Miniaturized Sulfite-Based Gold Bath for Controlled Electroplating of Zone Plate Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Design and Preparation
2.2. Electron-Beam Lithography
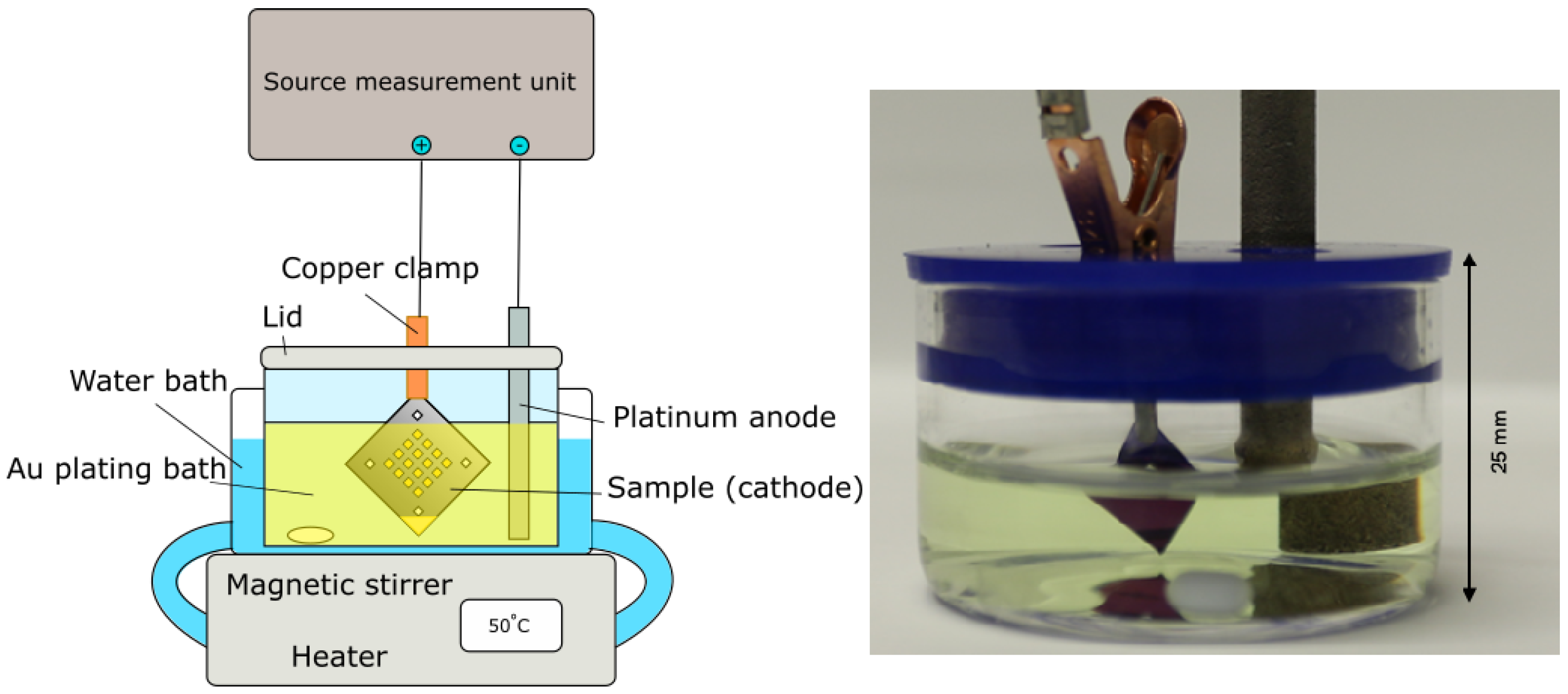
2.3. Electroplating
2.4. Calculating the Expected Plating Height
2.5. Analysis of Fabricated Gold Zone Plate Nanostructures
3. Results and Discussion
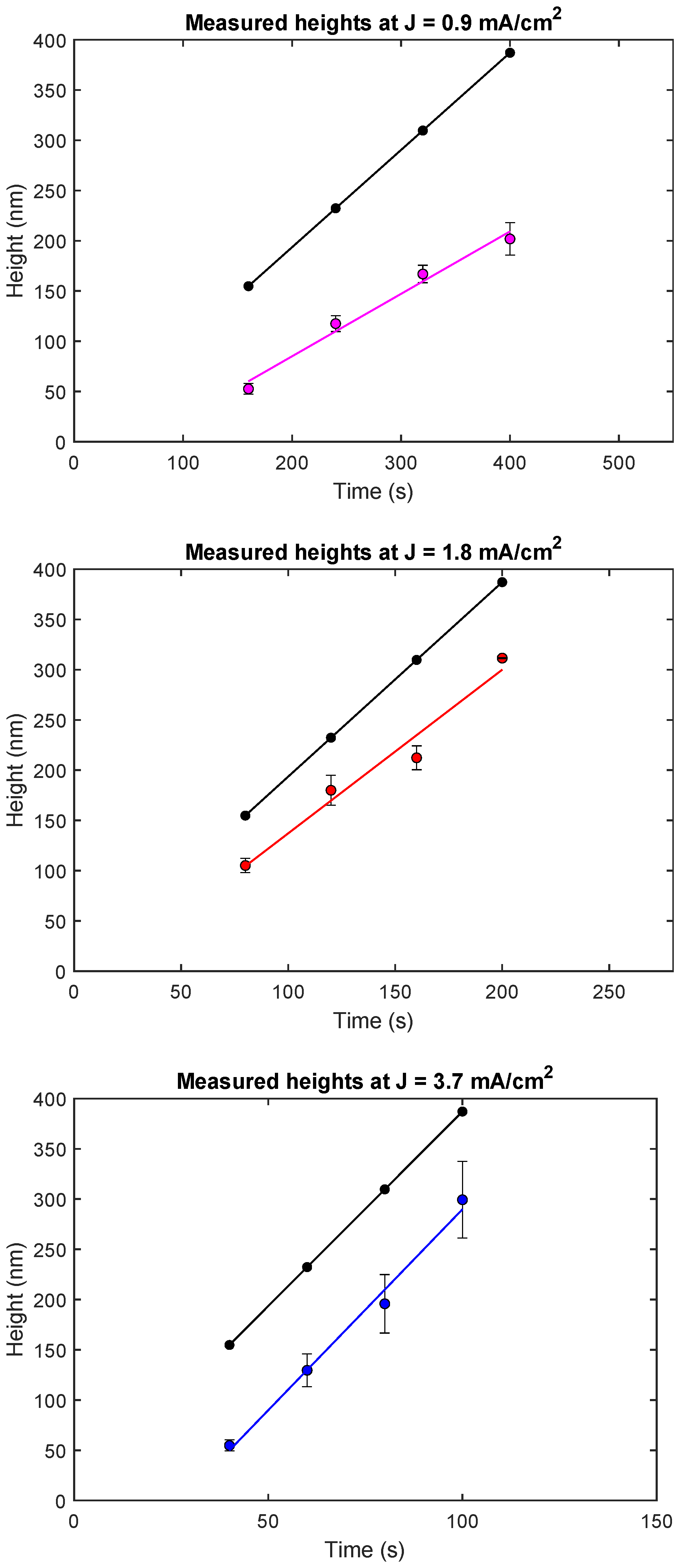
3.1. Plating Height as a Function of Current Density
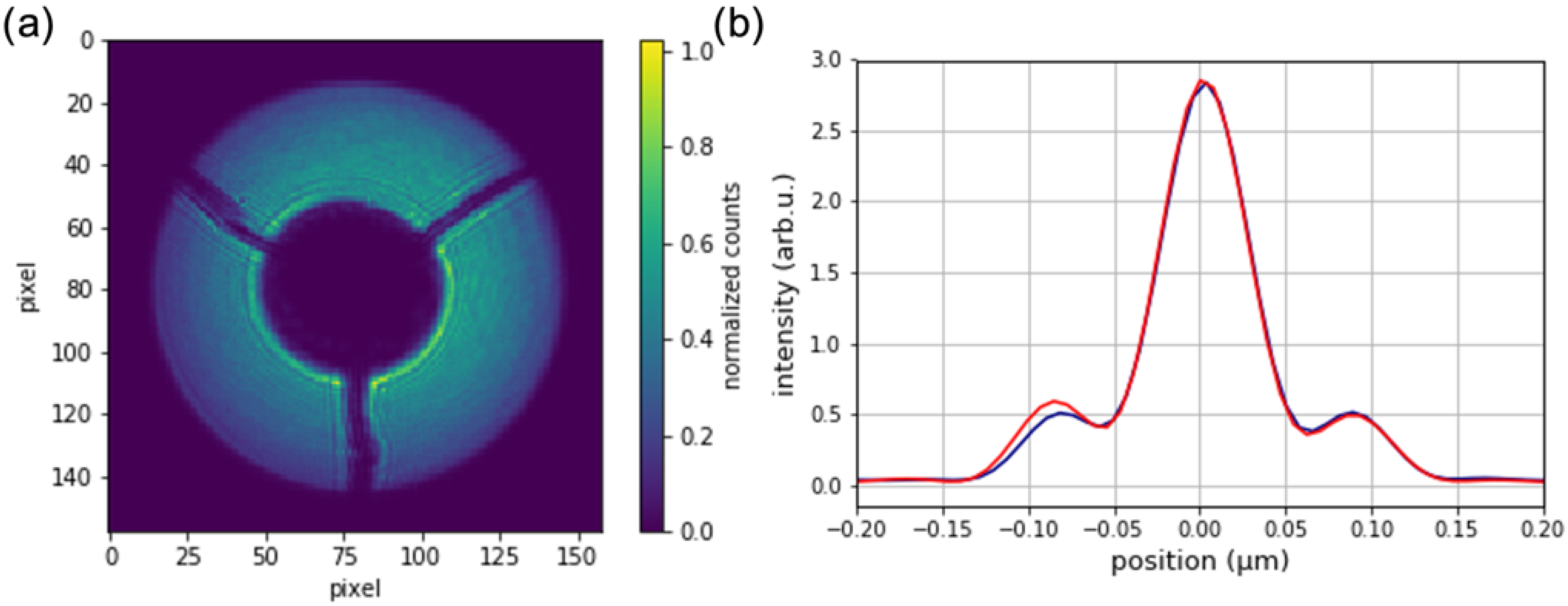
3.2. Imaging and Characterization of Fabricated Zone Plate Nanostructures
3.3. Plating Bath and Operation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Felix, E.; Muench, F.; Ensinger, W. Green plating of high aspect ratio gold nanotubes and their morphology-dependent performance in enzyme-free peroxide sensing. RSC Adv. 2014, 4, 24504. [Google Scholar] [CrossRef]
- Cavallotti, P.L.; Cojocaru, P.; Magagnin, L. Soft gold coatings: Influence of additives and pulse plating. Trans. Inst. Met. Finish. 2012, 90, 246–251. [Google Scholar] [CrossRef]
- Gamero, M.; Alonso, C. Deposition of nanostructurated gold on n-doped silicon substrate by different electrochemical methods. J. Appl. Electrochem. 2010, 40, 175–190. [Google Scholar] [CrossRef]
- Green, T.A.; Liew, M.J.; Roy, S. Electrodeposition of Gold from a Thiosulfate-Sulfite Bath for Microelectronic Applications. J. Electrochem. Soc. 2003, 150, C104. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Xie, S.; Zhang, L.; Wang, C.; Tai, R. Nanofabrication of 30 nm Au zone plates by e-beam lithography and pulse voltage electroplating for soft X-ray imaging. Microelectron. Eng. 2020, 225, 111254. [Google Scholar] [CrossRef]
- Gorelick, S.; Vila-Comamala, J.; Guzenko, V.A.; David, C. High aspect ratio nanostructuring by high energy electrons and electroplating. Microelectron. Eng. 2011, 88, 2259–2262. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yoshiba, M.; Nagoshi, T.; Chang, T.F.M.; Yamane, D.; Machida, K.; Masu, K.; Sone, M. Pulse electroplating of ultra-fine grained Au films with high compressive strength. Electrochem. Commun. 2016, 67, 51–54. [Google Scholar] [CrossRef]
- Hydes, P.C.; Middleton, H. The sulphito complexes of gold—Their chemistry and applications in gold electrodeposition. Gold Bull. 1979, 12, 90–95. [Google Scholar] [CrossRef]
- Liew, M.J.; Roy, S.; Scott, K. Development of a non-toxic electrolyte for soft gold electrodeposition: An overview of work at University of Newcastle upon Tyne. Green Chem. 2003, 5, 376–381. [Google Scholar] [CrossRef]
- Roy, S. Electrochemical Gold Deposition from Non-Toxic Electrolytes. ECS Trans. 2019, 16, 67–72. [Google Scholar] [CrossRef]
- Marathe, S.; Shi, X.; Wojcik, M.J.; Macrander, A.T.; Assoufid, L. Measurement of X-ray Beam Coherence along Multiple Directions Using 2-D Checkerboard Phase Grating. JoVE 2016, 116, e53025. [Google Scholar] [CrossRef] [PubMed]
- Znati, S.; Chedid, N.; Miao, H.; Chen, L.; Bennett, E.E.; Wen, H. Electrodeposition of gold to conformally fill high-aspect-ratio nanometric silicon grating trenches: A comparison of pulsed and direct current protocols. J. Surf. Eng. Mater. Adv. Technol. 2015, 5, 207–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johansson, U.; Carbone, D.; Kalbfleisch, S.; Björling, A.; Kahnt, M.; Sala, S.; Stankevic, T.; Liebi, M.; Rodriguez Fernandez, A.; Bring, B.; et al. NanoMAX: The hard X-ray nanoprobe beamline at the MAX IV Laboratory. J. Synchrotron Radiat. 2021, 28, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Björling, A.; Kalbfleisch, S.; Kahnt, M.; Sala, S.; Parfeniukas, K.; Vogt, U.; Carbone, D.; Johansson, U. Ptychographic characterization of a coherent nanofocused X-ray beam. Opt. Express 2020, 28, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Löchel, B.; Macioßek, A.; Trube, J.; Huber, H.L. Pulse plating of quarter micron gold patterns on silicon X-ray masks. Microelectron. Eng. 1990, 11, 279–282. [Google Scholar] [CrossRef]
- Davidovic, D.; Adzic, R. Electrocatalytic and Morphological Effects of Antimony Adsorbates on Deposition of Gold. Electrochem. Acta 1988, 33, 103–108. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Pradel, A.; Ribes, M.; Record, M.C. A voltammetric study of the underpotential deposition of cobalt and antimony on gold. J. Electroanal. Chem. 2014, 724, 55–61. [Google Scholar] [CrossRef]
- Lindblom, M.; Hertz, H.M.; Holmberg, A. Pulse reverse plating for uniform nickel height in zone plates. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2006, 24, 2848–2851. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohlin, H.; Frisk, T.; Åstrand, M.; Vogt, U. Miniaturized Sulfite-Based Gold Bath for Controlled Electroplating of Zone Plate Nanostructures. Micromachines 2022, 13, 452. https://doi.org/10.3390/mi13030452
Ohlin H, Frisk T, Åstrand M, Vogt U. Miniaturized Sulfite-Based Gold Bath for Controlled Electroplating of Zone Plate Nanostructures. Micromachines. 2022; 13(3):452. https://doi.org/10.3390/mi13030452
Chicago/Turabian StyleOhlin, Hanna, Thomas Frisk, Mattias Åstrand, and Ulrich Vogt. 2022. "Miniaturized Sulfite-Based Gold Bath for Controlled Electroplating of Zone Plate Nanostructures" Micromachines 13, no. 3: 452. https://doi.org/10.3390/mi13030452
APA StyleOhlin, H., Frisk, T., Åstrand, M., & Vogt, U. (2022). Miniaturized Sulfite-Based Gold Bath for Controlled Electroplating of Zone Plate Nanostructures. Micromachines, 13(3), 452. https://doi.org/10.3390/mi13030452





