrossRef]
- Hangody, L.; Vásárhelyi, G.; Hangody, L.R.; Sükösd, Z.; Tibay, G.; Bartha, L.; Bodó, G. Autologous osteochondral grafting—Technique and long-term results. Injury 2008, 39, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.J.; Mosich, G.M.; Williams, R.J. Fresh precut osteochondral allograft core transplantation for the treatment of femoral cartilage defects. Arthrosc. Tech. 2018, 7, e791–e795. [Google Scholar] [CrossRef]
- Kang, D.-G.; Lee, D.H.; Im, J.-H. Osteochondritis dissecans of the metacarpal head in a soldier treated with osteochondral autograft transplantation surgery: A case report. Medicine 2023, 102, e32563. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, C.M.; Nuelle, C.W.; Ray, T.; Sherman, S.L. Osteochondral Autograft for Treatment of Small Cartilage Injuries. In Cartilage Injury of the Knee: State-of-the-Art Treatment and Controversies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2021; pp. 253–260. [Google Scholar]
- Biswas, P.; Sikander, S.; Kulkarni, P.; Song, S.-E. A Robotic Nondestructive Osteochondral Tissue Harvesting for Autograft Transplantation. EPiC Ser. Health Sci. 2019, 3, 36–39. [Google Scholar]
- Song, S.-E.; Biswas, P. Nondestructive Autograft Extracting Device for Autologous Osteochondral Transplantation. U.S. Patent No. 11,432,825, 6 September 2022. [Google Scholar]
- Hangody, L.; Berta, Á. Surgical techniques in cartilage repair surgery: Osteochondral autograft transfer (OATS, Mosaicplasty). In Techniques in Cartilage Repair Surgery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; pp. 131–140. [Google Scholar]
- Erggelet, C.; Mandelbaum, B.R. Principles of cartilage repair; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Munavalli, J.R.; Sankpal, P.R.; Sumathi, A.; Oli, J.M. Introduction to Brain–Computer Interface: Applications and Challenges. In Brain-Computer Interface: Using Deep Learning Applications; Wiley Publishing: Hoboken, NJ, USA, 2023; pp. 1–24. [Google Scholar]
- Lal, A.; White, R.M. Micromachined silicon needle for ultrasonic surgery. In Proceedings of the 1995 IEEE Ultrasonics Symposium. Proceedings. An International Symposium, Seattle, WA, USA, 7–10 November 1995; pp. 1593–1596. [Google Scholar]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Notarp, D.L.; Vogel, J.; Kieselstein, E.; Sommer, J.-P.; Brämer, K.; Großer, V.; Benecke, W.; Michel, B. Application of electroplating in MEMS-micromachining exemplified by a microrelay. Microsyst. Technol. 2001, 7, 196–202. [Google Scholar]
- Romankiw, L. A path: From electroplating through lithographic masks in electronics to LIGA in MEMS. Electrochim. Acta 1997, 42, 2985–3005. [Google Scholar] [CrossRef]
- Lambert, S. The influence of temperature on the efficiency of electroplating from various ionic liquids. Circuit World 2006, 32, 36–41. [Google Scholar] [CrossRef]
- Luo, J.; Flewitt, A.; Spearing, S.; Fleck, N.; Milne, W. Young’s modulus of electroplated Ni thin film for MEMS applications. Mater. Lett. 2004, 58, 2306–2309. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yoshiba, M.; Nagoshi, T.; Chang, T.-F.M.; Yamane, D.; Machida, K.; Masu, K.; Sone, M. Pulse electroplating of ultra-fine grained Au films with high compressive strength. Electrochem. Commun. 2016, 67, 51–54. [Google Scholar] [CrossRef]
- Yi, K.; Ma, R.; Xiang, S.; Liu, X.; Liu, C.; Zhang, X.; Fu, Y. Eliminating reversible hydrogen embrittlement in high-strength martensitic steel by an electric current pulse. Int. J. Hydrog. Energy 2022, 47, 17045–17055. [Google Scholar] [CrossRef]
- El-Sherik, A.; Erb, U.; Page, J. Microstructural evolution in pulse plated nickel electrodeposits. Surf. Coat. Technol. 1997, 88, 70–78. [Google Scholar] [CrossRef]
- Son, D.; Kim, J.J.; Kim, J.Y.; Kwon, D. Tensile properties and fatigue crack growth in LIGA nickel MEMS structures. Mater. Sci. Eng. A 2005, 406, 274–278. [Google Scholar] [CrossRef]
- Farrell, M.; Mathieson, A.; Chung, P.; Heller, J.; Clarke, S.P.; McDonald, M.K.; Cardoni, A. In vitro performance testing of two arcuate oscillating saw blades designed for use during tibial plateau leveling osteotomy. Vet. Surg. 2011, 40, 694–707. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Lamovec, J.; Jović, V.; Randjelović, D.; Aleksić, R.; Radojević, V. Analysis of the composite and film hardness of electrodeposited nickel coatings on different substrates. Thin Solid Film. 2008, 516, 8646–8654. [Google Scholar] [CrossRef]
- Gu, C.; You, Y.; Yu, Y.; Qu, S.; Tu, J. Microstructure, nanoindentation, and electrochemical properties of the nanocrystalline nickel film electrodeposited from choline chloride–ethylene glycol. Surf. Coat. Technol. 2011, 205, 4928–4933. [Google Scholar] [CrossRef]
- Zwierzak, I.; Baleani, M.; Viceconti, M. Microindentation on cortical human bone: Effects of tissue condition and indentation location on hardness values. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.K.; Guo, X.E.; Hoffler, C.E.; Moore, K.E.; Goldstein, S.A. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.T.; Burstein, A.H.; Frankel, V.H. The elastic modulus for bone. J. Biomech. 1974, 7, 271–275. [Google Scholar] [CrossRef]
- Antler, M. Sliding wear of metallic contacts. IEEE Trans. Compon. Hybrids Manuf. Technol. 1981, 4, 15–29. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, P.; Fasano, J.; Kim, Y.-C.; Song, S.-E.; Cho, H.J. Design and Fabrication of Micro Saw Enabling Root-Side Cutting of Bone. Micromachines 2023, 14, 856. https://doi.org/10.3390/mi14040856
Pathak P, Fasano J, Kim Y-C, Song S-E, Cho HJ. Design and Fabrication of Micro Saw Enabling Root-Side Cutting of Bone. Micromachines. 2023; 14(4):856. https://doi.org/10.3390/mi14040856
Chicago/Turabian StylePathak, Pawan, Jack Fasano, Young-Cheon Kim, Sang-Eun Song, and Hyoung Jin Cho. 2023. "Design and Fabrication of Micro Saw Enabling Root-Side Cutting of Bone" Micromachines 14, no. 4: 856. https://doi.org/10.3390/mi14040856
APA StylePathak, P., Fasano, J., Kim, Y.-C., Song, S.-E., & Cho, H. J. (2023). Design and Fabrication of Micro Saw Enabling Root-Side Cutting of Bone. Micromachines, 14(4), 856. https://doi.org/10.3390/mi14040856






